ABSTRACT
Moderation of polymorphonuclear neutrophils (PMNs) as part of a critical defense against invading pathogens may offer a promising therapeutic approach to supplement the antibiotic eradication of Pseudomonas aeruginosa infection in non-chronically infected cystic fibrosis (CF) patients. We have observed that egg yolk antibodies (IgY) harvested from White leghorn chickens that target P. aeruginosa opsonize the pathogen and enhance the PMN-mediated respiratory burst and subsequent bacterial killing in vitro. The effects on PMN phagocytic activity were observed in different Pseudomonas aeruginosa strains, including clinical isolates from non-chronically infected CF patients. Thus, oral prophylaxis with anti-Pseudomonas aeruginosa IgY may boost the innate immunity against Pseudomonas aeruginosa in the CF setting by facilitating a rapid and prompt bacterial clearance by PMNs.
Introduction
Cystic fibrosis (CF) is a hereditary disease characterized by progressive obstructive pulmonary damage accompanying high morbidity and premature death. The principal contributor is Pseudomonas aeruginosa, an opportunistic bacterial pathogen equipped with highly adaptive mechanisms that exploit the mucus-rich environment in the CF lung.Citation1 Early colonization with P. aeruginosa is a crucial determinant of disease prognosis and is associated with inferior lung function and increased morbidity and mortality.Citation2,3 The vicious nature of P. aeruginosa colonization demands constant attention to avoid chronic progression and the subsequent prolonged inflammatory response and the progressive development of P. aeruginosa-related lung disease provides a window of opportunity to eradicate the organism because CF patients become transiently infected prior to chronic infection. Several studies have emphasized the clinical benefit of employing anti-pseudomonal regimens to eradicate early infection, and antibiotic eradication therapy (AET) has substantially lowered the prevalence of P. aeruginosa in younger CF patients.Citation4,5,6,7 Thus, successful early eradication of P. aeruginosa is vital to prevent or postpone progressive chronic infection; moreover, CF patients who fail to eradicate P. aeruginosa after initial antibiotic treatment are at a higher risk of subsequent exacerbation.Citation8 However, failure of early eradication is observed in nearly 20% of cases.Citation9 Although AET does not promote antimicrobial resistance,Citation10 the subsequent aggressive chronic suppressive therapy is accompanied by the emergence of resistant bacteriaCitation11,12,13 and antibiotic-associated adverse effectsCitation14,15 and generally constitutes a substantive treatment burden for patients. Consequently, it would be favorable to complement antibiotics with other therapies to reduce colonization of P. aeruginosa in the airways of CF patients.
Passive immunotherapy is considered a valuable supplement to standard therapy against infectious diseases.Citation16 Egg yolk antibodies (IgY) targeting P. aeruginosa are proposed as such a complement because prophylactic oral treatment (gargling) with anti-Pseudomonas aeruginosa IgY antibodies reduces chronic colonization with P. aeruginosa in CF.Citation17
IgY antibodies originate from egg yolk and represent the avian homolog of mammalian IgG.Citation18 IgY is the predominant serum immunoglobulin in chickens (Gallus domesticus) and is generated to provide their offspring with an effective humoral immunity during maturation of the immune system. IgY is synthesized continuously and accumulates in the egg yolk after translocation from the blood.Citation19 By immunizing chickens with specific antigens, it is possible to purify high yields of antigen-specific antibodies in the egg yolk.Citation20,21 In addition to being amenable to rapid and effortless production methods, IgY antibodies offer some advantages over mammalian IgG antibodies.Citation22 These favorable properties of IgY antibodies offer a broad range of applications including therapeutic usages.
The mechanism of action of IgY is not yet completely understood, and the clinical impact of oral IgY prophylaxis on P. aeruginosa colonization demands further clarification. It is hypothesized that IgY interferes with bacterial colonization in the oropharynx by acting as an anti-adherence factor by inhibiting the interaction between P. aeruginosa and the epithelial lining. Indeed, an in vitro study demonstrated the adherence-obstructive ability of IgY.Citation23 In addition, IgY acts as a bacterial neutralizer through its strong reactivity with the immunogenic and virulent flagellar part of P. aeruginosa.Citation24 These mechanisms are thought to prevent P. aeruginosa colonization of the oropharynx, thus avoiding the establishment of P. aeruginosa infection and its dominance in the lungs.
Because effective host defense against bacterial lung infections relies on the clearance of pathogens in the airways by alveolar macrophages or recruited PMNs,Citation25 therapeutic approaches to maintain and augment their critical role in antibacterial defense are intriguing. An in vitro study demonstrated that IgY has bacterial opsonizing properties that improve bacterial phagocytosis.Citation26 Thus, the clinical impact of IgY prophylaxis is perhaps attributable to immunomodulatory mechanisms that enhance phagocytic activity and subsequent bacterial clearance in the airways. To further explore the plausible opsonizing capacity of anti-Pseudomonas aeruginosa IgY antibodies, in vitro assays evaluating the activity of phagocytes exposed to various strains of P. aeruginosa were established.
