ABSTRACT
Tetanus, diphtheria, and acellular pertussis (Tdap) vaccination is recommended for all women during each pregnancy to prevent pertussis in young infants. However, data on the safety of this protective measure are limited and conflicting. To assess maternal and infant outcomes associated with administration of this vaccine during pregnancy, we reviewed medical records of 1,759 women who delivered a singleton infant at a southeast Texas public hospital between November 1, 2012 and June 30, 2014. After excluding women who had inadequate prenatal care or who delivered at <27 weeks gestation, we used multivariable logistic regression analyses to compare 13 outcomes between those who did and did not receive the Tdap vaccine. We examined 6 maternal outcomes (chorioamnionitis, postpartum endometritis, preterm delivery, preterm premature rupture of membranes, induced labor, and mode of delivery) and 7 infant outcomes (low birth weight, very low birth weight, small for gestational age, 5-minute Apgar score, birth defects, and neonatal intensive care unit admission). Maternal Tdap vaccination was associated with decreased odds of cesarean delivery. No associations between maternal Tdap vaccination and infant outcomes were observed. This study demonstrates that Tdap vaccination during pregnancy does not increase the risk of adverse outcomes.
Introduction
Neonatal pertussis is a pressing public health concern due to the reemergence of this highly contagious respiratory disease.Citation1 Pertussis can lead to pneumonia and encephalopathy in infants and may be disabling or even fatal, particularly among infants <3 months of age.Citation2 The number of cases in the US has grown since the 1980s with a 50-year peak reported in 2012.Citation3 Infants are especially vulnerable to this disease because they do not complete the 3-dose series of pertussis vaccine until 6 months of age and are thus not fully protected until this time.Citation4 Therefore, young infants have in the past relied upon herd immunity as well as limited protection via maternal antibodies to protect them from developing this disease.
Unfortunately, herd immunity is not effective for preventing neonatal pertussis as only 14.2% of adults reported in 2012 receiving the tetanus, diphtheria, and acellular pertussis (Tdap) vaccine in the previous 7 y.Citation5 However, maternal transfer of pertussis antibodies does provide some protection, particularly when pregnant women receive the Tdap vaccine later in their pregnancy.Citation6 Thus, the Advisory Committee on Immunization Practices (ACIP) decided in October 2012 to recommend that all eligible women, regardless of prior vaccination status, be vaccinated with Tdap during each pregnancy preferably between 27 and 36 weeks gestation.Citation6 In June 2013, the American College of Obstetricians and Gynecologists announced its support of these guidelines.Citation7
Several studies have investigated the safety and immunogenicity of Tdap administration during pregnancy.Citation8-15 A small randomized controlled trial compared outcomes among 33 women vaccinated at 30–32 weeks gestation to 15 women not vaccinated during pregnancy.Citation8 This important study demonstrated that maternal vaccination resulted in higher antibody levels in infants, but its ability to examine safety issues was limited due to the small sample size. Overall, the vaccine has been found to be safe when administered during pregnancy, with only one study finding a small, but significant, increase in chorioamnionitis among vaccinated females.Citation12 This observation has not been found in subsequent studies, but still needs investigation due to the inability to conduct a clinical trial to examine this outcome in further detail. In general, many of the previous studies were limited by small sample sizes, biased observational groups, or limited ability to reliably observe more than 2 or 3 outcomes.
The primary purpose of this study was to compare several maternal and infant outcomes between women who did and did not receive the Tdap vaccine during pregnancy. A randomized study was not possible as Tdap vaccination is recommended for all pregnant women in the US. Evaluation of a greater number of outcomes that could be of concern to providers and patients who must decide on whether to be vaccinated with Tdap during pregnancy is needed. Thus, we selected a retrospective design, using a review of patient medical charts, which allowed us to more fully examine several outcomes that may not be as thoroughly documented in administrative billing records.
