ABSTRACT
Vaccine-preventable diseases still occur although measured coverage rates at 2 y of age are high. The occurrence of these diseases may be explained in part by untimely, that is, late vaccination. Our objective was to identify potentially dangerous vaccination delays for each dose of each vaccine in children younger than 2 y. A 3-round Delphi process was conducted by e-mail. We recruited 37 French experts in vaccines for children: 16 from the Infovac-France group and 21 from the French study group for pediatric infectious diseases. Items were generated by a literature review for the 10 vaccine doses recommended before 2 y of age. Item reduction in round 1 and 2 and any consensus in round 3 used a 70% consensus cutoff. The mean participation rate was 79%. Delays that should not be exceeded were identified for all vaccine doses. The 70% consensus was reached for 6 of the 10 vaccine doses: 15 d after the recommended date for the first 2 doses of the diphtheria-tetanus-acellular pertussis-inactivated polio vaccine/Haemophilus influenzae b vaccine and for the second dose of the pneumococcal conjugate vaccine, 1 month for the meningococcal C vaccine and for the first dose of the measles-mumps-rubella vaccine, and 11 y of age for completion of the hepatitis B vaccination. This Delphi process identified potentially dangerous vaccination delays for children to the age of 2 y. These can be used as new indicators in further studies of vaccine effectiveness and can help to improve the quality of vaccine protection in children.
Introduction
There are several available indicators of the effectiveness of vaccination programs. Among them, vaccine coverage is currently a useful indicator of vaccine uptake by the physicians and populations concerned. It is however a broad feature measured at ages beyond the recommended ages for vaccination. For example, its measurement at 2 y of age does not detect vaccination delays for vaccines recommended at various times before this age.Citation1 Vaccine-preventable diseases such as pertussis and invasive meningococcal or pneumococcal infections still cause severe illness, disabilities and death despite high levels of vaccine coverage.Citation2-7 Analysis of a pertussis outbreak in 2010 showed that 140/275 (51%) infants with pertussis could have had 1 dose of the vaccine and 22% at least 2 doses.Citation2 The main reason is a delay or failure to vaccinate some patients, resulting in the absence of protection.
Although vaccine coverage rates can be high for the main vaccines recommended for children under 2, giving vaccinations later than recommended can have 2 consequences. The first is an increased risk of vaccine-preventable infections for susceptible infants at the ages of greatest vulnerability, especially when the peak of incidence of the disease is very close to the recommended age of vaccination. A study in the United States a few years ago reported that 55% of children had not received all recommended doses by 24 months of age.Citation8 A study in low and middle-income countries showed substantial delays in primary routine vaccination (more than 2 months for more than 25% of children).Citation9 The second consequence of delaying vaccination is not completing the immunization schedule by domino effect;Citation10 this may explain outbreaks of vaccine-preventable infections in healthy children, adolescents and adults.Citation11,12
These consequences of delay underline the importance of timely vaccination and the potential impact of vaccinations administered later than scheduled. They can have important implications for the effect of new and established vaccines on the burden of these diseases. Potentially dangerous vaccination delays have never been defined. Different cutoffs have been suggested, ranging from 2 weeks to 6 months for the same vaccine.Citation10 This cutoff can moreover vary one vaccine to another and one dose to another for the same vaccine, depending on the epidemiology and severity of the vaccine-preventable disease and the immunization schedule. A quantitative definition of a potentially dangerous vaccination delay could be useful as a new indicator of vaccine effectiveness for children and could help to improve the quality of vaccine protection in children. The literature provides only sparse information on this topic.
The aim of this study was to apply a Delphi process to reach a consensus on a potentially dangerous vaccination delay for each dose of each vaccine recommended for children younger than 2 y of age, according to the French immunization schedule ().Citation13
Table 1. French 2015 vaccination schedule and recommendations for children younger than 2 years of age.
Results
Among the 37 experts identified in the 2 groups (French pediatric infectious disease group (GPIP), n=21; Infovac, n=16), the mean rate of participation was 79% with respectively 31, 29, and 28 experts participating in rounds 1, 2, and 3 (77% from Infovac and 81% from the GPIP). All were medical doctors, 26% had a PhD and 71% worked in a University hospital. Six percent had been practicing in the field of pediatric infectious diseases and vaccination for less than 10 years, 48% between 10 and 20 years, and 45% more than 20 y. Their principal fields of research were the epidemiology of infectious diseases, vaccine-preventable diseases, and meningitis.