Results
IgY antibodies increase the respiratory burst from phagocytizing PMNs
The respiratory burst assay explored the level of ROS generated by PMNs during phagocytosis of PA vaccine-strains () and clinical isolates from CF patients (). The anti-Pseudomonas aeruginosa IgY antibodies (S-IgY) augmented the chemiluminescence from PMNs that phagocytized PA vaccine-strains in a concentration-dependent manner. Except for strain PAO3, the lowest antibody concentration tested (0.5%) significantly increased the chemiluminescence compared to the PBS control (p<0.05). The non-specific IgY (C-IgY) also increased the chemiluminescence, although to a lesser extent than did S-IgY. In all strains aside from strains PAO3 and PAO9, there was a significant increase in chemiluminescence when the lowest concentration of S-IgY was added compared to when C-IgY was added (p < 0.05); however, all the assayed PA strains demonstrated elevated chemiluminescence when higher concentrations of IgY were added.
Figure 1. Chemiluminescence counts reflecting reactive oxygen species (ROS) production from PMNs challenged with Pseudomonas aeruginosa vaccine strains (PAO1, PAO3, PAO5, PAO6, PAO9, and PAO11). The chemiluminescence, given in counts per second (CPS), was measured using luminol in a microplate fluorescence reader (Wallac 1420 Victor2, Perkin Elmer) over 60 minutes of phagocytosis. Each panel shows the peak CPS ± SEM of experiments run in duplicate from 3 different donors. *Lowest concentration of S-IgY to give a significant increase in respiratory burst compared to the control (p < 0.05)
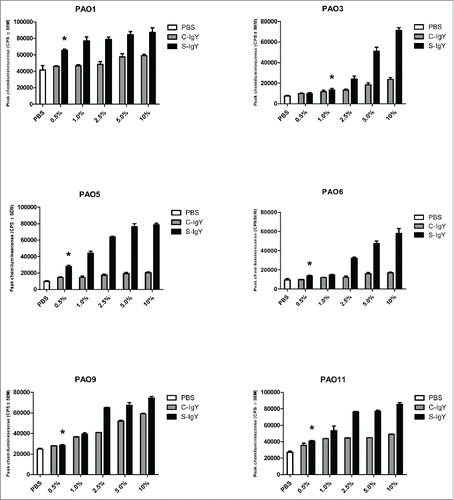
Figure 2. Chemiluminescence counts reflecting reactive oxygen species (ROS) production from PMNs challenged with non-mucoid Pseudomonas aeruginosa clinical strains (516/08, 5762C/07, 39014/06, 40542A/07, and 40542B/07) isolated from 4 non-chronically infected CF patients and a flagella mutant (PAO1172 TTN 183). The chemiluminescence, given in counts per second (CPS), was measured using luminol in a microplate fluorescence reader (Wallac 1420 Victor2, Perkin Elmer) over 60 minutes of phagocytosis. Each panel shows the peak CPS ± SEM of experiments run in duplicate from 3 different donors. *Lowest concentration of S-IgY to give a significant increase in respiratory burst compared to the control (p < 0.05)
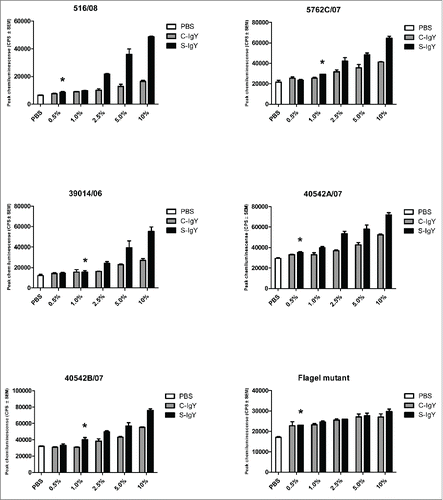
The chemiluminescences were similarly increased when the PMNs phagocytized the clinical PA strains (). In 2 of the 5 strains tested (516/08 and 40542A/07), the lowest S-IgY concentration that resulted in a significant increase in chemiluminescence was 0.5%. Likewise, C-IgY addition also increased the chemiluminescence, although generally at significantly reduced levels compared to that achieved with S-IgY addition.
IgY antibodies increase the respiratory burst from phagocytizing monocytes
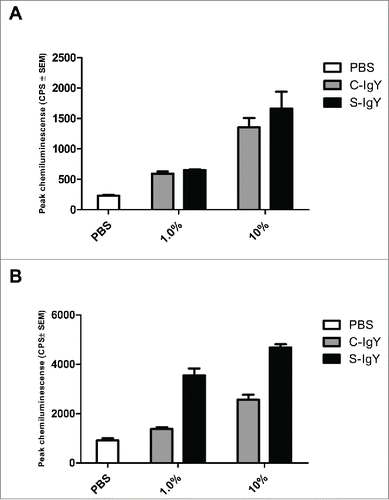
When monocytes were challenged with PAO1, the respiratory burst increased in the presence of IgY antibodies. Thus, the chemiluminescence counts were significantly elevated in the presence of both low (1% IgY) and high (10% IgY) concentrations compared to counts in control samples (). However, when the bacterial concentration was similar to that of the PMNs, there was no significant difference between S-IgY and C-IgY opsonization (). Conversely, when the monocytes phagocytized bacteria that were present at a higher concentration (with a monocyte-to-bacteria ratio of 1:10), S-IgY opsonization caused a significantly higher burst level compared to that caused by C-IgY opsonization.