Results
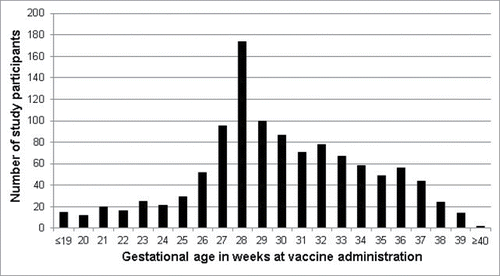
A total of 1,759 pregnant women met the inclusion criteria. The mean gestational age at vaccination was 30.3 ± 4.6 weeks (median 29.8 weeks, range: 1 week – 40 weeks), with 75.3% of women (835/1109) receiving the vaccine within the recommended interval of 27–36 weeks gestation (). Bivariate analyses showed that the mean maternal age was lower among those who received the vaccine (26.8 y vs 27.5 years, p = 0.02). A higher proportion of Hispanic mothers (68.7%) were vaccinated during pregnancy compared to women of other races/ethnicities (white: 60.0%, black: 56.6%; other: 52.9% p = <0.001). Vaccination occurred more frequently among mothers with a higher mean number of prenatal visits and who received the influenza vaccine during pregnancy (). Receipt of other vaccines during pregnancy was rare. Infants born to vaccinated mothers were heavier than those born to unvaccinated mothers (3264.3 ± 482.8 g vs 3175.5 ± 600.5 g, respectively, p = 0.001).
Table 1. Characteristics of pregnant women by Tdap vaccination status during pregnancy (N = 1,759)aFootnotea.
Birth defects were rare, and no significant differences were noted in frequency of birth defects by maternal vaccination status (). Four defects (Tetralogy of Fallot, rectal and large intestinal atresia/stenosis, reduction deformity of upper limbs, and gastroschisis) were not observed among the infant records in our sample. One stillbirth occurred and was born to an unvaccinated mother. Among infants admitted to the NICU, those in the vaccinated group spent fewer days in the unit and were less frequently admitted for preterm birth or anemia.
Table 2. Details on infant birth defects and intensive care admissions by maternal Tdap vaccination status.
In the fully adjusted multivariable logistic regression analyses, we found that there were no significant differences in frequency of the combined maternal or combined infant outcomes by maternal vaccination status (). None of the individual infant or maternal outcomes differed by maternal Tdap vaccination status in the adjusted models. We also found minor differences in mode of delivery, with vaccinated mothers less likely to deliver by cesarean compared to unvaccinated mothers.
Table 3. Maternal and infant outcomes by Tdap vaccination status (N = 1,759).
Sensitivity analyses that included all women with <4 prenatal visits had largely similar results to findings that included only women with ≥4 prenatal visits. There were 2 differences. When women with <4 prenatal visits were included in the analyses, vaccinated women were less likely to have an infant characterized as very low birth weight (OR: 0.13, 95% CI: 0.03–0.64) or have an infant admitted to the NICU (OR: 0.72, 95% CI: 0.52–0.98). These findings indicate that including women with <4 prenatal visits increased bias of the results toward poorer outcomes for unvaccinated mothers. Therefore, we presented our final results with only mothers who had adequate prenatal care (≥4 prenatal visits).
Discussion
This study adds to the reassuring evidence that the Tdap vaccine is safe in pregnancy, and addresses an early concern that linked vaccination with a small increase in risk of developing chorioamnionitis.Citation12 We examined the entire pregnancy record of a large number of women and their infants for complications and adjusted for likely confounders. Our use of a robust definition for chorioamnionitis ensured that the outcome was not a misdiagnosis, and provided an overall rate (3%) on par with national estimates (1–4% of all birthsCitation16) based on similar clinical symptoms. Overall, we did not detect any increased risk associated with Tdap administration during pregnancy for mothers or infants.