Results for the first round are presented in . After this first round, suggestions from some experts led to the introduction into round 2 of 2 additional delays: one of 7 d for the first dose of pneumococcal conjugate vaccine (PCV) and one of 1 month for the first diphtheria-tetanus-acellular pertussis-inactivated polio vaccine-Hib vaccine (DTaP-IPV-Hib) booster. shows the trends in the experts' choices from the first to the third round. After the second round, a consensus set a 1-month vaccination delay as the cutoff for the meningococcal C conjugate vaccine (MenC), (82.8%; 95% confidence intervals [CI]: 63.5–93.5).
Table 2. Potentially damaging vaccination delay for each dose of each recommended vaccine for children younger than 2 y according to the French immunization schedule: Round 1.
Figure 1. Development of experts' choices from round 1 to round 3 for each recommended injection of routine vaccines for children younger than 2 y.
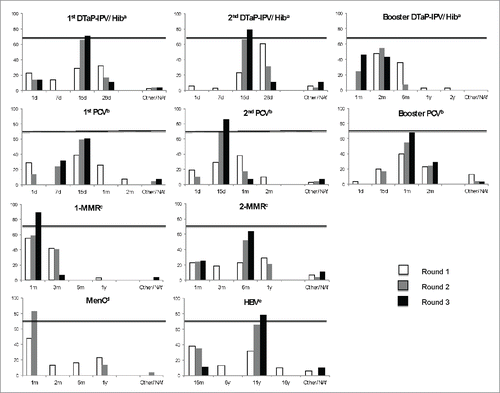
By the end of the third round, consensus was reached for most vaccines. A consensus was not reached for delays for 4 vaccinations: the first booster dose of DTaP-IPV-Hib vaccine, the first dose and the booster dose of PCV, and the second dose of measles-mumps-rubella (MMR) vaccine (). In these cases, the longest “finalist” vaccination delay, i.e., beyond which more than 70% of the experts time agreed that the delay is potentially dangerous, was chosen: 2 months for the first booster dose of DTaP-IPV-Hib, 15 d for the second PCV, 2 months for the PCV booster dose and 6 months for the second MMR. The sensitivity analysis did not result in any significant variation (data not shown).
Table 3. Potentially damaging vaccination delays for each dose of each vaccine in children younger than 2 y according to the French immunization schedule.
Discussion
This study provides for the first time definitions of potentially dangerous vaccine delays for routine vaccines in preschool children. They were established by experts in vaccination and infectious diseases in children, through a DELPHI process. For all doses of vaccines a longest tolerable delay was identified, beyond which it was potentially dangerous for each child individually. The 70% consensus was reached for 6 out of the 10 doses of vaccines recommended before the age of 2 y.
DTaP-IPV-Hib and pneumococcal conjugate vaccines are recommended early in life to protect infants against lethal diseases. Vaccination has substantially reduced the rates of diphtheria, tetanus and poliomyelitis, but pertussis and invasive pneumococcal diseases are still common early in life worldwide. A few experts initially proposed a cutoff delay of less than 15 d for the first doses of DTaP-IPV-Hib and PCV. The arguments were that 70% of pertussis cases occur under 3 months of age,Citation14 and incidence of pneumococcal meningitis peaks at 4 months of age.Citation15 Regardless of the epidemiological evidence, primary immunization with the DTaP-IPV-Hib and PCV vaccines usually takes place at the same time and involves the same risks when delayed. The experts agreed that a vaccination delay of 15 d or more after the recommended age for these primary immunizations is potentially dangerous. In France, immunization with the primary DTaP-containing combination and PCV has been scheduled at 2 and 4 months since 2013. Thus, this study suggests that delay past 15 d after the scheduled vaccination dose is potentially dangerous, regardless of the primary schedule (2–4 or 3–5 or 2-3-4 or 2-4-6 months). In countries where a third dose is proposed for primary vaccination, however, a tolerable delay for this third dose should be determined.