Figure 3. Chemiluminescence counts reflecting reactive oxygen species (ROS) production from monocytes challenged with the PAO1 vaccine strain. (A) depicts the respiratory burst level from monocytes phagocytizing 107 CFU/ml PAO1, which corresponds to a cell-to-bacteria ratio of 1:1. (B) shows the burst level from monocytes phagocytizing 108 CFU/ml PAO1, which corresponds to a cell-to-bacteria ratio of 1:10. The chemiluminescence, given in counts per second (CPS), was measured using luminol in a microplate fluorescence reader (Wallac 1420 Victor2, Perkin Elmer) over 60 minutes of phagocytosis. Each panel shows the peak CPS ± SEM of experiments run in duplicate from 3 different donors
IgY antibodies increase PMN-mediated bacterial killing
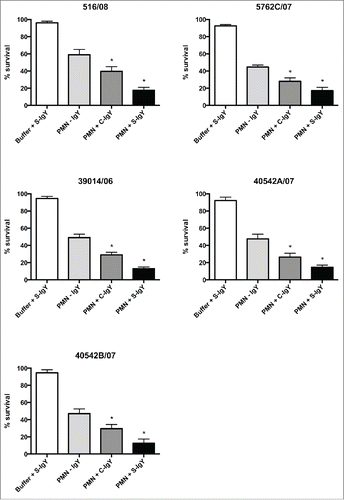
The bacterial killing assay explored the percentage of the initial inoculum of PA killed after 60 min of PMN-mediated phagocytosis (). The efficacy of IgY in increasing bacterial killing varied between the strains. S-IgY reduced the bacterial viability by 70% (PAO5) to 89% (PAO6) of the viability in non-IgY samples. S-IgY was similarly superior to C-IgY in its ability to reduce bacterial viability; S-IgY reduced bacterial viability by 50% (PAO3) to 79% (PAO6) compared to viability in samples with C-IgY. The non-pathogen-specific C-IgY increased bacterial killing by 26% (PAO5) to 69% (PAO1) compared to that in non-IgY samples. In the flagella mutant strain, there was no difference in bacterial killing between the S-IgY, C-IgY or non-IgY groups. When isolated PMNs were allowed to phagocytize clinical PA strains isolated from CF patients, the bacterial killing was likewise augmented by IgY (); however, the efficacy varied between the vaccine strains. S-IgY increased the percentage of bacterial killing in all the 5 strains tested compared to non-IgY and C-IgY. The bacterial viability was 62% (5762C/07) to 73% (40542A/07) lower in S-IgY samples compared to that in non-IgY control samples and reduced by 39% (5762C/07) to 58% (40542A/07) in S-IgY samples compared to in C-IgY samples. The presence of C-IgY also increased the PMN-mediated bacterial killing, and the bacterial viability was reduced by 33% (516/08) to 44% (39014/06) compared to that in non-IgY controls.
Figure 4. PMN-mediated bacterial killing depicted as the percentage of viable bacteria after 60 minutes of phagocytosis. PMNs were challenged with Pseudomonas aeruginosa vaccine strains (PAO1, PAO3, PAO5, PAO6, PAO9, and PAO11) and a flagella mutant (PAO1172 TTN 183), and the number of viable bacteria was determined by colony counting the next day. Each panel shows the % survival ± SEM of experiments run in duplicates. Significance was tested by one way ANOVA test with * representing comparison with PMN and non-IgY (-IgY) with p value < 0.05
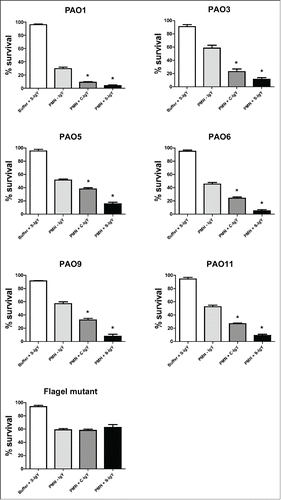
Figure 5. PMN-mediated bacterial killing depicted as the percentage of viable bacteria after 60 minutes of phagocytosis. PMNs were challenged with Pseudomonas aeruginosa clinical strains, and the number of viable bacteria was determined by colony counting the next day. Each panel shows the % survival ± SEM of experiments run in duplicates. Significance was tested by one way ANOVA test with * representing comparison with PMN and non-IgY (-IgY) with p value < 0.05
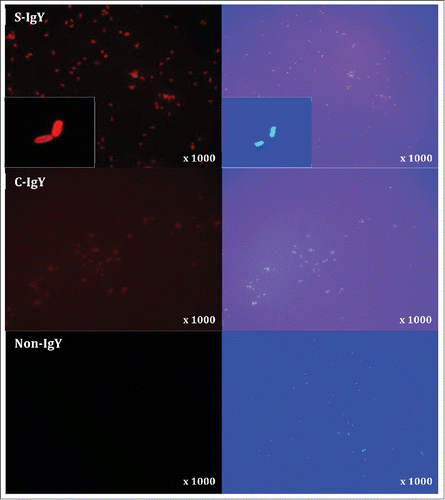
Immunofluorescent microscopy visualizes the IgY-mediated opsonization of Pseudomonas aeruginosa
The opsonization of P. aeruginosa (PAO1) with S-IgY antibodies was visualized by immunofluorescence microscopy (). PAO1 was initially incubated with S-IgY and subsequently stained with Texas Red-conjugated rabbit anti-chicken IgG secondary antibody, which allowed the IgY antibodies to fluoresce red. In the images, IgY antibodies appeared to enfold the entire bacteria; the subsequent DAPI stain confirmed the presence of bacteria. The IgY antibodies appeared to attach to the bacteria, thus substantiating the opsonizing properties of the egg yolk immunoglobulin. Control images visualize the lacking fluorescence of non-IgY and the reduced fluorescence intensity of C-IgY opsonized bacteria, which denotes a significant cross-reactivity of the immunoglobulin and a possible non-specific effect.
