ABSTRACT
Millions of doses of HPV vaccine have been administered globally. Inadvertent administration of HPV vaccine during pregnancy occurs given that the main recipients of the vaccine are fertile young women, who might be unaware of their pregnancy at the time of their vaccination. To investigate the subject of HPV vaccine and pregnancy , the databases of PubMed and Embase were searched to find the relevant literature published in English within the last 10 y. Most of the evidence pertaining to fetal adverse events following HPV vaccination relates to spontaneous miscarriage. None of the relevant studies found any significantly increased rate of spontaneous abortion in the overall analyses. There was no indication of other HPV vaccine-associated adverse events in pregnancy or immediately post-conception.
Introduction
The burden of disease
Human Papilloma Virus (HPV) has over 150 serotypes and can cause human infections in the squamous epithelium of skin and mucous membranes. Most conditions caused by HPV are asymptomatic, but some low-risk genotypes can cause papillomata, while high-risk genotypes can cause dysplasia and cancer of the anus, vulva, vagina and cervix. Currently, there is no treatment for HPV infection and most cases spontaneously resolve. In 2012, the worldwide estimated prevalence of cervical cancer was around 528,000 casesCitation1 and in the same year, the global disease specific mortality was 266,000. Both the incidence and mortality are highest in low-income countries where cervical screening programmes are unavailable. Accordingly, the need for a vaccine against HPV is greatest in these countries.
Introduction of HPV vaccination
Since 2006, a bivalent (2vHPV, CervarixTM) and a quadrivalent (4vHPV, Gardasil®) vaccine against HPV have been available. To date, these represent the only prophylactic anti-carcinogenic vaccines. The bivalent vaccine gives protection against HPV types 16 and 18, (which cause 70% of invasive cervical cancersCitation2) whereas the 4 vHPV vaccine provides additional protection against HPV types 6 and 11 (which cause vulvovaginal papillomatous warts). Both HPV vaccines are recombinant, containing virus-like particles (VLP's) enhanced by an adjuvant that triggers an immune response higher than a natural infection.Citation3 A 9-valent HPV vaccine (9vHPV, Gardasil 9, Merck & Co., Inc.) was licensed for use in the USA in December 2014. This vaccine protects against 5 additional oncogenic HPV types 31, 33, 45, 52 and 58 and may increase the protection against cervical dysplasia and cancer.Citation4
Full coverage by the HPV vaccine is obtained by 2 (administered at time 0 and after 6 months) or 3 doses (administered at time 0, after 1–2 months and after 6 months), depending on age,Citation5 but many girls do not receive all doses.Citation6 The repeat doses of vaccine are given to boost the immune system in order to achieve long-term duration of immunity. Recent data on the efficacy of the 2 vHPV up to 4 y after vaccination demonstrate a similar protection of one, 2 or 3 doses.Citation7 This provides the opportunity to optimize vaccine coverage in regions with lower socioeconomic levels and insufficient infrastructure. The quadrivalent vaccine is offered to female adolescents as part of a routine childhood immunization schedule in many high-income countries. In the UK, the vaccination program to prevent cervical cancer was implemented in 2008, and in 2012 the calculated rate of uptake was 81%.Citation8 Since January 2009 in Denmark, girls between 12 and 15 y of age have been offered free HPV vaccination and the rate of uptake after 1 y was 62%,Citation9 which increased to 80% in 2012.Citation10 In Australia, the rate of fully vaccinated females aged 12–13 y was 73% in 2007, which was the first year of the public vaccination program. At least one dose was administered to 83.2% of girls of this age.Citation11 In 2012 in the USA, the rate of uptake of HPV vaccine (3 doses) was 33.4%, and 53.8% of adolescent females aged 13–17 y who received at least one dose.Citation12 The aim of vaccination is to prevent the high mortality from cervical cancer and to lower the incidence of dysplasia and genital warts.