Questions about the timeliness of childhood immunization have been raised since the end of the 1980s. Previously published studies of national immunization rates focused on up-to-date vaccination, i.e., whether children have had the recommended number of vaccinations by a certain age. The gap between up-to-date and age-appropriate vaccination was first measured for DTaP primary vaccination in Baltimore among 525 inner-city children, at 69.9% and 32.8% respectively.Citation16 Similar results have been found for other vaccines regardless of differences between their immunization schedules. This new indicator of age-appropriate vaccination is currently used in immunization studies.Citation8,17–20 However, the acceptable delay beyond the age recommended for vaccination has generally been defined as 1 month for DT-IPV,Citation1,17,18,21 pertussis,Citation21–23 measles,Citation24 and hepatitis B vaccine (HBV).Citation21 That is, until now, timely vaccination has been defined a priori, without explanations or references.Citation25,26 Yadav et al. suggested that expert consensus define 2 weeks late as a vaccination delay for all vaccines.Citation27 Neither the a priori definitions nor that by Yadav et al. take the differences in terms of epidemiology and severity of diseases into account. Our study sought to define vaccination delays for each dose of each vaccine and thus to provide a new and more accurate indicator for French immunization studies. Varicella, meningococcal B, and rotavirus vaccination delays were not considered in this study because these vaccines were not recommended in France at that time.
The results of the study must be interpreted carefully. They are not a substitute for official or other recommended immunization schedules. Rather, they quantify unacceptable vaccination delays according to the immunization guidelines for the general population. In this study, the potentially dangerous HBV-delay in the general population was 11 y, because this population is not expected to be exposed to this infection in childhood. Because this infection is not only sexually transmitted, but can be also transmitted through other contacts by unscreened people (i.e., children born from undetected mothers, children adopted from endemic countries, refugees…), guidelines do recommend however that the HBV be administered early during infancy.Citation28 Moreover, neonates and infants born to mothers at the highest risk (from highly endemic countries, or who are drug users or sex workers, or with familial cases) must of course receive early vaccination; if indicated, both vaccination and serotherapy should begin at birth. We considered it important to define a potentially dangerous vaccination delay: immunization schedules cannot always be strictly followed for reasons including lack of physician availability, vacations, and other personal physician and parental constraints. Accordingly, many children experience vaccination delays,Citation8 which others have previously calculated as days under-vaccinated.Citation1 It can certainly be argued, as 24% to 31% of our experts suggested during the first round for the first dose of the PCV and DTaP-IPV-Hib vaccine (), that any day under-vaccinated is potentially dangerous. What our study sought to clarify was boundaries that must not be crossed for these delays. The experts thus had to consider the risk regarding the epidemiology of these vaccine-preventable diseases. A delay considered the longest tolerable was identified, beyond which individual children might be harmed by their lack of vaccination. Most potentially dangerous vaccine delays are very short —2 months or less. The existence of these delays might be used as evidence and arguments for parents and physicians about the importance of timeliness in vaccinations, and might thus improve overall vaccine protection.
This study may have some biases. The validity of the Delphi procedure relies on a panel of experts.Citation29 Limiting our panel of experts to those from Infovac (n=16) would probably have resulted in an insufficient number of responses and inadequate study power. Extending the study to experts in pediatric infectious diseases from the French GPIP, who frequently provide expertise and advice on vaccines or teach this subject, increased the set of participants without decreasing the quality of expertise. The 79% participation rate diminished participation bias. A fourth round was not conducted to avoid attrition bias. The experts' participation decreased from 81 to 76% between the first and third round and more reminders were needed to obtain that 76% rate. Finally, strict anonymity was not applied insofar as the investigators knew the panel of participating experts. However, quasi-anonymity according to McKenna was respected because each expert was individually screened and answers were anonymous.Citation30 This methodology ruled out subjectivity bias due to group effects and strong personalities.Citation31 Although informal discussions might enrich the debate, they might also impoverish it promoting polemics on controversial topics. Moreover, experts' comments and feedback helped us to ensure they understood the process and to adapt the delays offered in each round according to their advice.Citation32 Reproducibility of the Delphi technique has been questioned. Such a procedure was used in the lack of features in the literature, because of originality of the topic.Citation33 Moreover, we conducted a feasibility survey to test and improve the study tool.
