ABSTRACT
The Gram positive intracellular pathogen Listeria monocytogenes represents a promising vaccine or therapeutic DNA delivery vector that has been successfully administered to humans in clinical trials. However in generating Listeria mutants with therapeutic potential it is important to balance safety attenuation with efficacy. Here we show that L. monocytogenes mutants with a reduced capacity for murine gallbladder replication are capable of stimulating T cell responses in mice and protecting vaccinated animals from secondary challenge. Mutation of L. monocytogenes genes lmo2566 or lmo0598 resulted in significant attenuation in the murine model yet mutants retained a capacity for intracellular growth and stimulation of T cell responses against key Listeria epitopes (LLO91-99 and P60217-225). Importantly the mutants showed a reduced capacity for growth in the gallbladders of vaccinated mice as well as significantly reduced faecal shedding indicating that this approach generates live Listeria-based vector delivery systems with a reduced capacity for the spread of live genetically modified microorganisms into the natural environment.
The Gram positive pathogen Listeria monocytogenes is capable of intracytoplasmic growth in a variety of mammalian cell types (including antigen-presenting cells and epithelial cells).Citation1,2 A number of features of this intracellular life-cycle recommend the use of attenuated L. monocytogenes strains as therapeutic vectors for either vaccine or nucleic acid delivery.Citation3,4 Within the host cell cytoplasm of antigen presenting cells the pathogen accesses the cytoplasmic antigen processing pathway and is therefore a potent inducer of major histocompatability complex (MHC) class I-restricted protective CD8+ T cells.Citation5,6 The ability to proliferate within the cytoplasm significantly influences the magnitude of the resultant immune response as bacteria incapable of replication in this environment demonstrate markedly lower immunogenicity.Citation7,8 In addition, the ability to spread from cell to cell is likely to influence the efficacy of nucleic acid delivery to solid tumors and other tissues.Citation4,9 The pathogen is genetically tractable and it is possible to target specific genes in order to create mutants which are attenuated but retain vaccine potential.Citation10 Live attenuated L. monocytogenes delivery vectors expressing tumor-specific antigens have been administered to humans in phase I clinical trials and are generally safe and immunogenic.Citation11,12 Indeed, a recent clinical trial utilised an attenuated L. monocytogenes strain expressing the cancer antigen mesothelin as a component of a prime-boost immunotherapy in patients with pancreatic cancer with very promising results.Citation13 However there are risks associated with the use of live attenuated Listeria in this context as evidenced by the recent report of clinical disease (listeriosis) in a patient receiving the attenuated vaccine strain ADXS11-001.Citation14
During systemic infection of mice L. monocytogenes grows readily in the gallbladderCitation15,16 and is shed into the faeces.Citation17 Interestingly, highly attenuated mutants of L. monocytogenes generated through mutation of classical virulence factors are still capable of efficient growth in the gallbladder during murine infection.Citation15 In humans the pathogen is known to be a cause of cholecystitisCitation18 and has been detected in human faeces.Citation19 We have previously examined the genetic systems required for L. monocytogenes to grow in ex vivo gallbladder bile.Citation20 Here we demonstrate that mutants in the expression of two 2 separate loci encoding proteins involved in biotin metabolism (lmo0598) and a putative lipoate protein ligase (lmo2566) are also defective for infection in mice. The attenuated mutants stimulate significant anti-listerial immune responses but have reduced potential for replication in gallbladders and demonstrate significantly reduced faecal shedding. We suggest that this represents a novel approach to create attenuated Listeria mutants which retain immunogenicity but have reduced potential for environmental spread.