Vaccination in pregnancy
Since its introduction, millions of doses of HPV vaccine have been administered globally and inadvertent administration of HPV vaccine during pregnancy occurs because the main recipients of the vaccine are fertile young women. Fears of teratogenicity or other potentially harmful adverse effects (AE) to the unborn child such as miscarriage, preterm birth, congenital malformations, and fetal demise raises concerns among both recipients and health care providers. This fear is based on the fact that environmental factors such as drugs and medications theoretically might cause damage especially in the first trimester of pregnancy where the development of sensitive organs, e.g. the central nervous system and the heart, takes place. The World Health Organization as well as the vaccine manufacturers Merck and GlaxoSmithKline recommend avoiding HPV vaccination during pregnancy.Citation13 However, pregnancy testing before vaccination is not mandatory and no intervention is recommended in cases of accidental vaccination of pregnant women. The American College of Obstetricians and Gynecologists supports this approach.Citation14 Theoretically, pregnancy tests might have a negative effect on vaccine uptake since postponing vaccination might result in reduced compliance. Vaccination studies indicate a rate of unplanned pregnancy in young women of approximately 18 percent. Worldwide the rate of unplanned pregnancy is 41% of a total of 208 million pregnancies. In more developed regions, the rate is 47% and in less developed regions is 40 percent.Citation15 Accordingly, there is a need for examination of HPV vaccination administered immediately pre-conceptually or inadvertently during pregnancy.
Methodology
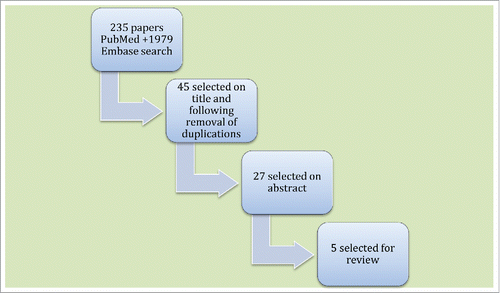
To investigate the subject of HPV vaccination and pregnancy, the databases of PubMed and Embase were searched to find the relevant literature published in English within the last 10 y. The keywords/MeSH terms used were the following: HPV; Human; human papilloma virus; human papillomavirus; human wart virus; papilloma, papilloma virus, human; papillomavirus; papillomavirus, human; pregnancies; pregnancy; pregnant; vaccination; vaccine; virus; virus, human; wart. The systematic search provided a total of 2214 citations and from these 2169 were excluded on title (not relevant) or duplication (). The relevancy of the citations was based on the subject of the article. Furthermore, case histories and conference abstracts were excluded. The abstracts of the 45 remaining citations were read and 18 were excluded as not being relevant. Of the remaining 27 citations the full manuscript was examined and 5Citation16-20 were chosen as relevant for the review (). Our aim was to provide an overview of the safety of HPV vaccination in pregnancy or immediately pre-conceptually.
Table 1. The 5 major studies included in the present review. *PATRICIA: PApilloma TRIal against Cancer In young Adults. #CVT: The Costa Rica Vaccine Trial. HAV: Hepatitis A-vaccine. HPV: HPV vaccine. VAERS: Vaccine Adverse Event Reporting System. LMP: Last menstrual period.
Results
Maternal safety
The safety of HPV vaccines for women is supported by a number of studies.Citation21-24 In a pooled analysis of 11 studies,Citation25 the incidence of serious AEs in 16,142 females who received at least one dose of the 2 vHPV was examined. Controls were 13,811 girls/women who received control vaccine and data for a total of 45,988 doses of vaccine were analyzed. The rate of medically significant conditions, new onset of chronic diseases or serious AEs in women receiving the HPV vaccine was no greater than in the controls. This applied to any age group (10–14 years, 15–25 years, and > 25 years) and any follow-up period (0–7 months, 7–12 months, and > 12 months). The European Medicines Agency (EMA) carried out a detailed scientific review of 2 reported possible vaccine side effects, postural orthostatic tachycardia syndrome and complex regional pain syndrome but no causal link to the vaccines was found.Citation26 In light of the EMA review it seems reasonable to assume that this also applies to the 4 vHPV vaccine.