This study could be useful for other countries that wish to analyze vaccination delays and improve routine vaccine protection in preschool children. Our results could be extended to other high-income countries where immunization schedules are very similar to that in France. Similar studies can be conducted with the same study design in countries with greater epidemiological differences. The results should enable the implementation of new public health strategies to improve age-appropriate vaccine protection in children and prevent disease outbreaks.
The timeliness of vaccine protection is a more precise indicator than vaccine coverage for appreciating the quality of immunization, and it varies for each vaccine-preventable disease according to its severity and epidemiology. Potentially dangerous vaccination delays have been identified through this Delphi process for each dose of each routine vaccine recommended for children younger than 2 y. These delays can be used as additional tools in future research to improve follow-up of immunization schedule guidelines.
Methods
Study design and definitions
A 3-round Delphi procedure for consensus was conducted in 2013 via an e-mail survey. The Delphi process was developed to perform a systematic synthesis of experts' opinions in situations where no consensus is available. It includes the following features: anonymity, independence of respondents, iteration with controlled feedback from one round to the next, and statistical aggregation of group responses until a sufficient consensus is reached.Citation31
Each potentially dangerous vaccination delay was defined by the time between the recommended age for vaccination (published in the official French national immunization schedule, updated yearlyCitation13) and the age at which it might be dangerous for individual children to have not been vaccinated.
Participants: investigators and experts
The study investigators had 3 roles in the Delphi process: (i) to define the concept of vaccination delay according to the literature, (ii) to identify and select the most relevant delays for each dose of each vaccine proposed to the experts at the start of the study and (iii) to analyze and interpret of the results for each round, and provide feedback of the survey to the experts for the next round by reducing the number of proposed vaccination delays.
The experts in vaccines for children were identified from members of the GPIP scientific committees and of the Infovac-France group, specialized in vaccination for children. Potential participants were asked about their willingness to participate and informed about the purpose of the study and the amount and type of work expected of them.
Delphi process
Pre-round: identification of delays and feasibility of the study
From December 2012 to January 2013, the investigators searched the literature to identify potentially relevant vaccination delays for children younger than 2 y and for the vaccines considered: DTaP-IPV-Hib, PCV, MenC, MMR, and HBV. We selected 4 initial potentially relevant vaccination delays for each dose of each vaccine after a discussion between the investigators based on the sparse literature data available about these delays, the potential severity of each disease, the incidence peak of each of these infections (if any), and the current immunization schedule. The proposed delays are available in . These choices took into account that DTaP-IPV-Hib, PCV, and MenC vaccines are designed to protect against life-threatening diseases occurring early in life:Citation2,3 younger than 1 year for invasive MenC and Hib diseases,Citation34,35 younger than 6 months for invasive pneumococcal diseases,Citation15 and younger than 3 months for pertussis.Citation36 It was considered that MMR diseases are less life-threatening in children and that the incidence of measles is relatively constant for children >1 y of age and adolescents.Citation37 HBV vaccine protects against serious chronic diseases with a low incidence in childhood.Citation38 A feasibility survey was performed in January 2013 with 5 pediatricians not involved in the Delphi process and minor changes were made based on their feedback.
Three-round Delphi process
The first round of the Delphi process was e-mailed to the experts along with a document explaining the study background. They received a table presenting the 4 delays selected by the investigators. The table included also one column for an open answer if the expert did not find appropriate any of the delays proposed and space for comments where the experts were encouraged to give explanations about their choice or other various comments. An answer was expected within 2 weeks and an e-mail reminder was sent 2 d before the due date.
The investigators reduced the number of choices for vaccine delays according to the previous round and experts' comments. In each subsequent round, the experts received a revised table presenting the selected vaccination delays and a qualitative summary of comments and results from the previous round. The results were collected within 4 weeks after each round, after 3 e-mail reminders.