Prior data on the safety of Tdap administration during pregnancy have been limited and conflicting. A few earlier studies suggested no increased risk for adverse outcomes following maternal Tdap vaccination. However, these studies had small sample sizes or lacked a control group for comparison.Citation9-11 Only 4 studies have examined the safety of Tdap vaccination during pregnancy among a large sample of US women. In contrast to our findings, an insurance claims study of 123,494 births, of which 26,229 occurred among women vaccinated during pregnancy, observed a small, but significant, increased risk of chorioamnionitis among women vaccinated against Tdap during pregnancy compared to those not vaccinated.Citation12 Since these women did not experience an increased risk of preterm delivery, an expected sequela of chorioamnionitis, the authors stated that this finding could have resulted from confounding. In that study, only 2 maternal outcomes (chorioamnionitis and hypertensive disorders) and 2 infant outcomes (preterm and small for gestational age) were examined. Another study, based on a chart review of 7,378 women in Dallas, Texas, did not find an association between chorioamnionitis and Tdap vaccination, but did find that unvaccinated women were more likely to deliver prematurely, have infants who were small for gestational age, or have infants that required longer hospital stays.Citation13 Although our initial analysis suggested that women who declined the Tdap vaccine gave birth to babies of lower weight compared to those born to mothers who were vaccinated, this effect did not persist after we accounted for various potential confounders. Thus, our findings suggests that the difference in infant size found by the Dallas study may have been due to unaccounted for confounding factors. Moreover, only 3% of women in the Dallas study sample were unvaccinated, of whom 92% had been referred to high-risk obstetrics clinics.Citation13 Thus, the unvaccinated women may have been at higher risk for an adverse outcome. In contrast, vaccinated and unvaccinated mothers in our study were more representative of a general patient sample, with both groups including women with both healthy and at-risk pregnancies. Two more recent studies with larger sample sizes examined limited outcomes for mothers (fever and acute reactions) and infants (preterm birth, low birth weight, and small for gestational age).Citation14,15 Like our study, these 2 prior studies did not find an association between Tdap vaccination and maternal or infant outcomes.
Our study only included women with adequate prenatal care. This was done to avoid bias as inadequate prenatal care is associated with adverse outcomes, such as premature birth and infants who are small for gestational age.Citation17 Our sample had a lower proportion of low birth weight infants (6.8%) compared to the national average (8.0%), which was likely due to including only women with adequate prenatal care. In fact, our sensitivity analysis comparing results from models both including and excluding women with adequate prenatal care demonstrated that including these women would bias the results. We also excluded those who delivered prior to 27 weeks gestation, which allowed women sufficient time to receive the vaccine, and further reduced the possibility of inclusion bias. Differences in methodology may explain why our findings differ from some prior studies.Citation12,13
We cannot determine why vaccinated women in our study were less likely to deliver by cesarean section, but it is unlikely that this was directly related to receiving the Tdap vaccine during pregnancy, and was due instead to unknown confounders. It is possible that patients who were anxious about their pregnancies were more reluctant to be vaccinated due to fear of complications; however, medical records would not reflect subtle worries and interactions between patient and provider. Nonetheless, in a survey study regarding Tdap vaccination during pregnancy, mothers reported that the safety of the baby was the most important consideration surrounding the mother's decision to be vaccinated during pregnancy.Citation18 This indicates that if the mother had any hesitation about how the vaccine might affect her infant, she may choose not to vaccinate. Her anxiety may have led to a request for an elective cesarean delivery. As of yet, no information is available on how mothers who have a healthy pregnancy feel about Tdap vaccination compared to those who are concerned about how their pregnancy is progressing. Further, future studies should address outcomes among pregnancies after the mother has received multiple doses of the Tdap vaccine in a short period of time.
The strengths of our study included the thorough review of hundreds of detailed electronic medical records (EMRs) of women who did and did not receive the Tdap vaccine during pregnancy. Our review of EMRs allowed us to examine a variety of outcomes, and did not rely on records that only contained ICD-9 codes. Furthermore, our study included low-income women from minority backgrounds who have been shown to be at higher risk for adverse outcomes.Citation19 Nearly 85% of women who receive care through UTMB's prenatal clinics have an annual family income under $30,000 and 40% earn less than $15,000 annually.Citation20 In our study population, 45.9% of mothers were Hispanic women, 31.3% were non-Hispanic white women, and 18.0% were non-Hispanic black women. Thus, our study shows that vaccination is safe in women from vulnerable backgrounds who may be at higher risk of poor birth outcomes even though the results may not be fully generalizable to the whole US pregnant population (In 2014, 20.1% of births (not including Asian, Pacific Islander, American Indian, or Alaska Native births) were to Hispanic women, 66.9% were to non-Hispanic white women, and 13% were to non-Hispanic black women.Citation21). In addition, our study included only complications which occurred after vaccination to increase the validity of this study.