Fetal safety
Spontaneous miscarriage
Most of the evidence pertaining to fetal AE of HPV vaccination relates to spontaneous miscarriage ().Citation16-20 A total of 1,119 miscarriages occurred in the 8,092 pregnancies exposed to the HPV vaccine giving a miscarriage rate ranging from 6.6%Citation19 to 18.2%.Citation18 In 4 of the studies,Citation16-18,20 the exposed pregnancies were compared to either Hepatitis A vaccine (HAV) exposedCitation16-18 or placebo exposed pregnanciesCitation20 and one study included both an HAV group and a placebo group.Citation17
Table 2. Overview of data on spontaneous abortions. NA: Not applicable because of no control/unvaccinated group. RR: Relative risk (HPV over Control). 95% CI: exact 95% confidence interval for relative risk.
None of the studies found a significantly increased rate of spontaneous abortion in the overall analyses. Looking at subgroups (e.g., age, interval of estimated time of conception and nearest vaccination, number of vaccinations) one studyCitation16 detected a significant increase in the risk of miscarriage (14.7% vs. 9.2%, P = 0.031). The authors claimed this to be compatible with chance but acknowledged a concern with this finding. It is notable that the studies concerning spontaneous abortion associated with HPV vaccination did not match the women for parity, race, or socioeconomic status. In summary, these findings suggest that the increased risk of miscarriage due to HPV vaccine is negligible.
Other fetal outcomes
Following administration of the 4 vHPV vaccine in the month immediately prior to the last menstrual period before pregnancy or during the pregnancy itself, major birth defects were found in 2.2% (10/517) of live born babies compared to 2.7% in the general population (95% CI 1.05–4.05). Of 517 cases reported, 7 were of fetal deaths > 20 weeks gestation and 1 early neonatal death in the vaccination group. This was slightly higher than in the general population but the authors concluded that no increased risk of AE in pregnancy associated with vaccines was demonstrated.Citation19 In another study, no significant differences were found with respect to live births, ectopic pregnancies, or congenital anomalies. The only parameter that showed a statistically significantly increased risk for women vaccinated with Gardasil, was caesarean section (P = 0.015). In both groups, the majority of caesarean sections were due to failure to progress, dystocia, repeat or elective caesarean section.Citation18
Discussion
Most vaccine programs recommend HPV vaccination before sexual debut, at the age of 9–13 y in order to optimize the effect of this prophylactic vaccine. In addition, it is sufficient to administer 2 doses of HPV vaccine 6 months apart in this age group compared to 3 doses to females older than 15 y of age.Citation27 However, in many cases and for many different reasons, the vaccine may be administered during a woman's fertile life and the risk of inadvertent vaccination during or immediately prior to pregnancy exists. This review suggests that the risk of AE during pregnancy is unrelated to HPV vaccination before or during pregnancy. This is valuable information for healthcare professionals and the women who receive HPV vaccination in or immediately prior to pregnancy. The vast majority of data we found were from studies that were conducted for regulatory purposes by the manufacturers of the HPV vaccines. These studies were designed to monitor the general side effects of the vaccines and not specifically those associated with pregnancy, which might bias the conclusions. Furthermore, the control groups are in many cases women vaccinated with HAV which by itself may cause AE's and thereby reduce the difference in AE's between the HPV and HAV vaccinated groups. Some of the pregnancies included in the cited studies might occur more than once due to overlap of the included papers. However, this bias is not possible to detect or describe from the published data and the amount of data seems sufficient to provide a stabile background for a conclusion. Currently, studies in which HPV vaccine is deliberately administered to pregnant women are not ethically feasible. Approval of administration of the vaccines to pregnant women has not yet been achieved Although the studies in this review have not documented any signs of increased risk of HPV vaccine-associated adverse events, even a small adverse effect could cause harm to thousands of pregnancies globally. Larger numbers of inadvertently vaccinated pregnant females will increase the external validity of the present review but present data seem reassuring. The new data on similar rates of efficacy of one dose compared to 2 or 3 doses open a new perspective on vaccination of pregnant women, although it must be stressed that the full duration of protective immunity achieved following a single vaccine dose remains to be proven.