Item reduction and analysis
The most commonly used threshold in Delphi studies is 70%.Citation39 Vaccination delays were selected for the following round and defined as consensual according to 2 criteria: i) agreement by at least 70% of experts about a vaccination delay was defined as a consensus; ii) otherwise, the delays with the best responses with a sum of responses rate higher or equal to 70% were selected for the next round. When the 70% threshold was not obtained, delays that should not be exceeded were defined as the maximum delays tolerated by at least 70% of the experts. When experts provided 2 answers instead of 1, both answers were excluded from the main analysis. A sensitivity analysis was then performed in taking into account the 2 answers in turn and comparing them with the main results. Statistical analyses were performed with R Commander Software (version 1.9–5). Percentages of participants and vaccination delays were calculated with their 95% CIs when appropriate.
List of the French experts from Infovac-France group and the GPIP
F. Angoulvant, P. Backhache, J. Beytout, M. Chalumeau, R. Cohen, M.A. Dommergues, V. Dufour, X. Durrmeyer, A. Faye, V. Gajdos, J. Gaudelus, Y. Gillet, C. Gras-Leguen, E. Grimprel, N. Guerin, H. Haas, I. Hau, V. Hentgen, E. Launay, M. Lorrot, F. Madhi, P. Minodier, F. Moulin, D. Pinquier, L. de-Pontual, N. Remus, O. Romain, G. Thiebault, F. Vié le Sage, B. Virey, C. Weil-Olivier.
Abbreviations
| CI | = | confidence interval |
| DTaP-IPV-Hib | = | diphtheria-tetanus-acellular-pertussis-poliomyelitis-Haemophilus influenzae b |
| GPIP | = | French pediatric infectious disease group |
| HBV | = | hepatitis B vaccine |
| MMR | = | measles-mumps-rubella |
| PCV | = | pneumococcal conjugate vaccine |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Akmatov MK, Mikolajczyk RT. Timeliness of childhood vaccinations in 31 low and middle-income countries. J Epidemiol Community Health 2012; 66:e14; PMID:21551179; http://dx.doi.org/10.1136/jech.2010.124651
- Winter K, Glaser C, Watt J, Harriman K. Centers for Disease Control and Prevention (CDC). Pertussis epidemic–California, 2014. MMWR Morb Mortal Wkly Rep 2014; 63:1129-32; PMID:25474033
- Cantey JB, Sánchez PJ, Tran J, Chung W, Siegel JD. Pertussis: a persistent cause of morbidity and mortality in young infants. J Pediatr 2014; 164:1489-92; PMID:24565424; http://dx.doi.org/10.1016/j.jpeds.2014.01.023
- Wormsbecker AE, Wonga K, Jamieson FB, Crowcroft NS, Deeks SL. Epidemiology of serogroup C and Y invasive meningococcal disease (IMD) in Ontario, 2000–2013: Vaccine program impact assessment. Vaccine 2015; 33:5678-83; PMID:26299749; http://dx.doi.org/10.1016/j.vaccine.2015.08.023
- Olarte L, Barson WJ, Barson RM, Lin PL, Romero JR, Tan TQ, Givner LB, Bradley JS, Hoffman JA, Hultén KG, et al. Impact of the 13-Valent Pneumococcal Conjugate Vaccine on Pneumococcal Meningitis in US Children. Clin Infect Dis 2015; 61:767-75; PMID:25972022; http://dx.doi.org/10.1093/cid/civ368
- Levy C, Varon E, Bingen E, Lécuyer A, Boucherat M, Cohen R, Bacterial Meningitis Study Group. Pneumococcal meningitis in French children before and after the introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J 2011; 30:168-70; PMID:21298818; http://dx.doi.org/10.1097/INF.0b013e3181f4cf69
- Levy C, Varon E, Béchet S, Cohen R. Effect of the 13-Valent pneumococcal conjugate vaccine on pneumococcal meningitis in children. Clin Infect Dis 2016; 62:131-2; PMID:26265497; http://dx.doi.org/10.1093/cid/civ692
