ABSTRACT
Respiratory syncytial virus (RSV) is a significant cause of lower respiratory tract infections resulting in bronchiolitis and even mortality in the elderly and young children/infants. Despite the impact of this virus on human health, no licensed vaccine exists. Unlike many other viral infections, RSV infection or vaccination does not induce durable protective antibodies in humans. In order to elicit high titer, neutralizing antibodies against RSV, we investigated the use of the adjuvant Advax™, a novel polysaccharide adjuvant based on delta inulin microparticles, to enhance antibody titers following vaccination. BALB/c mice were vaccinated intramuscularly with live RSV as a vaccine antigen in combination with one of two formulations of Advax™. Advax-1 was comprised of the standard delta inulin adjuvant and Advax-2 was formulated delta inulin plus CpG oligodendronucleotides (ODNs). An additional group of mice were either mock vaccinated, immunized with vaccine only, or administered vaccine plus Imject Alum. Following 3 vaccinations, mice had neutralizing antibody titers that correlated with reduction in viral titers in the lungs. Advax-1 significantly enhanced serum RSV-specific IgG1 levels at week 6 indicative of a Th2 response, similar to titers in mice administered vaccine plus Imject Alum. In contrast, mice vaccinated with vaccine plus Advax-2 had predominately IgG2a titers indicative of a Th1 response that was maintained during the entire study. Interestingly, regardless of which AdvaxTM adjuvant was used, the neutralizing titers were similar between groups, but the viral lung titers were significantly lower (∼10E+3pfu/g) in mice administered vaccine with either AdvaxTM adjuvant compared to mice administered adjuvants only. The lung pathology in vaccinated mice with AdvaxTM was similar to Imject Alum. Overall, RSV vaccine formulated with AdvaxTM had high neutralizing antibody titers with low lung viral titers, but exacerbated lung pathology compared to unvaccinated mice.
KEYWORDS:
Introduction
For infants less than 2 y of age, ∼5% of all hospitalizations are due to respiratory syncytial virus (RSV) infections. For pre-term infants (<30 weeks gestation), hospitalization rates may be as high as 18.7%.Citation1 To reduce the incidence of hospitalization from RSV infections, high-risk individuals, including infants and the elderly, can be administered palivizumab. This humanized mouse monoclonal antibody binds to the RSV fusion F protein, inhibits viral fusion to target cells and thereby neutralizes RSV.Citation2,3 Unfortunately, the expense of palivizumab reduces its cost-effectiveness for use in healthy full-term infants, children under 5 y of age or the elderly, and subsequently leaves these populations at risk for RSV infections, particularly through nosocomial exposure.Citation4-8 There are currently no licensed RSV vaccines. Development of new RSV vaccines has been impeded after an alum-adjuvanted formalin-inactivated RSV vaccine caused enhanced respiratory disease (ERD) complications during clinical trials.Citation9-14 The formalin-inactivated RSV vaccine enhanced RSV infection by eliciting non-neutralizing antibodies and inducing a highly Th2-polarized immune response within the respiratory tract.Citation9,11-13,15-17 Part of the problem may have been that formalin inactivation can alter virus epitopes resulting in elicitation of pathogenic instead of protective immune responses.Citation13,18-20 Formalin-inactivated influenza virus, measles virus, and severe acute respiratory syndrome (SARS) coronavirus vaccines have similarly elicited aberrant immune responses following vaccination.Citation21-23 Aluminum adjuvants appear to contribute to respiratory pathology. Finally, pre-term infants and young children may have polarized Th2 responses, exacerbating allergic and asthmatic responses,Citation24-26 and may therefore be more susceptible to damage induced by vaccine-induced Th2-dominant immune responses.Citation27 As such, there is still a need for vaccines against RSV and other viruses that are able to elicit antiviral protection without inducing adverse vaccine-induce immunopathology after virus exposure.