Conclusion
Considering the large amount of data on pregnancies conceived in relation to HPV vaccination, little or no evidence has been found to demonstrate a correlation between vaccination and adverse outcomes of pregnancy. It is important to continue to report and follow-up these inadvertent administrations during or immediately prior to pregnancy. It is also important to stress to women who conceive around the time of a HPV vaccination and to health care providers that based on the current evidence, there is no reason for concern with respect to the potential AEs for the pregnancy. Therapeutic termination should not be considered leaving the pregnancy to be continued with a standard level of observation and health care. However, it should be emphasized that the true safety of vaccination in pregnancy has not yet been formally established by a randomized controlled trial.
Abbreviations
| AE | = | Adverse event |
| EMA | = | European Medicines Agency |
| HAV | = | Hepatitis A vaccine |
| HPV | = | Human papillomavirus |
| VLP | = | Virus-like particles |
| 2vHPV | = | Bivalent HPV vaccine |
| 4vHPV | = | Quadrivalent HPV vaccine |
| 9vHPV | = | Nonavalent HPV vaccine |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- al. Fe. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International journal of Cancer 2015; 136(5):E359-86. http://dx.doi.org/10.1002/ijc.29210; Epub 2014 Oct 9.
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048-56; PMID:20952254; http://dx.doi.org/10.1016/S1470-2045(10)70230-8
- Poland GA, Jacobson RM, Koutsky LA, Tamms GM, Railkar R, Smith JF, Bryan JT, Cavanaugh PF, Jr., Jansen KU, Barr E. Immunogenicity and reactogenicity of a novel vaccine for human papillomavirus 16: a 2-year randomized controlled clinical trial. Mayo Clin Proc 2005; 80:601-10; PMID:15887427; http://dx.doi.org/10.4065/80.5.601
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED, Jr., Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015; 372:711-23; PMID:25693011; http://dx.doi.org/10.1056/NEJMoa1405044
- Human papillomavirus vaccines: WHO position paper, October 2014. Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations 2014; 89(43): 465-91.
- Liu G, Kong L, Du P. HPV vaccine completion and dose adherence among commercially insured females aged 9 through 26 years in the US. Papillomavirus Res 2016; 2:1-8; PMID:26623444; http://dx.doi.org/10.1016/j.pvr.2015.10.001
- Kreimer AR, Struyf F, Del Rosario-Raymundo MR, Hildesheim A, Skinner SR, Wacholder S, Garland SM, Herrero R, David MP, Wheeler CM. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol 2015; 16:775-86; PMID:26071347; http://dx.doi.org/10.1016/S1470-2045(15)00047-9
- Division PHWHP. Vaccine uptake in Children in Wales, April to June 2012: COVER report 103, August 201. Cardiff, Public Health Wales. 2012.
- Widgren K, Simonsen J, Valentiner-Branth P, Molbak K. Uptake of the human papillomavirus-vaccination within the free-of-charge childhood vaccination programme in Denmark. Vaccine 2011; 29:9663-7; PMID:22015392; http://dx.doi.org/10.1016/j.vaccine.2011.10.021
- 2013. SSIAJ. EPI-NEWS HPV-vaccination - coverage 2012. Available from: http://www.ssidk/English/News/EPI-NEWS/2013/No%2020%20-%202013aspx
- Barbaro B, Brotherton JM. Assessing HPV vaccine coverage in Australia by geography and socioeconomic status: are we protecting those most at risk? Aust N Z J Public Health 2014; 38:419-23; PMID:24962721; http://dx.doi.org/10.1111/1753-6405.12218
- Human Papillomavirus Vaccination Coverage Among Adolescent Girls, 2007-2012, and Postlicensure Vaccine Safety Monitoring, 2006-2013, United States. 2015
- http://www.cdc.gov/vaccines/pubs/preg-guide.htm - hpv
- Human Papillomavirus Vaccination - ACOG. 2015.