Discussion
Promising data on the potential use of pathogen-specific IgY as a prophylactic passive immunotherapy to protect against infectious diseases are currently accumulating. Treatment and prevention of infections in various animal species with IgY have been assessed in several studies.Citation27 Human clinical studies on the efficacy of prophylactic IgY therapy have primarily targeted gastrointestinal infections. Oral pathogens causing dental caries,Citation28 periodontitisCitation29 and oral candidiasisCitation30 have been successfully reduced with pathogen-specific IgY. Infectious diseases caused by important gastrointestinal pathogens like Helicobacter pyloriCitation31 and rotavirusCitation32 are diminished clinically with oral passive IgY immunotherapy. Although IgY antibodies are fairly sensitive to the gastric pH and hydrolysis by gastric enzymes, IgY seems to keep its immunological function during the passage from the stomach to the small intestine,Citation33 which together with the effortless oral administration of IgY favors pursuing gastrointestinal pathogens. In the CF setting, however, IgY must exert its protective effect in the upper airways, which is accomplished by gargling an aqueous IgY solution.Citation34 The stability and protective effect of IgY prophylaxis in airway compartments seem to be adequate, as IgY antibodies targeting influenza virus in murine lung models are promising.Citation35,36 Accordingly, oral prophylaxis with anti-Pseudomonas aeruginosa IgY antibodies may assist antibiotics in reducing colonization of P. aeruginosa in the oropharynx.Citation17
The potential IgY-mediated effect on PMN action is the focus of the present in vitro studies. An effective host response in the lungs allows rapid clearance of the microorganism from the airways. PMN phagocytosis is the fundamental determinant of an appropriate innate immune response to combat invading bacterial pathogens, and its malfunction causes excessive bacterial colonization and ultimately parenchymal infection advancing to sepsis.
When PMNs were allowed to phagocytize 6 different P. aeruginosa vaccine strains in the presence of specific IgY antibodies, the respiratory burst produced by PMNs during phagocytosis was significantly increased (). The specific IgY-mediated increase in respiratory burst was accompanied by augmented PMN-facilitated bacterial killing (). Intense reactivity of IgY to the P. aeruginosa vaccine strains is somewhat expected because the vaccinated chickens develop a competent humoral immune response. However, when PMNs were challenged with clinical P. aeruginosa strains isolated from non-chronically infected CF patients in the presence of IgY antibodies, the augmented PMN-mediated respiratory burst () and subsequent bacterial killing () were sustained, probably caused by a comprehensive activity of the polyclonal antibody. Thus, in vitro specific IgY appears to enhance the phagocytic competence of PMNs and consequently results in a more rapid bacterial clearance. Although IgY antibodies are polyclonal and recognize various bacterial antigens, these properties of IgY are not observed with a flagella mutant, which is in line with the previously observed strong reactivity of IgY to flagellin.Citation24 Addition of IgY increases the respiratory burst of monocytes challenged with PAO1 (); however, the burst is minimal compared to observed levels from PMNs, implying that monocytes/macrophages play a minor role in phagocytosing bacteria – rather, the cells are probably crucial for recruiting PMNs during infection.Citation37
Invading bacterial pathogens are opsonized by specific IgG antibodies, priming the bacteria to be killed by competent phagocytic cells through Fc-receptor mediated engulfment.Citation38 The immunofluorescence images in reveal that specific IgY antibodies bind to P. aeruginosa, suggesting an opsonizing mechanism. However, IgY antibodies do not activate Fc-receptors, so the observed IgY-enhanced PMN phagocytosis is possibly not triggered by the conventional receptor-mediated engulfment of opsonized bacteria. Indeed, the improved phagocytosis may be due to recognition of IgY by receptors resembling avian IgY receptorsCitation39 or non-receptor-mediated mechanisms like alterations in physio-chemical conditions of the bacteria, facilitating a more effortless and rapid phagocytosis.
The use of isolated PMNs from healthy donors may be controversial in the setting of CF. The CFTR protein is present in human PMNs, and its dysfunction in CF may attenuate phagocytic competence.Citation40 Various aggravating mechanisms have been proposed, for instance, reduced phagocytic priming of PMNs recruited to the lungs,Citation41 defective production of intraphagosomal bactericidal hypochlorous acid (HOCl)Citation42 or the presence of high levels of human neutrophil peptides in sputum, which counteracts the phagocytic activity of PMNs in a negative feedback manner.Citation43 Nevertheless, the observed reduced phagocytic activity of CF PMNs is controversial, and while the ROS production by murine CFTR deficient alveolar macrophages is attenuated,Citation44 the intrinsic production of ROS by PMNs derived from CF patients is normal.Citation45 Interestingly, the imperative IgG receptor Fcγ RIII (CD16) is downregulated in PMNs isolated from CF sputum,Citation44 suggesting that non-Fc receptor-mediated phagocytosis by CF PMNs may be important. Thus, the effect of specific IgY antibodies on the phagocytic competence of PMNs from healthy donors may be even more prominent in CF conditions because of the possible diminished bactericidal activity of PMNs from CF patients.