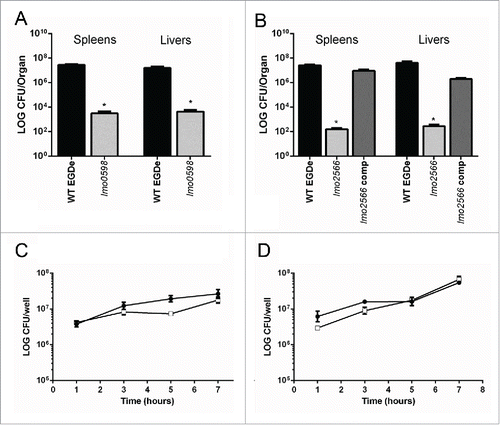
We previously utilised a transposon mutagenesis approach to isolate L. monocytogenes mutants with reduced potential for growth in porcine gallbladder bile.Citation20 We performed significant in vitro characterization of these mutants and the study indicated that particular bacterial systems for amino acid metabolism, enzymatic activity and purine metabolism are necessary for growth in this environment.Citation20 Further work demonstrated that 4 out of the 6 transposon mutants defined as deficient in growth in bile also demonstrated attenuated virulence in mice (unpublished data). Of these a mutant deficient in biotin metabolism (Tnlmo0598) demonstrated a 4-log reduction in capacity to replicate in murine liver and spleen (). As Tnlmo0598 can be chemically complemented through addition of biotin to bile or defined media we did not genetically complement this strain.Citation20 A mutant in a putative lipoate protein ligase (Tnlmo2566) demonstrated a 5-log reduction in virulence potential, a phenotype that can be restored through genetic complementation (). Both strains carry a single copy of the transposon.Citation20 Interestingly both mutants were capable of intracellular growth in an in vitro J774 macrophage assay () that utilises a standard gentamicin protection protocol as described previously.Citation21 In contrast a mutant deficient for purine metabolism (TnpurB) was incapable of growth in macrophages (unpublished data).
Figure 1. Listeriamutants are attenuated for infection of mice but retain an ability to replicate in macrophages. Listeria monocytogenes CFU from livers and spleens of infected mice 3 d post i.p. infection with 3 × 105 CFU. (A) Wild-type (EGDe) and biotin auxotroph Tnlmo0598 levels in livers and spleens. (B) Wild-type (EGDe), Tnlmo2566 and genetically complemented strain (lmo2566 Comp) in livers and spleens. *P < 0.05 by the Kruskal Wallis one way ANOVA, with post hoc comparison using Dunn's method. Differences are shown relative to the wild-type. Error bars represent the mean ± SEM (C) Intracellular growth of wild-type (•) and Tnlmo0598 (□) in J774 macrophage cells using a gentamicin protection assay. (D) Intracellular growth of wild-type (•) and Tnlmo2566 (□) in J774 macrophage cells using a gentamicin protection assay. No statistical significance observed.
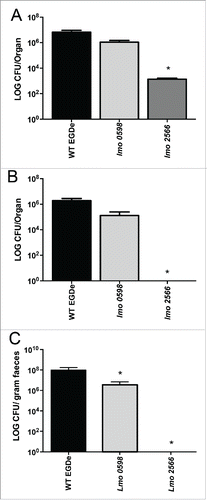
Given that the mutants were attenuated in vivo yet capable of intracellular growth we tested the ability of these strains to vaccinate mice against secondary infection (as an index of immunogenicity). Animal studies were carried out following institutional review and under license from the Irish Department of Health. Balb/c mice were vaccinated using intra-peritoneal (i.p.) inoculation with 3 × 104 CFU of wild-type L. monocytogenes EGDe (a sub-lethal dose) or 1 × 106 CFU of the attenuated strains (Tnlmo0598 or Tnlmo2566) or a sham inoculation (PBS) as a negative control (). Three days following injection of live bacteria 5 mice in each group were euthanized and analyzed for bacterial loads in the spleen, gallbladder or faeces (). The data indicate that even when utilised at a 100-fold higher dose than the wildtype, the Tnlmo2566 mutant in particular was significantly attenuated for replication in the spleen and demonstrated reduced capacity to replicate in the gallbladder and to be shed in faeces ().
Figure 2. Overview of the vaccination study. Balb/c mice received an initial primary vaccination via the i.p. route with live wild-type bacteria (3 × 104 CFU) or attenuated vaccine strains (Tnlmo0598 or Tnlmo2566) (1 × 106 CFU) at time 0. Mice received a vaccination booster on day 14 with the same vaccination doses. Sham inoculated control mice received sterile PBS. A subgroup of mice received a challenge dose of wild type (EGDe) bacteria at day 35. Subgroups of mice were analyzed at day 3 to determine the organ load of the initial vaccination dose and at day 35 for efficacy in generating T cells (by ELISPOT). Protection from secondary infection by L. monocytogenes was determined on day 37 in mice challenged with wild-type bacteria.