There are some limitations. While we had adequate power to evaluate combined outcomes, our relatively small sample size led to low power to detect significant differences for individual outcomes with low frequencies. We also included only conditions that providers noted on their patients' medical charts, and considered the outcome absent if not noted, which may have resulted in some underestimation of some outcomes. However, it is unlikely that this would contribute to bias, as omissions are likely to have been random. Moreover, some birth defects may not have been detected prior to hospital discharge and thus may have been underestimated. Similar to the omissions, there is no reason why this would have occurred preferentially in one group. Also, we included all women who were vaccinated regardless of whether they received the vaccine during the recommended window (27–36 weeks gestation). Although in a controlled trial all vaccines would be given in this timeframe, our study examines all of the vaccinated women, and is more likely to reflect real world conditions. Finally, it is possible that our retrospective study design resulted in residual confounding by including categories that were too broad or other unaccounted for factors.
Overall, these data suggest that Tdap vaccination during pregnancy does not result in adverse outcomes for mothers or infants. Given the urgent need to protect newborns from possible pertussis infections, these findings should be shared with pregnant women concerned about the safety of this vaccine.
Methods
Study sample
We conducted a retrospective review of EMRs of all women from Galveston County who delivered a singleton infant at the University of Texas Medical Branch at Galveston (UTMB) between November 1, 2012 and June 30, 2014, including 178 (9.2%) that delivered in 2012, 1,169 (60.0%) that delivered in 2013, and 599 (30.8%) that delivered during the period examined in 2014. We chose to review records starting in November 2012 because this reflected the early period of time after the recommendation to vaccinate all pregnant women was made. The EMRs of these 1,946 women were reviewed by 4 specially trained study coordinators. Data were recorded onto a data collection form developed by the first author (A.B.B.). Ten percent of data was validated by a second study coordinator and the margin of error between reviewers' extractions of individual data points was <2% (range: 0% – 9.4%; standard deviation ±1.7%). Any errors were resolved by re-reviewing the chart for further clarification. Coordinators then entered mother and infant data into an electronic database using SPSS Statistics software, Version 22 (IBM, Armonk, New York). Frequent database auditing was performed to identify outliers and other inconsistencies, which were subsequently reviewed and corrected or confirmed. This study was approved by UTMB's institutional review board.
The final analyses included singleton pregnancies delivered at ≥27 weeks gestation. This gestational age was chosen because the Tdap vaccine is recommended between 27–36 weeks gestation.Citation6 Thus, almost all women who delivered prior to 27 weeks were in the unvaccinated group and would have biased the results. To avoid confounding due to scanty prenatal care, women with <4 clinic visits during pregnancy (identified as inadequate prenatal care by the World Health Organization)Citation22 were excluded in the final analyses. Mothers' charts were reviewed up to the date of discharge from that delivery and again at first postpartum checkup while infant charts were reviewed up to the date of initial hospital discharge. While some infant data were available on the mother's chart (eg, birth weight and Apgar score), data such as neonatal intensive care unit (NICU)-admission status and birth defects were extracted from the corresponding infant charts.