The use of Th1 polarizing adjuvant(s) can prevent vaccine-associated lung immunopathology. Currently, only a limited number of adjuvants are approved for use in human vaccines due to issues such as poor tolerability, toxicity, or poor antigen compatibility.Citation28-32 The majority of RSV vaccines that have been tested pre-clinically or clinically have contained alum based adjuvants such as aluminum hydroxideCitation33,34 or Imject Alum.Citation35-37 Aluminum-based adjuvants skew T cell responses in a Th2 direction with IgE immune responses,Citation38,39 and therefore are relatively contraindicated for RSV vaccines leaving a need for alternative adjuvants free of Th2 bias. Recently, a plant-derived delta inulin polysaccharide adjuvant (Advax™) provided antigen-sparing and enhanced immunogenicity for several experimental viral vaccines.Citation40-43 Advax™ is described as a Th0 adjuvant that works by enhancing antigen presenting cell function and is able to elicit both Th1 and Th2 responses while inducing minimal inflammation at the site of injection or systemically.Citation40
Previously, AdvaxTM adjuvant combined with a Toll-like receptor 9 (TLR9) agonist (CpG oligonucleotide) when combined with the recombinant spike protein or inactivated SARS coronavirus prevented eosinophilic lung immunopathology after SARS challenge,Citation44 thus exemplifying how a Th1-inducing adjuvant formulation may be helpful in preventing undue vaccine Th2 bias and associated lung pathology. This study therefore sought to compare the protective efficacy and ability to prevent vaccine-associated lung immunopathology of an RSV vaccine formulated with AdvaxTM adjuvant in place of the Th2-biasing alum adjuvant. To minimize potential formalin-associated alterations of the RSV F and G surface glycoproteins, intramuscular injection of live RSV virus was used as the vaccine antigen,Citation18 based on previous studies that showed, for example, that intramuscular vaccination with live influenza virus did not induce lung infection but was instead immunogenic and protective.Citation45
Materials and methods
Purification of RSV line 19
HEp2 cells (ATCC, CCL23), a human carcinoma HeLa-derivative, were grown in Dulbecco's Modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and penicillin-streptomycin (P/S) and maintained in a 5% CO2 humidified enclosure kept at 37°C. Line 19 respiratory syncytial virus (RSV) is an antigenic subgroup A strain and was propagated in semi-confluent HEp2 cells in FBS-free OptiMEM media until cytopathic effect was 70-80% visible. Cell supernatant containing RSV was semi-purified through a 30% glycerol layer as described previously.Citation46 In brief, pre-clarified viral supernatant supplemented with 0.1 volumes of 1 M MgSO4, was loaded onto 8.2 mL of a glycerol layer (0.22 μm-filtered buffer of 30% glycerol in 0.1 M MgSO4 and 50 mM HEPES pH 7.5, stored at 4°C) and viral particles were pelleted by centrifuging at 11,600 rpm (24,000g) in SureSpin 630 rotor for 3 hr at 4°C. Pellet was resuspended in pre-cooled viral resuspension buffer (0.22 μm-filtered 50mM HEPES (pH 7.5), 0.1 M MgSO4, and 150 mM NaCl), aliquoted, and snap-frozen over dry ice. Virus stocks were quantified using plaque assay as described below. To serve as a mock vaccine, HEp2 cells were mock-infected and grown in FBS-free OptiMEM and processed the same manner as the semi-purified virus solution.
Immunostaining plaque assay
HEp2 cells were seeded at density of 400,000 cells per well in a 24-well plate in complete growth media to achieve even, confluent monolayers the following day. Serial dilutions from virus stocks or lung homogenates were made in FBS-free DMEM and volumes of 100 μl were used to inoculate the monolayers for 1 h at room temperature (RT), with occasional rocking. Plates were incubated at 37°C for an additional 15 min then inoculum was replaced with 0.8% methylcellulose overlay composed of 5% FBS DMEM, supplemented with L-glutamine, P/S and amphotericin B. 4-5 d post-infection, when plaques were visible, methylcellulose was discarded into Virkon S solution and plates were gently rinsed with phosphate buffered saline twice. Monolayers were fixed in 80% methanol overnight at 4°C in preparation for immunostaining to detect RSV F.Citation47,48 Non-specific epitopes were blocked with 5% non-fat milk in PBS, then plaques were immunostained with monoclonal mouse anti-RSV F antibody (AbDSerotec, MCA490), followed by a horseradish peroxidase-conjugated anti-mouse secondary antibody, and visualized with 4CN substrate (Kirkegaard and Perry Laboratories).