- Singh S, Sedgh G, Hussain R. Unintended Pregnancy: Worldwide Levels, Trends, and Outcomes. Stud Fam Plann 2010; 41:241-50; PMID:21465725; http://dx.doi.org/10.1111/j.1728-4465.2010.00250.x
- Wacholder S, Chen BE, Wilcox A, Macones G, Gonzalez P, Befano B, Hildesheim A, Rodriguez AC, Solomon D, Herrero R, et al. Risk of miscarriage with bivalent vaccine against human papillomavirus (HPV) types 16 and 18: pooled analysis of two randomised controlled trials. BMJ 2010; 340:c712; PMID:20197322; http://dx.doi.org/10.1136/bmj.c712
- Panagiotou OA, Befano BL, Gonzalez P, Rodriguez AC, Herrero R, Schiller JT, Kreimer AR, Schiffman M, Hildesheim A, Wilcox AJ, et al. Effect of bivalent human papillomavirus vaccination on pregnancy outcomes: long term observational follow-up in the Costa Rica HPV Vaccine Trial. BMJ 2015; 351:h4358; PMID:26346155; http://dx.doi.org/10.1136/bmj.h4358
- Garland SM, Ault KA, Gall SA, Paavonen J, Sings HL, Ciprero KL, Saah A, Marino D, Ryan D, Radley D, et al. Pregnancy and infant outcomes in the clinical trials of a human papillomavirus type 6/11/16/18 vaccine: a combined analysis of five randomized controlled trials. Obstet Gynecol 2009; 114:1179-88; PMID:19935017; http://dx.doi.org/10.1097/AOG.0b013e3181c2ca21
- Dana A, Buchanan KM, Goss MA, Seminack MM, Shields KE, Korn S, Cunningham ML, Haupt RM. Pregnancy outcomes from the pregnancy registry of a human papillomavirus type 6/11/16/18 vaccine. Obstet Gynecol 2009; 114:1170-8; PMID:19935016; http://dx.doi.org/10.1097/AOG.0b013e3181c2a122
- Angelo MG, David MP, Zima J, Baril L, Dubin G, Arellano F, Struyf F. Pooled analysis of large and long-term safety data from the human papillomavirus-16/18-AS04-adjuvanted vaccine clinical trial programme. Pharmacoepidemiol Drug Saf 2014; 23:466-79; PMID:24644063; http://dx.doi.org/10.1002/pds.3554
- Scheller NM, Pasternak B, Svanstrom H, Hviid A. Quadrivalent human papillomavirus vaccine and the risk of venous thromboembolism. JAMA 2014; 312:187-8; http://dx.doi.org/10.1001/jama.2014.2198
- Scheller NM, Svanstrom H, Pasternak B, Arnheim-Dahlstrom L, Sundstrom K, Fink K, Hviid A. Quadrivalent HPV vaccination and risk of multiple sclerosis and other demyelinating diseases of the central nervous system. JAMA 2015; 313:54-61; http://dx.doi.org/10.1001/jama.2014.16946
- Arnheim-Dahlstrom L, Pasternak B, Svanstrom H, Sparen P, Hviid A. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. BMJ 2013; 347:f5906; PMID:24108159.
- Vichnin M, Bonanni P, Klein NP, Garland SM, Block SL, Kjaer SK, Sings HL, Perez G, Haupt RM, Saah AJ, et al. An Overview of Quadrivalent Human Papillomavirus Vaccine Safety: 2006 to 2015. Pediatr Infect Dis J 2015; 34:983-91; PMID:26107345; http://dx.doi.org/10.1097/INF.0000000000000793
- Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T, Dubin G. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin 2009; 5:332-40; PMID:19221517; http://dx.doi.org/10.4161/hv.5.5.7211
- European Medicines Agency - News and Events - Review concludes evidence does not support that HPV vaccines cause CRPS or POTS. 2015
- Toh ZQ, Licciardi PV, Fong J, Garland SM, Tabrizi SN, Russell FM, Mulholland EK. Reduced dose human papillomavirus vaccination: An update of the current state-of-the-art. Vaccine 2015; 33:5042-50; PMID:26271829; http://dx.doi.org/10.1016/j.vaccine.2015.07.102