In CF, the devastating respiratory decline is caused by the returning colonization by microbial pathogens and subsequent inflammation, creating a deteriorating vicious circle. Chronic infection with P. aeruginosa is an important contributor and is complemented by an irrational activity of PMNs that promotes pulmonary insult. Therapeutic approaches to moderate the critical role of PMNs in the defense against invading pathogens are intriguing, especially at an early stage of disease prior to the unsuitable accelerated activity of PMNs. Thus, in non-chronically infected CF patients, the entering pathogens may be promptly cleared by PMNs in the presence of IgY antibodies by the observed IgY-mediated enhancement of PMN phagocytosis. The previously described mechanism of action of IgY as a bacterial neutralizer with anti-adherence properties seems plausible given the efficacy of IgY in preventing epithelial-related infections, e.g. influenza. Nonetheless, the present in vitro results imply that the opsonizing capacity of IgY and enhanced phagocytosis of P. aeruginosa are fundamental to the observed clinical impact of anti-pseudomonas aeruginosa IgY.
Materials and methods
Anti-Pseudomonas aeruginosa IgY
Solution containing specific (S-IgY) or non-specific (C-IgY) IgY antibodies was purchased from ImmunSystem I.M.S. (Uppsala, Sweden). The anti-Pseudomonas aeruginosa IgY antibodies employed in these experiments were harvested from egg yolks of White leghorn chickens immunized with 6 different Pseudomonas aeruginosa O-antigen strains (O1, O3, O5, O6, O9, and O11) and manufactured according to the standard procedure described by Kollberg et al.Citation46 producing doses of 70 ml volume containing 50 mg IgY. Control IgY (C-IgY) (Immunsystem I.M.S. AB, Uppsala, Sweden) originates from naive (non-immunized) chickens and serves as chicken IgY polyclonal isotype control and specifically tested not to cross react with P. aeruginosa.
Pseudomonas aeruginosa strains
The vaccine strains (PAO1, PAO3, PAO5, PAO6, PAO9 and PAO11) were kindly provided by the manufacturer of the IgY antibodies (ImmunSystem I.M.S.) and grown in LB broth. Subsequently, the flagella mutant strain (172 TTN 183) and clinical strains (40542A/07, 40542B/07, 39014/06, 516/08, and 5762C/07) isolated from sputum samples of non-chronically infected CF patients were propagated in LB broth. Stationary phase organisms were obtained from overnight cultures at 37°C. Exponential phase bacteria were from a dilution of an overnight culture in fresh LB broth, shaken at 37°C for 2 h.
Neutrophil isolation
Full blood from normal human volunteers was used for all the experiments. All donors were physically well and not taking any medication. Informed consent was obtained from each donor and guidance for the approval process for the study was received from the local ethics committee for research in human subjects in accordance with the Declaration of Helsinki.
Erythrocytes were allowed to sediment in 5% dextran solution, and the leukocyte-enriched plasma was layered on Lymphoprep (Axis-Shield, Norway) and centrifuged. After hypertonic lyses, the purified PMNs were resuspended in Krebs-Ringer buffer supplemented with 10 mM glucose and 10% (v/v) normal human AB positive sera at a density of 107 PMNs/ml.
Monocyte isolation
Monocytes were isolated from human peripheral blood mononuclear cells (PBMC) using magnetic cell separation (MACS) and the principles of negative selection (depletion of non-monocytes). PBMC were isolated from human whole blood by density gradient centrifugation using Ficoll-Paque. After wash steps, including removal of platelets, the PBMC were resuspended in buffer (PBS + 2% fetal bovine serum) and applied to magnetic labeling followed by magnetic separation according to the manufacturer's protocol (Miltenyi Biotec Inc., Auburn, USA). The purity of monocytes as evaluated by FACS was on average 85%.
Respiratory burst assay
The production of reactive oxygen species (ROS) during oxidative burst was measured by luminol-enhanced chemiluminescence. Aliquots of isolated PMNs (1 × 107 cells/ml) or monocytes (1 × 107 cells/ml) were added to each well in a microtiter plate (Nunc). Phagocytosis was commenced by adding P. aeruginosa (108 CFU/ml) to the wells containing PMNs in the presence of C-IgY or S-IgY at various concentrations. Lastly, luminol (30 nM) was added to each well, and the luminol-enhanced chemiluminescence indicating the presence of phagocyte-derived reactive oxygen species (ROS) was measured using a luminometer (Wallac 1420 Victor2, Perkin Elmer) at 37°C for 1 hour.
Bactericidal assay
To evaluate PMN-mediated bacterial killing, the loss of bacterial viability over time was measured by mixing non-IgY-opsonized bacteria and PMNs, plating diluted samples, incubating overnight, and counting bacterial colonies. P. aeruginosa bacteria of the different strains were washed and diluted in Krebs-Ringer Buffer supplemented with a final concentration of 10 mM glucose and with 10% human AB positive serum +/− S-IgY or C-IgY, such that the bacteria were present at a concentration of 5 × 107 CFU/ml. The mixture was rotated at 120 rpm for 30 min at 37°C, and a suspension of PMNs was added to give a final bacterium-to-PMN ratio of 5 to 1. Serum was added to bring the final concentration of serum to 10%. At time-points 0 and 60 min after the mixing of PMNs and bacteria, 50 µl of the reaction mixture was removed and was added to 2.45 ml H2O (pH 11), which initiated cellular lysis. The sample was incubated for 5 min before being vigorously vortexed and plated onto modified Conradi-Drigalski plates, which were then incubated overnight at 37°C. Colony counts were performed the next day to determine the PMN-mediated bacterial killing by comparing the loss of bacterial viability during 60 min of phagocytosis.