Vaccination of mice
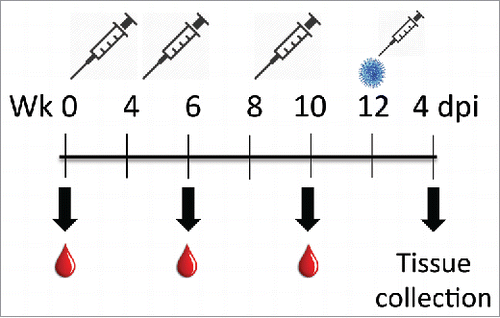
Female BALB/cJ mice (5-8 weeks of age) were purchased from The Jackson Laboratories and maintained in specific-pathogen free (SPF) conditions in accordance with the policies of the Institutional Animal Use and Care Committee. Submandibular blood collection using 5 mm sterile lancets yielded ∼250 μl blood, which was clotted for 1 hr at RT, then centrifuged for 10 min at 6k rpm. Sera aliquots were stored at −20°C until use for ELISA or plaque reduction neutralization test (PRNT). Intramuscular vaccination with adjuvanted-live RSV line 19 (10e+6 pfu) or mock-solution occurred on weeks 0, 4, and 8, and blood was collected at week 0, 6, and 10 (). Three immunizations were administered to elicit maximum antibody titers prior to live RSV challenge. Vaccine formulations were made at a 1:1 ratio between adjuvant and antigen such that 25 μl contained 106 pfu. The live RSV cell supernatant was mixed immediately before use with equal volume with one of the following adjuvants: Advax-1 or Advax-2 (Vaxine Pty Ltd., Adelaide, Australia) or Imject Alum (Thermo Scientific). Advax™ formulations contain a microparticulate suspension of delta inulin polysaccharide.Citation44,49 Advax-1™ was made up of delta inulin adjuvant alone whereas Advax-2™ was comprised of delta inulin suspension to which CpG ODN dissolved in PBS had been added by simple admixture. A total volume of 50 μl was injected into the right rear thigh using a 30-gauge needle.
Figure 1. Schematic of vaccination formulations and schedule for serum analysis. Animals were administered vaccine or mock solutions intramuscularly on weeks 0, 4, and 8 and bled via the submandibular cheek on weeks 6 and 8. On week 12, animals were subjected to an intranasal high-dose, high volume infection with Line 19 (blue dot). Animals were monitored for clinical symptoms up to 4 d post-infection, followed by humane euthanasia and collection of tissues for analysis.
RSV challenges
To infect, mice were anesthetized with 2-5% vaporized isoflourane then a total volume of 100ul was administered drop wise to the nares such that 10e+7 plaque forming units (pfu) of virus were used to intranasally infect each mouse (18-24 g in weight). This high-dose/high-volume (HD/HV) used was adapted from a previous use of RSV line 19 in BALB/c mice.Citation50 Animals were monitored for up to 4 d post-infection, followed by euthanasia by CO2. The left lung was gently inflated with 10% formalin, while the right lung was diced, portioned into collection tubes, then snap-frozen on dry ice. Lung homogenate was obtained by gently pressing samples of lung through a 70 μM mesh filter using a rubber plunger in a 10X weight/volume (wt/vol) of FBS-free DMEM. Cellular debris was removed by brief centrifugation and supernatant was used to infect monolayers to obtain lung viral load titers.
Plaque reduction neutralization test (PRNT)
The following approach was adapted from previously described standard RSV PRNT methodologyCitation51 and modified for 48-well format for rapid plaque counting. HEp2 cells (200,000 per well) were seeded 24 h prior to obtain even semi-confluent monolayers in 48-well plates. Heat inactivated mouse antisera was initially diluted in PBS 1:20, then serially diluted 4-fold in a 96-well plate. Virus was added to sera such that 50-80 pfu/ well. The virus-sera mixture was incubated for 1 h at 37°C. Media was removed from cell monolayers and virus-sera mixture (80 μl) was used to infect cells for 1 h at RT with rocking, followed by 15 min incubation at 37°C. Inoculum was replaced with methylcellulose overlay and plates were incubated for 2 d at 37°C, 5% CO2. Methylcellulose was removed, plates were washed gently then plaques were immunostained for the detection of RSV F. Plates were scanned and plaques counted using CTL-ImmunoSpot® S6 FluoroSpot Line Analyzer.