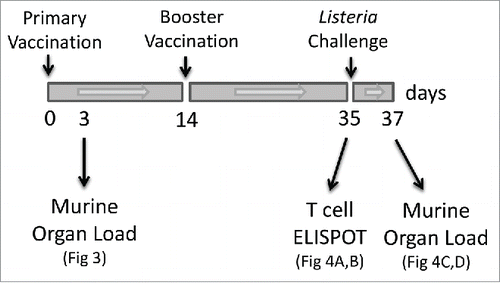
Figure 3. L. monocytogenes vaccine strains fail to significantly colonise mouse gallbladders and show reduced faecal excretion. Mice were inoculated via the i.p. route with wild-type (strain EGDe) bacteria (at 3 × 104 CFU) or attenuated vaccine strains (Tnlmo0598 or Tnlmo2566) (1 × 106 CFU) and levels in internal organs were assessed 3 d later. (A) Levels of wild-type (strain EGDe) or attenuated strains Tnlmo0598 or Tnlmo2566 in spleens of infected mice. (B) Levels of wild-type (strain EGDe) or attenuated strains Tnlmo0598 or Tnlmo2566 in gallbladders of infected mice. (C) Levels of wild-type (strain EGDe) or attenuated strains Tnlmo0598 or Tnlmo2566 in faeces of infected mice. *P < 0.05 by the Kruskal Wallis one way ANOVA, with post hoc comparison using Dunn's method. Differences are shown relative to the wild-type. Significant differences are indicated relative to the wild-type. Error bars represent the mean ± SEM.
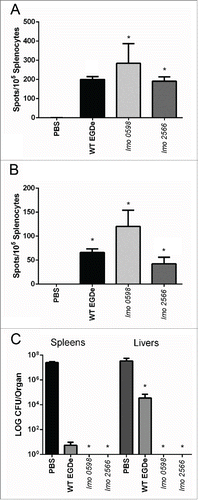
The remaining mice were administered a vaccine booster at 14 days d following the primary inoculations and at day 35 were either analyzed for T cell responsiveness using an enzyme-linked immunospot (ELISPOT) assay or were challenged with a challenge dose of wild-type L. monocytogenes EGDe to determine levels of protective immunity (). We allowed 21 d between the vaccine booster and further immunological analyses in order to eliminate the possibility of non-specific immune effects associated with administration of the booster.Citation22 We used a standard ELISPOT approach to determine the levels of interferon gamma-producing CD8+ T cells reactive to known H2-Kd restricted epitopes of the major L. monocytogenes antigens LLO and P60 (epitopes LLO91-99 and P60217-225). The assay was carried out as described previously.Citation8,23,24 The data indicated that both Tnlmo0598 and Tnlmo2566 strains were capable of eliciting a potent Listeria-specific T cell response that was comparable to that elicited by the wild-type bacterium and significantly higher than that seen in naïve (sham inoculated) mice (, B). In addition mice that had undergone the prime and boost vaccination were fully protected against challenge with wild-type L. monocytogenes EGDe ().
Figure 4. L. monocytogenes vaccine strains stimulate anti-Listeria T cells and protect mice against secondary infection. Mouse groups were vaccinated as outlined with a primary inoculation on day 0 and a booster at day 14. At day 35 mice were examined for T-cells responsive to (A) LLO91–99 or (B) P60217–225 using peptide pulsed P815-1-1 cells. *P < 0.05 by the Kruskal Wallis one way ANOVA, with post hoc comparison using Dunn's method. Significant differences are shown relative to the sham (PBS) inoculated animals. No significant differences were evident between Tnlmo0598 or Tnlmo2566 vaccinated animals. Error bars represent the mean ± SEM (C) A subgroup of vaccinated mice were challenged with L. monocytogenes wild type (EGDe) (3 × 105 CFU via the i.p. route). Mice vaccinated with wild-type (strain EGDe), attenuated strains Tnlmo0598 or Tnlmo2566 or sham-inoculated mice were analyzed for organ load 2 d post-infection. *P < 0.05 by the Kruskal Wallis one way ANOVA, with post hoc comparison using Dunn's method. Significant differences are shown for protection relative to the sham (PBS) inoculated animals. Error bars represent the mean ± SEM.