Indirect immunofluorescence microscopy
The P. aeruginosa PAO1 strain was grown overnight in LB broth and washed twice in PBS. After standardization and re-suspension in PBS, 30-µl aliquots were applied on microscope slides and routinely fixed in formalin, then blocked with PBS with 1% bovine albumin serum (BAS) for 10 min and incubated with S-IgY at 1:200, 1:400 and 1:800 dilutions with 1% BAS overnight at 4°C. After washing twice with PBS, Texas Red-conjugated rabbit anti-chicken IgG secondary antibody (Abcam plc, Cambridge, UK; diluted 1:500 with PBS with 1% BAS), was added. The cells were then incubated at 37°C for 1 h on a platform rocker. Slides were washed twice with PBS and air-dried, then stained with a drop of DAPI stain and mounted with a coverslip. Immunofluorescent micrographs of specimens were obtained using a confocal laser scanning microscope (Zeiss Imager.Z2, LSM 710 CLSM) and the accompanying software (Zeiss Zen 2010 v. 6.0, Zeiss, Germany).
Statistics
Chemiluminescence data were logged automatically into Microsoft Excel. The software GraphPad Prism (version 5.00) was used for statistical analyses. The non-parametric Mann-Whitney U-test was used to determine differences between groups when the D'Agostino-Pearson test demonstrated that not all datasets were normally distributed. In case of normally distributed data sets, the parametric t-test was applied. One-way ANOVA test was employed to determine significant differences between groups. A level of p≤0.05 was used for assigning statistical significance.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by the European Union FP7 support for the IMPACTT Project (Grant agreement no: 261095).
References
- Koch C, Høiby N. Pathogenesis of cystic fibrosis. Lancet 1993 Apr 2,4; 341(8852):1065–9; http://dx.doi.org/10.1016/0140-6736(93)92422-P
- Schaedel C, de Monestrol I, Hjelte L, Johannesson M, Kornfält R, Lindblad A, Strandvik B, Wahlgren L, Holmberg L. Predictors of deterioration of lung function in cystic fibrosis. Pediatr Pulmonol 2002 Jun; 33(6):483–91; PMID:12001283; http://dx.doi.org/10.1002/ppul.10100
- Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 2002 Aug; 34(2):91–100; PMID:12112774; http://dx.doi.org/10.1002/ppul.10127
- Frederiksen B, Koch C, Høiby N. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr Pulmonol 1997 May; 23:330–5; PMID:9168506; http://dx.doi.org/10.1002/(SICI)1099-0496(199705)23:5%3c330::AID-PPUL4%3e3.0.CO;2-O
- Hansen CR, Pressler T, Høiby N. Early aggressive eradication therapy for intermittent Pseudomonas aeruginosa airway colonization in cystic fibrosis patients: 15 years experience. J Cyst Fibros 2008 Nov; 7:523–30; PMID:18693078; http://dx.doi.org/10.1016/j.jcf.2008.06.009
- Taccetti G, Campana S, Festini F, Mascherini M, Döring G. Early eradication therapy against Pseudomonas aeruginosa in cystic fibrosis patients. Eur Respir J 2005 Sep; 26:458–61; PMID:16135728; http://dx.doi.org/10.1183/09031936.05.00009605
- Gibson RL, Emerson J, McNamara S, Burns JL, Rosenfeld M, Yunker A, Hamblett N, Accurso F, Dovey M, Hiatt P, et al. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am J Respir Crit Care Med 2003 Mar 15; 167:841–9; http://dx.doi.org/10.1164/rccm.200208-855OC
- Döring G, Flume P, Heijerman H, Elborn JS, Consensus Study Group. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros 2012 Dec; 11:461–79; PMID:23137712; http://dx.doi.org/10.1016/j.jcf.2012.10.004
- Mayer-Hamblett N, Kronmal RA, Gibson RL, Rosenfeld M, Retsch-Bogart G, Treggiari MM, Burns JL, Khan U, Ramsey BW, EPIC Investigators. Initial Pseudomonas aeruginosa treatment failure is associated with exacerbations in cystic fibrosis. Pediatr Pulmonol 2012 Feb; 47:125–34; PMID:21830317; http://dx.doi.org/10.1002/ppul.21525
- Ho SA, Lee TW, Denton M, Conway SP, Brownlee KG. Regimens for eradicating early Pseudomonas aeruginosa infection in children do not promote antibiotic resistance in this organism. J Cyst Fibros 2009 Jan; 8:43–6; PMID:18829398; http://dx.doi.org/10.1016/j.jcf.2008.08.001
- Bosso JA, Allen JE, Matsen JM. Changing susceptibility of Pseudomonas aeruginosa isolates from cystic fibrosis patients with the clinical use of newer antibiotics. Antimicrob Agents Chemother 1989 Apr; 33:526–8; PMID:2499252; http://dx.doi.org/10.1128/AAC.33.4.526
- Mouton JW, den Hollander JG, Horrevorts AM. Emergence of antibiotic resistance amongst Pseudomonas aeruginosa isolates from patients with cystic fibrosis. J Antimicrob Chemother 1993 Jun; 31:919–26; PMID:8360129; http://dx.doi.org/10.1093/jac/31.6.919
- Ciofu O, Giwercman B, Pedersen SS, Høiby N. Development of antibiotic resistance in Pseudomonas aeruginosa during two decades of antipseudomonal treatment at the Danish CF Center. APMIS 1994 Sep; 102:674–80.