Histopathology
During tissue collection, left lungs were gently inflated with 10% formalin and stored for paraffin embedding. Formalin-fixed paraffin embedded tissue was cut into 6−μm sections and stained with hematoxylin and eosin. Two blinded pathologists visually scored histological slides based on the percentage of total lung involvement (0 = <10 %, 1 = 10-25%, 2 = 26-50%, 3 = >51 %) and bronchial and alveolar severity (0 = normal, 1 = mild, 2 = moderate, 3 = severe).
Detection of anti-RSV F antibodies
A high-affinity, 96-well flat bottom ELISA plate was coated with 100ng/well of recombinant baculovirus-derived RSV F (11049-V08B-20, Sino Biologicals, Inc., North Wales, PA, USA) in ELISA carbonate buffer (50mM carbonate buffer, pH 9.5) overnight at 4°C on a rocker. As a recombinant protein, the highly stable post-fusion RSV F conformation is expected to be the dominant antigen present.Citation52 The next morning, plates were washed PBS with 0.05% Tween-20 (PBST), then non-specific epitopes were blocked with 1% bovine serum albumin (BSA) in PBST solution for 1 h at RT. Buffer was removed then serial dilutions of antisera from vaccinated animals were added to plate and incubated for 1 h at 37°C. Plates were washed and probed with anti-mouse IgG horseradish-peroxidase-conjugated secondary antibodies (total IgG, IgG1, IgG2a, IgG2b, IgG3, SouthernBiotech) at dilution of 1:4000 for 1 h at 37°C. Plates were washed then freshly prepared o-phenylenediamine dihydrochloride (OPD) (P8287, Sigma, St. Louis, MO, USA) substrate in citrate buffer (P4922, Sigma) was added to wells, followed by 1N H2SO4 stopping reagent. Plates were analyzed with 492 nm absorbance using a Biotek microplate reader and background from negative wells was subtracted. All endpoint dilutions titers were normalized in reference to the mock-vaccinated animals, which did not have detectable specific mouse anti-RSV G IgG titers greater than background (data not shown). Optical density (O.D.) values were analyzed for statistical significance using 2-way multiple comparisons ANOVA and significance was assumed when p < 0.05.
Results
Vaccination with high-dose live RSV induces specific anti-RSV F antibodies
Mice were vaccinated intramuscularly three times at 4-week intervals with live RSV antigen (10e+6 pfu) alone or formulated with Imject alum or Advax-1 or −2 adjuvants (). Mice were then bled at week 6 (2 weeks post-second immunization) and week 10 (2 weeks post-third immunization) for assessment of total IgG responses, before being challenged intranasally with RSV to assess protection. Mice vaccinated with adjuvant only had undetectable (<1 :200) endpoint dilution titers at all time points (). Although mice vaccinated with RSV alone (no adjuvant) induced detectable antibody titers, these titers were ∼4-fold lower (1:49,355) after two vaccinations than those seen in animals vaccinated with RSV formulated with Imject alum or Advax-1 or −2 adjuvants, and they did not rise after the third vaccination. Mice vaccinated with RSV had similar average anti-RSV F antibody titers, regardless of the adjuvant formulation. Mice vaccinated with RSV plus any of the three adjuvants had an anti-RSV F titer ranging from 1:181,483 to 1:275,617 following 2 vaccinations and 1:1:377,421 to 1:486,000 following 3 vaccinations (). However, different IgG1 and IgG2a antibody profiles were observed based upon the adjuvant administered (). Mice vaccinated with HD RSV plus Advax-2 had higher IgG2a than IgG1 titers at week 6, indicating a dominant T-helper-1 (Th1) vaccine response () (p < 0 .05, both * and **). By contrast, mice vaccinated with Imject Alum had significantly higher IgG1 than IgG2a antibody titers indicative of a dominant Th2 vaccine response (). Mice that received HD RSV plus Advax-1 at week 10 had equal amounts of IgG1 and IgG2a, consistent with its previous description as a balanced Th0-adjuvant ().