Herein we demonstrate that L. monocytogenes mutants Tnlmo0598 or Tnlmo2566, initially selected for an inability to grow in gallbladder bile,Citation20 are also attenuated for virulence during murine infection yet retain an efficient capacity for intracellular replication and stimulation of the host immune system. To our knowledge this is the first study that links reduced gallbladder replication (rather than reduced intracellular replication) to reduced virulence potential in mice. The work therefore demonstrates the importance of the gallbladder phase of infection for murine virulence. Importantly strain Tnlmo2566 in particular was undetectable in the gallbladder or faeces of mice inoculated with a high vaccinating dose (1 × 106 CFU). We propose that the inability to replicate in the gallbladder results in significantly reduced faecal excretion of the vaccine strain, a hypothesis strengthened by previous studies.Citation17 While further study is required, we suggest that a mutant strain lacking lmo2566 could have applications as a vaccine against listeriosis in domestic animals. Alternatively mutation of lmo2566 could potentially be utilised alongside mutations in key virulence factors Citation10 to enhance safety and prevent gallbladder growth in existing live therapeutic Listeria vector systems. The current work therefore represents a novel approach to biological containment of live Listeria-based vector delivery systems with reduced capacity for the spread of live genetically modified microorganisms into the natural environment.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest
Funding
The authors acknowledge the funding of the APC Microbiome Institute by the Science Foundation of Ireland Centers for Science, Engineering and Technology (CSET) program (Grant Number SFI/12/RC/2273). CGMG is in receipt of funding from the EU FP7 Industry-Academia Partnerships and Pathways (IAPP) through funding of the Vaccines and Imaging Partnership (ViP).
References
- Hamon M, Bierne H, Cossart P. Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol 2006; 4:423-434; PMID:16710323; http://dx.doi.org/10.1038/nrmicro1413
- Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev 2001; 14:584-640; PMID:11432815; http://dx.doi.org/10.1128/CMR.14.3.584-640.2001
- Shahabi V, Maciag PC, Rivera S, Wallecha A. Live, attenuated strains of Listeria and Salmonella as vaccine vectors in cancer treatment. Bioeng Bugs 2010; 1:235-243; PMID:21327055; http://dx.doi.org/10.4161/bbug.1.4.11243
- Tangney M, van Pijkeren JP, Gahan CG. The use of Listeria monocytogenes as a DNA delivery vector for cancer gene therapy. Bioeng Bugs 2010; 1:284-287; PMID:21327062; http://dx.doi.org/10.4161/bbug.1.4.11725
- Kursar M, Bonhagen K, Kohler A, Kamradt T, Kaufmann SH, Mittrucker HW. Antigen-specific CD8+ T cell responses in intestinal tissues during murine listeriosis. Microbes Infect 2004; 6:8-16; PMID:14738888; http://dx.doi.org/10.1016/j.micinf.2003.10.004
- Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol 2004; 4:812-823; PMID:15459672; http://dx.doi.org/10.1038/nri1461
- von Koenig CH, Finger H, Hof H. Failure of killed Listeria monocytogenes vaccine to produce protective immunity. Nature 1982; 297:233-234; PMID:6176874; http://dx.doi.org/10.1038/297233a0
- Bahey-El-Din M, Casey PG, Griffin BT, Gahan CG. Lactococcus lactis-expressing listeriolysin O (LLO) provides protection and specific CD8(+) T cells against Listeria monocytogenes in the murine infection model. Vaccine 2008; 26:5304-5314; PMID:18691625; http://dx.doi.org/10.1016/j.vaccine.2008.07.047
- van Pijkeren JP, Morrissey D, Monk IR, Cronin M, Rajendran S, O'Sullivan GC, Gahan CG, Tangney M. A novel Listeria monocytogenes-based DNA delivery system for cancer gene therapy. Hum Gene Ther 2010; 21:405-416; PMID:20105075; http://dx.doi.org/10.1089/hum.2009.022
- Brockstedt DG, Giedlin MA, Leong ML, Bahjat KS, Gao Y, Luckett W, Liu W, Cook DN, Portnoy DA, Dubensky TW, Jr. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci U S A 2004; 101:13832-13837; PMID:15365184; http://dx.doi.org/10.1073/pnas.0406035101
- Radulovic S, Brankovic-Magic M, Malisic E, Jankovic R, Dobricic J, Plesinac-Karapandzic V, Maciag PC, Rothman J. Therapeutic cancer vaccines in cervical cancer: phase I study of Lovaxin-C. J BUON 2009; 14 Suppl 1:S165-168; PMID:19785060
- Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine 2009; 27:3975-3983; PMID:19389451; http://dx.doi.org/10.1016/j.vaccine.2009.04.041
- Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 2015; 33:1325-1333; PMID:25584002; http://dx.doi.org/10.1200/JCO.2014.57.4244
- Sacco JJ, Evans M, Harrington KJ, Man S, Powell N, Shaw RJ, Jones TM. Systemic listeriosis following vaccination with the attenuated Listeria monocytogenes therapeutic vaccine, ADXS11-001. Hum Vaccin Immunother 2015:0. [Epub ahead of print].