- Prayle A, Watson A, Fortnum H, Smyth A. Side effects of aminoglycosides on the kidney, ear and balance in cystic fibrosis. Thorax 2010 Jul; 65:654–8.; http://dx.doi.org/10.1136/thx.2009.131532
- Parmar JS, Nasser S. Antibiotic allergy in cystic fibrosis. Thorax 2005 Jun; 60:517–20; PMID:15923254; http://dx.doi.org/10.1136/thx.2004.027953
- Keller MA, Stiehm ER. Passive immunity in prevention and treatment of infectious diseases. Clin Microbiol Rev 2000 Oct; 13:602–14; PMID:11023960; http://dx.doi.org/10.1128/CMR.13.4.602-614.2000
- Nilsson E, Larsson A, Olesen HV, Wejåker PE, Kollberg H. Good effect of IgY against Pseudomonas aeruginosa infections in cystic fibrosis patients. Pediatr Pulmonol 2008 Sep; 43:892–9; PMID:18680179; http://dx.doi.org/10.1002/ppul.20875
- Warr GW, Magor KE, Higgins DA. IgY: clues to the origins of modern antibodies. Immunol Today 1995 Aug; 16:392–8; PMID:7546196; http://dx.doi.org/10.1016/0167-5699(95)80008-5
- Patterson R, Youngner JS, Weigle WO, Dixon FJ. Antibody production and transfer to egg yolk in chickens. J Immunol 1962 Aug; 89:272–8; PMID:14484407
- Rose ME, Orlans E, Buttress N. Immunoglobulin classes in the hen's egg: their segregation in yolk and white. Eur J Immunol 1974 Jul; 4:521–3; PMID:4213170; http://dx.doi.org/10.1002/eji.1830040715
- Bhanushali JK, Gilbert JM, McDougald LR. Simple method to purify chicken immunoglobulin G. Poult Sci 1994 Jul; 73:1158–61; PMID:7937478; http://dx.doi.org/10.3382/ps.0731158
- Dias da Silva W, Tambourgi DV. IgY: a promising antibody for use in immunodiagnostic and in immunotherapy. Vet Immunol Immunopathol 2010 Jun 15; 135:173–80; PMID:20083313; http://dx.doi.org/10.1016/j.vetimm.2009.12.011
- Carlander D, Sundstrom J, Berglund A, Larsson A, Wretlind B, Kollberg H. Immunoglobulin Y(IgY)-A new tool for the prophylaxis against Pseudomonas aeruginosa in cystic fibrosis patients. Pediatr Pulmonol Suppl 1999; 19:241.
- Nilsson E, Amini A, Wretlind B, Larsson A. Pseudomonas aeruginosa infections are prevented in cystic fibrosis patients by avian antibodies binding Pseudomonas aeruginosa flagellin. J Chromatogr B Analyt Technol Biomed Life Sci 2007; 856:75–80; PMID:17581799; http://dx.doi.org/10.1016/j.jchromb.2007.05.029
- Jensen PØ, Moser C, Kobayashi O, Hougen HP, Kharazmi A, Høiby N. Faster activation of polymorphonuclear neutrophils in resistant mice during early innate response to Pseudomonas aeruginosa lung infection. Clin Exp Immunol 2004 Sep; 137:478–85.; http://dx.doi.org/10.1111/j.1365-2249.2004.02554.x
- Zhen YH, Jin LJ, Guo J, Li XY, Li Z, Fang R, Xu YP. Characterization of specific egg yolk immunoglobulin (IgY) against mastitis-causing Staphylococcus aureus. J Appl Microbiol 2008 Nov; 105:1529–35; PMID:19146490; http://dx.doi.org/10.1111/j.1365-2672.2008.03920.x
- Xu Y, Li X, Jin L, Zhen Y, Lu Y, Li S, You J, Wang L. Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: a review. Biotechnol Adv 2011 Nov–Dec; 29:860–8; PMID:21787857; http://dx.doi.org/10.1016/j.biotechadv.2011.07.003
- Hatta H, Tsuda K, Ozeki M, Kim M, Yamamoto T, Otake S, Hirasawa M, Katz J, Childers NK, Michalek SM. Passive immunization against dental plaque formation in humans: effect of a mouth rinse containing egg yolk antibodies (IgY) specific to Streptococcus mutans. Caries Res 1997; 31:268–74; PMID:9197932; http://dx.doi.org/10.1159/000262410
- Yokoyama K, Sugano N, Shimada T, Shofiqur RA, Ibrahim el-SM, Isoda R, Umeda K, Sa NV, Kodama Y, Ito K. Effects of egg yolk antibody against Porphyromonas gingivalis gingipains in periodontitis patients. J Oral Sci 2007 Sep; 49:201–6; http://dx.doi.org/10.2334/josnusd.49.201
- Takeuchi S, Motohashi J, Kimori H, Nakagawa Y, Tsurumoto A. Effects of oral moisturising gel containing egg yolk antibodies against Candida albicans in older people. Gerodontology 2014; http://dx.doi.org/10.1111/ger.12139
- Horie K, Horie N, Abdou AM, Yang JO, Yun SS, Chun HN, Park CK, Kim M, Hatta H. Suppressive effect of functional drinking yogurt containing specific egg yolk immunoglobulin on Helicobacter pylori in humans. J Dairy Sci 2004 Dec; 87:4073–9; PMID:15545368; http://dx.doi.org/10.3168/jds.S0022-0302(04)73549-3