Figure 2. Vaccination with high-dose live RSV eliminates detectable viral loads in lungs. Viral load was assessed by methylcellulose-plaque titration of lung homogenates and presented in a Log10 pfu/g of lung tissue. Titers were performed in triplicate and the mean titer for each animal (n=5) is indicated by the group symbol. The minimum level of detection of RSV viral loads in the lungs is 100 pfu/g and samples that did not yield plaques are indicated as none detected (N/D). *p>0 .01.
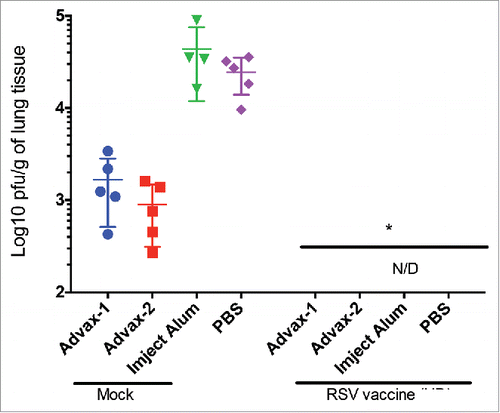
Figure 3. The IgG isotype of the anti-RSV antisera. The specific IgG isotype was assayed from the pooled serum collected at week 8 from each mouse group at a dilution of 1:1000 and reported as specific O.D. P-values were calculated between the IgG1 and IgG2a titers within a single group. Groups indicated with (*) have p < 0 .05 and the ratio of IgG1:IgG2a is <1 ; Groups indicated with (**) also have p < 0 .05 and ratio of IgG1:IgG2a is >1 . Panel A: week 6; Panel B: week 10.
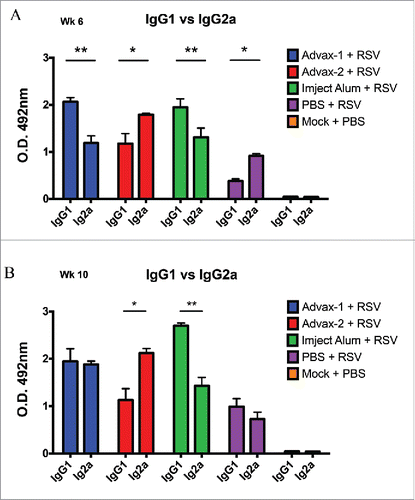
Table 1. Anti-RSV F specific IgG titers. Total mouse anti-RSV IgG responses were assayed by endpoint-dilution in ELISA assay. Each sample was performed in triplicate and average endpoint dilution was calculated from average of all mice within the same treatment group (n = 5). The IgG responses elicited by mock- or low-dose virus vaccine were below the background cut-off and are not shown.
Table 2. IgG Isotype Ratios. A ratio of IgG1 to IgG2a is provided and ratios less than 1 are indicative of enhanced Th1 responses, while ratios greater than 1 are skewed toward Th2. Subjects that have neither a Th1 or Th2 dominant profile are given values of 1. The O.D. values were averages of a group of mice (n = 5) when performed in triplicate.
Plaque reduction neutralization titers
Mice vaccinated with vaccine alone had PRNT titers at week 10 that were the same as mice vaccinated with vaccine plus Advax-1 (). However, mice administered vaccine plus Advax-2 or Imject alum had higher PRNT titers compared to mice vaccinated with vaccine plus Advax-1. Mice administered adjuvant only had no detectable PRNT antibodies.
Table 3. Anti-RSV responses elicited are neutralizing in plaque reduction neutralization test (PRNT) at week 10. Serum was examined for neutralizing activity in a modified PRNT assay and endpoint dilutions of sera were determined for a 50% or 80% reduction in the initial 50-80 pfu inoculated in each well. Serum samples were diluted 4-fold and performed in triplicate and average representative values are shown.