- Luman ET, McCauley MM, Stokley S, Chu SY, Pickering LK. Timeliness of childhood immunizations. Pediatrics 2002; 110:935-9; PMID:12415033; http://dx.doi.org/10.1542/peds.110.5.935
- Clark A, Sanderson C. Timing of children's vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet 2009; 373:1543-9; PMID:19303633; http://dx.doi.org/10.1016/S0140-6736(09)60317-2
- Guerra FA. Delays in immunization have potentially serious health consequences. Paediatr Drugs 2007; 9:143-8; PMID:17523694; http://dx.doi.org/10.2165/00148581-200709030-00002
- Antona D, Lévy-Bruhl D, Baudon C, Freymuth F, Lamy M, Maine C, Floret D, Parent du Chatelet I. Measles elimination efforts and 2008–2011 outbreak, France. Emerg Infect Dis 2013; 19:357-64; PMID:23618523; http://dx.doi.org/10.3201/eid1903.121360
- Feikin DR, Lezotte DC, Hamman RF, Salmon DA, Chen RT, Hoffman RE. Individual and community risks of measles and pertussis associated with personal exemptions to immunization. JAMA 2000; 24:3145-50; http://dx.doi.org/10.1001/jama.284.24.3145
- Institut de veille sanitaire. 2013 vaccination schedule and recommendations from the « Haut Conseil de la santé publique » in France. Bull Epidemiol Hebdom 2013; 14–15:129-158 Available at: http://www.invs.sante.fr/Publications-et-outils/BEH-Bulletin-epidemiologiquehebdomadaire/Archives/2013/BEH-n-14-15-2013 [last access August 8rd, 2014].
- Bonmarin I, Guiso N, Rosso M, Levy-Bruhl D. Renacoq: hospital surveillance of pertussis in 2008. [Internet]. Bull Epidemiol Hebdom 2010; 31–32:336-8 Available at: http://www.invs.sante.fr/beh/2010/31_32/index.htm [last access August, 8th 2014]
- Varon E, Janoir C, Gutmann L. Pneumococcus National Reference Center. 2012 activity report. Available at: http://cnr-pneumo.com/ [last access August, 8th 2014]
- Bolton P, Hussain A, Hadpawat A, Holt E, Hughart N, Guyer B. Deficiencies in current childhood immunization indicators. Public Health Rep WashDC 1998; 113:527-32
- Dombkowski KJ, Lantz PM, Freed GL. The need for surveillance of delay in age-appropriate immunization. Am J Prev Med 2002; 23:36-42; PMID:12093421; http://dx.doi.org/10.1016/S0749-3797(02)00442-7
- Dayan GH, Shaw KM, Baughman AL, Orellana LC, Forlenza R, Ellis A, Chaui J, Kaplan S, Strebel P. Assessment of delay in age-appropriate vaccination using survival analysis. Am J Epidemiol 2006; 163:561-70; PMID:16421238; http://dx.doi.org/10.1093/aje/kwj074
- Akmatov MK, Kretzschmar M, Krämer A, Mikolajczyk RT. Determinants of childhood vaccination coverage in Kazakhstan in a period of societal change: implications for vaccination policies. Vaccine 2007; 25:1756-63; PMID:17229498; http://dx.doi.org/10.1016/j.vaccine.2006.11.030
- Dominguez SR, Parrott JS, Lauderdale DS, Daum RS. On-time immunization rates among children who enter Chicago public schools. Pediatrics 2004; 114:e741-7; PMID:15574606; http://dx.doi.org/10.1542/peds.2004-1053
- Luman ET, Chu SY. When and why children fall behind with vaccinations: missed visits and missed opportunities at milestone ages. Am J Prev Med 2009; 36:105-11; PMID:19062241; http://dx.doi.org/10.1016/j.amepre.2008.09.035
- Tanaka M, Vitek C, Brian P, Bisgard K, Tate J, Murphy T. Trends in pertussis among infants in the United States, 1980–1999. JAMA 2003; 22:2968-75; http://dx.doi.org/10.1001/jama.290.22.2968
- Grant C, Roberts M, Scragg R, Stewart J, Lennon D, Kivell D, Ford R, Menzies R. Delayed immunisation and risk of pertussis in infants: unmatched case-control study. BMJ 2003; 326:852-3; PMID:12702617; http://dx.doi.org/10.1136/bmj.326.7394.852