- Rahman S, Higo-Moriguchi K, Htun KW, Taniguchi K, Icatlo FC Jr, Tsuji T, Kodama Y, Van Nguyen S, Umeda K, Oo HN, et al. Randomized placebo-controlled clinical trial of immunoglobulin Y as adjunct to standard supportive therapy for rotavirus-associated diarrhea among pediatric patients. Vaccine 2012 Jun 29; 30:4661–9; PMID:22575165; http://dx.doi.org/10.1016/j.vaccine.2012.04.091
- Yokoyama H, Peralta RC, Sendo S, Ikemori Y, Kodama Y. Detection of passage and absorption of chicken egg yolk immunoglobulins in the gastrointestinal tract of pigs by use of enzyme-linked immunosorbent assay and fluorescent antibody testing. Am J Vet Res 1993 Jun; 54:867–72; PMID:8323054
- Carlander D, Kollberg H, Larsson A. Retention of specific yolk IgY in the human oral cavity. BioDrugs 2002; 16:433–7; PMID:12463766; http://dx.doi.org/10.2165/00063030-200216060-00004
- Wallach MG, Webby RJ, Islam F, Walkden-Brown S, Emmoth E, Feinstein R, Gronvik KO. Cross-protection of chicken immunoglobulin Y antibodies against H5N1 and H1N1 viruses passively administered in mice. Clin Vaccine Immunol 2011 Jul; 18:1083–90; PMID:21613458; http://dx.doi.org/10.1128/CVI.05075-11
- Nguyen HH, Tumpey TM, Park HJ, Byun YH, Tran LD, Nguyen VD, Kilgore PE, Czerkinsky C, Katz JM, Seong BL, et al. Prophylactic and therapeutic efficacy of avian antibodies against influenza virus H5N1 and H1N1 in mice. PLoS One 2010 Apr 13; 5:e10152; PMID:20405007; http://dx.doi.org/10.1371/journal.pone.0010152
- Cheung DO, Halsey K, Speert DP. Role of pulmonary alveolar macrophages in defense of the lung against Pseudomonas aeruginosa. Infect Immun 2000 Aug; 68:4585–92; PMID:10899859; http://dx.doi.org/10.1128/IAI.68.8.4585-4592.2000
- Anderson CL, Shen L, Eicher DM, Wewers MD, Gill JK. Phagocytosis mediated by three distinct Fc gamma receptor classes on human leukocytes. J Exp Med 1990 Apr 1; 171:1333–45; http://dx.doi.org/10.1084/jem.171.4.1333
- Schreiner B, Viertlboeck BC, Göbel TW. A striking example of convergent evolution observed for the ggFcR:IgY interaction closely resembling that of mammalian FcR:IgG. Dev Comp Immunol 2012 Mar; 36:566–71; PMID:21986582; http://dx.doi.org/10.1016/j.dci.2011.09.013
- Alexis NE, Muhlebach MS, Peden DB, Noah TL. Attenuation of host defense function of lung phagocytes in young cystic fibrosis patients. J Cyst Fibros 2006 Jan; 5:17–25. Epub 2005 Dec 13; http://dx.doi.org/10.1016/j.jcf.2005.11.001
- Morris MR, Doull IJ, Dewitt S, Hallett MB. Reduced iC3b-mediated phagocytotic capacity of pulmonary neutrophils in cystic fibrosis. Clin Exp Immunol 2005 Oct; 142:68–75; http://dx.doi.org/10.1111/j.1365-2249.2005.02893.x
- Painter RG, Valentine VG, Lanson NA Jr, Leidal K, Zhang Q, Lombard G, Thompson C, Viswanathan A, Nauseef WM, Wang G, et al. CFTR Expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry 2006 Aug 29; 45:10260–9; PMID:16922501; http://dx.doi.org/10.1021/bi060490t
- Voglis S, Quinn K, Tullis E, Liu M, Henriques M, Zubrinich C, Peñuelas O, Chan H, Silverman F, Cherepanov V, et al. Human neutrophil peptides and phagocytic deficiency in bronchiectatic lungs. Am J Respir Crit Care Med 2009 Jul 15; 180:159–66; PMID:19406984; http://dx.doi.org/10.1164/rccm.200808-1250OC
- Del Porto P, Cifani N, Guarnieri S, Di Domenico EG, Mariggi∫ MA, Spadaro F, Guglietta S, Anile M, Venuta F, Quattrucci S, et al. Dysfunctional CFTR alters the bactericidal activity of human macrophages against Pseudomonas aeruginosa. PLoS One 2011; 6:e19970; PMID:21625641; http://dx.doi.org/10.1371/journal.pone.0019970
- McKeon DJ, Cadwallader KA, Idris S, Cowburn AS, Pasteur MC, Barker H, Haworth CS, Bilton D, Chilvers ER, Condliffe AM. Cystic fibrosis neutrophils have normal intrinsic reactive oxygen species generation. Eur Respir J 2010 Jun; 35:1264–72; PMID:19840964; http://dx.doi.org/10.1183/09031936.00089709
- Kollberg H, Carlander D, Olesen H, Wejåker PE, Johannesson M, Larsson A. Oral administration of specific yolk antibodies (IgY) may prevent Pseudomonas aeruginosa infections in patients with cystic fibrosis: a phase I feasibility study. Pediatr Pulmonol 2003 Jun; 35:433–40; PMID:12746939; http://dx.doi.org/10.1002/ppul.10290