Reduction in viral lung titers in Advax vaccinated mice
Mice vaccinated with RSV alone or in combination with either Advax-1 or −2 or Imject alum control adjuvant formulations had no virus that was detectable in the lungs at 4 d post-RSV challenge (). Interestingly, mice vaccinated only with either AdvaxTM adjuvant alone (no vaccine) also had reduced lung viral titers compared to either mice administered Imject alum adjuvant alone or mock-vaccinated animals.
Lung histopathology
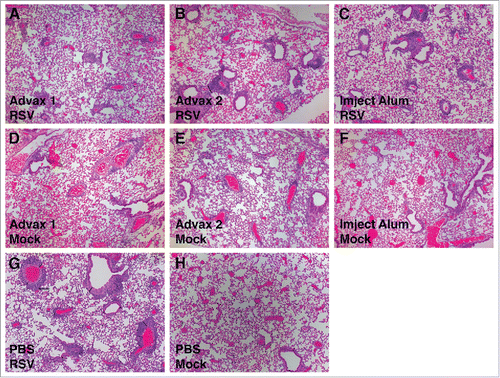
Lungs were harvested for histology 4 d post-infection from mice challenged intranasally with RSV. Mock-vaccinated mice challenged with RSV had little histopathological lung damage despite having high lung virus titers suggesting pneumonia was at nascent stages. Conversely, mice that were administered three vaccinations of RSV vaccine, and with high RSV neutralizing antibody titers, had more severe lung pathology with moderate bronchial and minor alveolar or interstitial inflammation (). This is consistent with previous reports that RSV F antigen elicits neutralizing titers, but is also associated with increased lung immunopathology post challenge.Citation53 There was a consistent trend to lower lung pathology scores in the mice that received RSV vaccine formulated with Advax-1 (). These lower lung scores were also observed in mice immunized with Advax-1 adjuvant alone.
Figure 4. Histopathology Scores. Histopathologic analysis of RSV-infected lung. Hematoxylin and eosin–stained sections of lungs that were collected at day 4 post-infection. Pneumonia severity was assessed by evaluating the percentage of lung involvement (A), and scoring the severity of bronchial (B) and alveolar or interstitial (C) pneumonia. Scoring of percentage of lung involvement: 0 = <10 %, 1 = 10-25%, 2 = 26–50%, 3 = >51 %. Scoring of bronchial and alveolar severity 0 = normal, 1 = mild, 2 = moderate, 3 = severe.
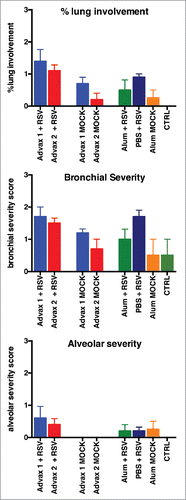
Figure 5. Histopathology images of lungs after RSV challenge. BALB/c mice were vaccinated with RSV vaccine with various adjuvants and infected with the RSV line 19 at 4 weeks after the final vaccination. Lungs were collected 4 d post-infection, formalin fixed, and paraffin embedded. Representative images from hematoxylin-and-eosin-stained sections: RSV vaccine plus Advax-1 (A), RSV vaccine plus Advax-2 (B), RSV vaccine plus Imject Alum (C), Mock vaccine plus Advax-1 (D), Mock vaccine plus Advax-2 (E), Mock vaccine plus Imject Alum (F), RSV vaccine only (G), and Mock vaccinated with mock adjuvant (H).
Discussion
Bronchiolitis and lower respiratory tract infections associated with RSV remain a significant threat to preterm infants, the elderly and immunocompromised individuals. The presence of serum- and mucosal-neutralizing antibody titers is correlated with reduced RSV infection and disease severity,Citation54,55 however natural infection fails to elicit long-term immunity.Citation56,57 In this report, we used different formulations of delta-inulin to elicit either T helper (Th) type 1 (Th1) or type 2 (Th2) immune responses induced by RSV viral particles administered intramuscularly as a vaccine. The elicited immune responses were compared to controls groups of mice vaccinated with vaccine only or vaccine plus aluminum salts. All mice vaccinated with vaccine plus adjuvants, regardless whether inulin- or aluminum-based, enhanced anti-RSV F antibody titers as compared to vaccine without adjuvant (). Mice vaccinated with vaccine without adjuvant still had protective anti-RSV antibody titers and moderate lung pathology (). In contrast, adjuvants alone failed to elicit protective anti-RSV F antibody titers in either total IgG or the ability to neutralize virus in in vitro assays.