Measures
Outcomes of women who received the Tdap vaccine anytime during their pregnancy were compared to those who did not receive this vaccine during pregnancy, using a dichotomous variable indicating the presence or absence of an outcome. As this study consisted of a chart review, if an outcome of interest was not noted on the patients' medical chart, it was considered to be an indication that the patient did not have the outcome. Outcomes were measured anytime during pregnancy to create standardization between vaccinated and unvaccinated groups. The value or number and type of those outcomes that occurred after vaccination were also assessed for the Tdap-vaccinated group. Mean age at the time of birth and the mean of the total number of prenatal visits were compared between vaccinated and unvaccinated mothers. Maternal health outcomes included: chorioamnionitis, postpartum endometritis, preterm premature rupture of membranes, and preterm delivery (delivery <37 weeks gestation).Citation23 Other details examined included whether labor was induced and mode of delivery (vaginal or cesarean). We re-reviewed all maternal charts with a physician diagnoses of chorioamnionitis or endometritis to confirm that they met published criteria for these infections.Citation24,25 Chorioamnionitis was considered to be present only if a patient's chart included a physician's diagnosis, a fever ≥100.4°F, and at least 2 of the following clinical findings: uterine tenderness, maternal tachycardia, fetal tachycardia, maternal leukocytosis, or foul-smelling vaginal discharge. Endometritis was considered present if a patient's chart included a physician's diagnosis, a fever ≥100.4°F, and at least 1 of the following: uterine tenderness, foul-smelling vaginal discharge, or maternal leukocytosis.
Infant outcomes included: low birth weight, very low birth weight, small for gestational age, Apgar score <8 at 5 minutes of life,Citation26 admission to the neonatal intensive care unit (NICU), and any one of the following birth defects (spina bifida, transposition of great arteries, tetralogy of Fallot, atrioventricular septal defect, cleft palate, cleft lip, rectal and large intestinal atresia/ stenosis, reduction deformity of upper limbs, gastroschisis, or diaphragmatic hernia). These birth defects were selected because they are the 10 most commonly encountered birth defects reported by the Centers for Disease Control and Prevention.Citation27 We decided to examine birth defects because this is a concern to the public for any medication given to pregnant women, although the vaccine is unlikely to play an etiological role in any of these birth defects because they usually develop early in gestation, before the vaccine would have been administered. Other infant outcomes included mean birth weight and length of hospital stay for those infants admitted to the NICU.
We also evaluated maternal and infant outcomes by combining all possible adverse maternal outcomes (chorioamnionitis, postpartum endometritis, preterm premature rupture of membranes, and preterm delivery) and all possible adverse infant outcomes (low birth weight, small for gestational age, Apgar <8 at 5 minutes, NICU admission, and presence of birth defect) into binary outcomes (1 = any outcome, 0 = no outcomes). Medical records were also reviewed to assess self-reported race/ethnicity, recorded history of prior sexually transmitted infections, date and type of all vaccines administered during the current pregnancy, gestational age at first prenatal visit, total number of previous pregnancies (full-term and premature), previous spontaneous abortions, and previous ectopic pregnancies. These variables were assessed as possible confounders in the administration of the Tdap vaccine.
Statistical analysis
This study was designed to evaluate whether outcomes were equivalent among women and infants by maternal Tdap vaccination status. We estimated that 1,600 women in total (around 800 in each group) would provide at least 80% power at the 2-sided 0.05 significance level to assume outcomes were equivalent, with a margin of δ = 5% if outcome rates remained within 10 ± 5 %. Our final study yielded 1,109 vaccinated women and 650 unvaccinated women, which may have increased bias for normal outcomes among the vaccinated. Chi-square test was used for bivariate categorical comparisons; where expected cells had counts <5, 2-sided Fisher's exact test was used. Differences in means were evaluated using Student's t test for continuous variables. Multivariable logistic regression was used to determine whether there was an association between maternal Tdap vaccination and adverse maternal and infant outcomes after adjusting for potential confounders, including: mother's age, race, number of prenatal visits, influenza vaccination received, number of previous infants born prematurely, and whether the first prenatal visit occurred before 12 weeks of gestation. Maternal outcomes and infant outcomes were modeled both separately, and then as 2 combined outcomes, respectively. We also conducted a sensitivity analysis that included all women with <4 prenatal visits to make sure that this exclusion criterion was not biasing our results. A p-value <0.05 was considered significant. Analyses were conducted using SAS software, version 9.3 (SAS Institute Inc., Cary, NC).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Kimberly S. Carlson, RN, Maria D. Garcia, MS, Margarita Morgado, MA, and Didi Rivas assisted with data collection (UTMB). Karry K. McCarty, BS, assisted with data management (UTMB). Susan Y. Rojahn, PhD, assisted with manuscript preparation (UTMB). The findings of this study were presented at the 9th Vaccine & ISV Congress held October 18–20, 2015 in Seoul, Korea.