- Akmatov MK, Kretzschmar M, Krämer A, Mikolajczyk RT. Timeliness of vaccination and its effects on fraction of vaccinated population. Vaccine 2008; 26:3805-11; PMID:18565626; http://dx.doi.org/10.1016/j.vaccine.2008.05.031
- Lernout T, Theeten H, Hens N, Braeckman T, Roelants M, Hoppenbrouwers K, Van Damme P. Timeliness of infant vaccination and factors related with delay in Flanders, Belgium. Vaccine 2014; 32:284-9; PMID:24252698; http://dx.doi.org/10.1016/j.vaccine.2013.10.084
- Tozzi AE, Piga S, Corchia C, Di Lallo D, Carnielli V, Chiandotto V, Fertz MC, Miniaci S, Rusconi F, Cuttini M. Timeliness of routine immunization in a population-based Italian cohort of very preterm infants: results of the ACTION follow-up project. Vaccine 2014; 32:793-9; PMID:24397902; http://dx.doi.org/10.1016/j.vaccine.2013.12.044
- Yadav K, Srivastava R, Kumar R, Chinnakal P, Rai SK, Krishnan A. Significant vaccination delay can occur even in a community with very high vaccination coverage: evidence from Ballabgarh, India. J Trop Pediatr 2012; 58:133-8; PMID:21742766; http://dx.doi.org/10.1093/tropej/fmr059
- Strikas RA, Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices (ACIP); ACIP Child/Adolescent Immunization Work Group. Advisory Committee on Immunization Practices Recommended Immunization Schedules for Persons Aged 0 Through 18 Years — United States, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:93-4; PMID:25654610
- Keeney S, Hasson F, McKenna HP. A critical review of the Delphi technique as a research methodology for nursing. Int J Nurs Stud 2001; 38:195-200; PMID:11223060; http://dx.doi.org/10.1016/S0020-7489(00)00044-4
- McKenna HP. The Delphi technique: a worthwhile research approach for nursing? J Adv Nurs 1994; 19:1221-5; PMID:7930104; http://dx.doi.org/10.1111/j.1365-2648.1994.tb01207.x
- Goodman C. The Delphi technique: a critique. J Adv Nurs 1987; 6(12):729-34; http://dx.doi.org/10.1111/j.1365-2648.1987.tb01376.x
- McDonnel J, Meijler A, Kahan JP, Bernstein SJ, Rigter H. Panellist consistency in the assessment of medical appropriateness. Health Policy 1996; 3:139-52; http://dx.doi.org/10.1016/S0168-8510(96)90021-4
- Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995; 311:376-80; PMID:7640549; http://dx.doi.org/10.1136/bmj.311.7001.376
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27(Suppl 2):B51-63; PMID:19477562; http://dx.doi.org/10.1016/j.vaccine.2009.04.063
- Georges S, Lepoutre A, Dabernat H, Levy-Bruhl D. Impact of Haemophilus influenzae type b vaccination on the incidence of invasive Haemophilus influenzae disease in France, 15 years after its introduction. Epidemiol Infect 2013; 141:1787-96; PMID:23425638; http://dx.doi.org/10.1017/S0950268813000083
- Amirthalingam G. Strategies to control pertussis in infants. Arch Dis Child 2013; 98:552-5; PMID:23698594; http://dx.doi.org/10.1136/archdischild-2012-302968
- Leuridan E, Sabbe M, Van Damme P. Measles outbreak in Europe: susceptibility of infants too young to be immunized. Vaccine 2012; 30:5905-13; PMID:22841972; http://dx.doi.org/10.1016/j.vaccine.2012.07.035
- Behre U, Bleckmann G, Crasta PD, Leyssen M, Messier M, Jacquet JM, Hardt K. Long-term anti-HBs antibody persistence and immune memory in children and adolescents who received routine childhood hepatitis B vaccination. Hum Vaccines Immunother 2012; 8:813-8; http://dx.doi.org/10.4161/hv.19898
- Salmond S. Orthopaedic nursing research priorities: a Delphi study. Orthop Nurs 1994; 2:31-45; http://dx.doi.org/10.1097/00006416-199403000-00006