Table 4. Summary of results. The adjuvant and vaccine groups are listed below with concluding results in plaque reduction neutralization titers, viral plaque titers obtain from lung homogenates, and histopathological scores. The (-) value indicates the titers are below the levels of detection, while (+++) or High indicate the groups that had the greatest overall values in each criteria.
Mice administered vaccine plus delta-inulin (Advax-1) had a Th2 response as indicated by the predominance of IgG1 anti-F antibody titers at week 6. An immunoglobulin class switch occurs following the expression of a new downstream heavy chain constant region (e.g. Cγ1, Cγ2b, Cγ2a, Cε) in a B-cell that already has a recombined immunoglobulin variable (VDJ) segment. This gene rearrangement of heavy chain constant regions depends on the cytokine environment at the time of the class switch.Citation58 Therefore, the detected IgG isotype is an indication of the cell type that was stimulated by the vaccine/adjuvant in order to secret the appropriate cytokine(s) to induce the particular gene rearrangement. Immunizations with aluminum salts or delta-inulin (Advax-1) induced significantly higher IgG1 titers at week 6 (), suggesting the presence of IL-4 producing Th2 cells. Aluminum salts have been used to enhance antibody responses to vaccines for almost 100 y.Citation59 Inclusion of aluminum salts as an adjuvant increased anti-F antibody responses to similar levels as all the AdvaxTM adjuvants. However, only mice vaccinated with the RSV vaccine plus Imject alum maintained a Th2 phenotype 4 weeks following the 3rd vaccination. By week 10, mice vaccinated with Advax-1 had a mixed IgG1/IgG2a isotype response (). The addition of CpG-oligonucleotides (CpG-ODN) to the delta-inulin adjuvant (Advax-2) during vaccination altered the IgG isotype profile resulting in the elicitation of predominantly IgG2a antibody titers against RSV F at week 6. This is indicative of a CD4+ T helper (Th) type 1 (Th1) response and hence an environment rich in IFN-γ. Following a third vaccination, mice vaccinated with the RSV vaccine plus Advax-2 maintained the Th1 phenotype, but mice vaccinated with Advax-1 adjuvants had a mixed Th1/Th2 phenotype (). IgG1 and IgG2a have non-redundant functions with IgG1 associated with virus neutralization and IgG2a associated with respiratory syncytial viral clearance.Citation56,60 Therefore, an adjuvant that elicits a mixed IgG response, and therefore, a mixed CD4+ T helper response may be more effective at reducing RSV lung titers and reducing disease severity.
T-cell-dependent humoral immune responses play an important role in the clearance and prevention of several virus infections. These immune responses require the critical interaction of antigen-specific TCRαβ+ CD4+ T-cells [CD4+ T-helper (Th) cells] with activated B-cells.Citation61 The “help” provided by CD4+ helper T-cell includes signaling through surface molecules (CD40 ligand and CD28, on the surface of the T-cell; CD40 and B7 (CD80/CD86) on the surface of B-cells) and the production of multiple cytokines (e.g., IL-4, IFN-γ, TGF-β, TNF-α, IL-5, IL-13, and others).Citation62 The consequences of this “help” are antibody production, induction of immunoglobulin class switch (IgG, IgA and IgE) and differentiation of immature B-cells into plasma B-cells.Citation63
The mechanism of action by Advax-1 is not yet understood. Advax-1 is a novel polysaccharide adjuvant derived from polyfructofuranosyl-D-glucose (delta inulin).Citation49 AdvaxTM adjuvant enhances immune responses and provides antigen-sparing in vaccines against a variety of pathogens in multiple animals.Citation64-67 As observed previously, formulation of the RSV vaccine with AdvaxTM adjuvant increased both Th1 (IgG2a) and Th2 (IgG1) antibody responses. Rather than skewing the immune response to a vaccine in either a Th1 or Th2 direction, mice immunized with vaccine containing Advax-1 have increased Th1 (IL-2, IFN-γ) and Th2 (IL-5, IL-6) cytokines produced by splenocytes. By contrast, aluminum-based adjuvants skew toward Th2 immune responses (36), whereas toll-like receptor (TLR) agonists typically skew toward Th1 responses (17-18). Given the absence of Th1 or Th2 polarization, AdvaxTM may best be described as a Th0 adjuvant. MF59 has similarly been described as a Th0 adjuvant (36). Despite their very different chemistries, AdvaxTM adjuvant being a polysaccharide particle and MF59 being a squalene oil emulsion, the effects of AdvaxTM adjuvant and MF59 adjuvant on influenza vaccine immunogenicity were similar in respect of the ability to increase influenza IgG titers, T-cell proliferation, and protection one year post-immunization. The results presented in this report with an RSV vaccine and AdvaxTM formulations elicited enhanced immunogenicity over the vaccine without an adjuvant, but did not sufficiently prevent lung pathology despite the use of live RSV and adjuvants that would promote either Th0 or Th1 responses.