- Hardy J, Francis KP, DeBoer M, Chu P, Gibbs K, Contag CH. Extracellular replication of Listeria monocytogenes in the murine gall bladder. Science 2004; 303:851-853; PMID:14764883; http://dx.doi.org/10.1126/science.1092712
- Bron PA, Monk IR, Corr SC, Hill C, Gahan CG. Novel luciferase reporter system for in vitro and organ-specific monitoring of differential gene expression in Listeria monocytogenes. Appl Environ Microbiol 2006; 72:2876-2884; PMID:16597994; http://dx.doi.org/10.1128/AEM.72.4.2876-2884.2006
- Hardy J, Margolis JJ, Contag CH. Induced biliary excretion of Listeria monocytogenes. Infect Immun 2006; 74:1819-1827; PMID:16495556; http://dx.doi.org/10.1128/IAI.74.3.1819-1827.2006
- Charlier C, Fevre C, Travier L, Cazenave B, Bracq-Dieye H, Podevin J, Assomany D, Guilbert L, Bossard C, Carpentier F, et al. Listeria monocytogenes-associated biliary tract infections: a study of 12 consecutive cases and review. Medicine (Baltimore) 2014; 93:e105; PMID:25319439; http://dx.doi.org/10.1097/MD.0000000000000105
- Gahan CG, Hill C. Listeria monocytogenes: survival and adaptation in the gastrointestinal tract. Front Cell Infect Microbiol 2014; 4:9; PMID:24551601; http://dx.doi.org/10.3389/fcimb.2014.00009
- Dowd GC, Joyce SA, Hill C, Gahan CG. Investigation of the mechanisms by which Listeria monocytogenes grows in porcine gallbladder bile. Infect Immun 2011; 79:369-379; PMID:20937762; http://dx.doi.org/10.1128/IAI.00330-10
- McLaughlin HP, Xiao Q, Rea RB, Pi H, Casey PG, Darby T, Charbit A, Sleator RD, Joyce SA, Cowart RE, et al. A putative P-type ATPase required for virulence and resistance to haem toxicity in Listeria monocytogenes. PLoS One 2012; 7:e30928; PMID:22363518; http://dx.doi.org/10.1371/journal.pone.0030928
- Bahey-El-Din M, Casey PG, Griffin BT, Gahan CG. Efficacy of a Lactococcus lactis DeltapyrG vaccine delivery platform expressing chromosomally integrated hly from Listeria monocytogenes. Bioeng Bugs 2010; 1:66-74; PMID:21327128
- Bahey-El-Din M, Casey PG, Griffin BT, Gahan CG. Expression of two Listeria monocytogenes antigens (P60 and LLO) in Lactococcus lactis and examination for use as live vaccine vectors. J Med Microbiol 2010; 59:904-912; PMID:20488938; http://dx.doi.org/10.1099/jmm.0.018770-0
- Bahey-El-Din M, Gahan CG. Vaccination studies: detection of a Listeria monocytogenes-specific T cell immune response using the ELISPOT technique. Methods Mol Biol 2014; 1157:263-274; PMID:24792565; http://dx.doi.org/10.1007/978-1-4939-0703-8_22