Funding
JMH is supported by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women's Health Program–BIRCWH; Principal Investigator: Berenson) from the Office of Research on Women's Health, the Office of the Director, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health. The work was also supported by the John Sealy Foundation Memorial Endowment Fund via the Center for Interdisciplinary Research in Women's Health at UTMB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Swamy GK, Heine RP. Vaccinations for pregnant women. Obstet Gynecol 2015; 125(1):212-26; PMID:25560127; http://dx.doi.org/10.1097/AOG.0000000000000581
- Swamy GK, Wheeler SM. Neonatal pertussis, cocooning and maternal immunization. Expert Rev Vaccines 2014; 13(9):1107-14; PMID:25075629; http://dx.doi.org/10.1586/14760584.2014.944509.
- National Notifiable Diseases Surveillance System, Centers for Disease Control and Prevention. Pertussis (Whooping Cough) (Bordetella pertussis) 2014 Case Definition. Available from: http://wwwn.cdc.gov/nndss/conditions/pertussis/case-definition/2014/
- Strikas RA. Advisory committee on immunization practices recommended immunization schedules for persons aged 0 through 18 years - United States, 2015. MMWR Morb Mortal Wkly Rep 2015; 64(4):93-4; PMID:25654610
- Williams WW, Lu PJ, O'Halloran A, Bridges CB, Pilishvili T, Hales CM, Markowitz LE, Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults - United States, 2012. MMWR Morb Mortal Wkly Rep 2014; 63(5):95-102; PMID:24500288
- Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP) Summary Report, October 24–25, 2012. Available from: http://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-oct12.pdf
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 558: Integrating immunizations into practice. Obstet Gynecol 2013; 121(4):897-903; PMID:23635707; http://dx.doi.org/10.1097/01.AOG.0000428788.74725.90
- Munoz FM, Bond NH, Maccato M, Pinell P, Hammill HA, Swamy GK, Walter EB, Jackson LA, Englund JA, Edwards MS, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA 2014; 311(17):1760-9; PMID:24794369; http://dx.doi.org/10.1001/jama.2014.3633
- Talbot EA, Brown KH, Kirkland KB, Baughman AL, Halperin SA, Broder KR. The safety of immunizing with tetanus-diphtheria-acellular pertussis vaccine (Tdap) less than 2 years following previous tetanus vaccination: Experience during a mass vaccination campaign of healthcare personnel during a respiratory illness outbreak. Vaccine 2010; 28(50):8001-7; PMID:20875487; http://dx.doi.org/10.1016/j.vaccine.2010.09.034
- Zheteyeva YA, Moro PL, Tepper NK, Rasmussen SA, Barash FE, Revzina NV, Kissin D, Lewis PW, Yue X, Haber P, et al. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women. Am J Obstet Gynecol 2012; 207(1):59-7; PMID:22727350
- Shakib JH, Korgenski K, Sheng X, Varner MW, Pavia AT, Byington CL. Tetanus, diphtheria, acellular pertussis vaccine during pregnancy: pregnancy and infant health outcomes. J Pediatr 2013; 163(5):1422-6; PMID:23896191; http://dx.doi.org/10.1016/j.jpeds.2013.06.021
- Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Klein NP, Cheetham TC, Naleway A, Omer SB, Hambidge SJ, Lee GM, Jackson ML, et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. JAMA 2014; 312(18):1897-904; PMID:25387187; http://dx.doi.org/10.1001/jama.2014.14825
- Morgan JL, Baggari SR, McIntire DD, Sheffield JS. Pregnancy outcomes after antepartum tetanus, diphtheria, and acellular pertussis vaccination. Obstet Gynecol 2015; 125(6):1433-8; PMID:26000515; http://dx.doi.org/10.1097/AOG.0000000000000862
- Sukumaran L, McCarthy NL, Kharbanda EO, Weintraub ES, Vazquez-Benitez G, McNeil MM, Li R, Klein NP, Hambidge SJ, Naleway AL, et al. Safety of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis and Influenza Vaccinations in Pregnancy. Obstet Gynecol 2015; 126(5):1069-74; PMID:26444109; http://dx.doi.org/10.1097/AOG.0000000000001066
- Sukumaran L, McCarthy NL, Kharbanda EO, McNeil MM, Naleway AL, Klein NP, Jackson ML, Hambidge SJ, Lugg MM, Li R, et al. Association of Tdap Vaccination With Acute Events and Adverse Birth Outcomes Among Pregnant Women With Prior Tetanus-Containing Immunizations. JAMA 2015; 314(15):1581-7; PMID:26501534; http://dx.doi.org/10.1001/jama.2015.12790
- Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010 Jun; 37(2):339-54; http://dx.doi.org/10.1016/j.clp.2010.02.003
- VanderWeele TJ, Lantos JD, Siddique J, Lauderdale DS. A comparison of four prenatal care indices in birth outcome models: comparable results for predicting small-for-gestational-age outcome but different results for preterm birth or infant mortality. J Clin Epidemiol 2009; 62(4):438-45; PMID:18945589; http://dx.doi.org/10.1016/j.jclinepi.2008.08.001
- Healy CM, Rench MA, Montesinos DP, Ng N, Swaim LS. Knowledge and attitiudes of pregnant women and their providers towards recommendations for immunization during pregnancy. Vaccine 2015; 33(41):5445-51; PMID:26307234; http://dx.doi.org/10.1016/j.vaccine.2015.08.028
- Kim D, Saada A. The social determinants of infant mortality and birth outcomes in Western developed nations: a cross-country systematic review. Int J Environ Res Public Health 2013; 10(6):2296-335; PMID:23739649; http://dx.doi.org/10.3390/ijerph10062296
- Davlin SL, Berenson AB, Rahman M. Correlates of HPV knowledge among low-income minority mothers with a child 9–17 years of age. J Pediatr Adolesc Gynecol 2015; 28(1):19-23; PMID:25444051; http://dx.doi.org/10.1016/j.jpag.2014.01.109
- Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ. Births: Final Data for 2014. Natl Vital Stat Rep 2015 Dec; 64(12):1-64.
- World Health Organization. Global Health Observatory data: Antenatal care. (Accessed February 11, 2015, at http://www.who.int/gho/maternal_health/reproductive_health/antenatal_care_text/en/).
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol 2012; 119(6):1308-17; PMID:22617615; http://dx.doi.org/10.1097/AOG.0b013e31825af2f0
- Czikk MJ, McCarthy FP, Murphy KE. Chorioamnionitis: from pathogenesis to treatment. Clin Microbiol Infect 2011; 17(9):1304-11; PMID:21672080; http://dx.doi.org/10.1111/j.1469-0691.2011.03574.x
- Chaim W, Bashiri A, Bar-David J, Shoham-Vardi I, Mazor M. Prevalence and clinical significance of postpartum endometritis and wound infection. Infect Dis Obstet Gynecol 2000; 8(2):77-82; PMID:10805361; http://dx.doi.org/10.1155/S1064744900000053
- Iliodromiti S, Mackay DF, Smith GC, Pell JP, Nelson SM. Apgar score and the risk of cause-specific infant mortality: a population-based cohort study. Lancet 2014; 384(9956):1749-55; PMID:25236409; http://dx.doi.org/10.1016/S0140-6736(14)61135-1
- Centers for Disease Control and Prevention. Birth Defects - Data and Statistics. Available from: http://www.cdc.gov/ncbddd/birthdefects/data.html