While the exact mechanism of action of AdvaxTM adjuvant is not yet fully known, this adjuvant appears to act primarily through enhancement of antigen presenting cell function with this leading to enhanced B- and T-cell activationCitation40 and not through activation of innate inflammatory responses. Injection of AdvaxTM adjuvant alone without antigen did not provide significant non-specific protection against RSV in this study or other pathogens, such as influenza or Japanese encephalitis virusesCitation64 although viral lung titers were 10-fold reduced compared to aluminum salts or mock vaccine (). Although incompletely understood, there is a potential for priming of the innate immune system through use of adjuvants, which may enhance pathogen recognition or pathogen clearance by monocyte/macrophages or natural killer (NK) cells described in recent reviewsCitation68; the addition of Advax™ delta inulin adjuvant to a Listeria monocytogenes gold-nanoparticle vaccine reportedly increased total splenic NK numbersCitation69 yet mechanisms for delta-inulin related NK enhancement require further investigation. Administering the live RSV vaccine intramuscularly, all mice were protected against RSV infection regardless of the use of adjuvant during vaccination. These mice had high neutralizing antibody titers and had no viral titers detected in the lungs 4 d post-infection. This differs greatly from FI-RSV, which fails to elicit neutralizing antibody titers and will result in detectable viral loads in the lungs upon RSV challenge.Citation70
At lower doses of vaccine, the additional of CpG ODNs was more effective at reducing viral lung titers than delta-inulin only (Advax-1), which was statistically similar to mock vaccinated mice (data not shown). The effectiveness of CpG ODN as an adjuvant may be due to its ability to directly activate B cells (30, 43) and macrophagesCitation71 to induce secretion of Th1 cytokines such as IFN-γ and IL-12. In addition, through interactions with the Toll-like receptor 9 (TLR-9) of dendritic cells (17), CpG ODNs upregulate the expression of costimulatory molecules including class II MHC and B7 to produce Th1 polarizing cytokines and thus increase antigen presenting dendritic cells (1, 7, 25, 61). Despite reported advantages associated with immune activation through use of CpG ODNs, these nucleic acdis may also activate innate inflammatory response pathways and enhance susceptibility to some pathogens.Citation72 Importantly, another study observed enhanced lung immunopathology when administered in combination with a RSV F vaccine delivered intranasally.Citation73 As observed in this study, CpG ODN skew serum antibodies predominantly to the IgG2a isotype and yet failed to prevent lung immunopathology following RSV challenge. This suggests RSV vaccine-enhanced immunopathology could involve additional mechanisms beyond Th2-bias phenotypes and vaccine-enhanced lung disease may not be adequately predicted through IgG isotyping or microneutralization. Further examination is necessary to identify non-neutralizing antibodies or complement components that could be elevated, concurrently with neutralizing anti-RSV F titers, which could lead to immune complexes associated with ERD.Citation70
Absence of an effective vaccine leaves preterm infants, the elderly and immunocompromised populations susceptible and treatment associated with RSV disease persists as an economic and medical burden. The 1960s formalin-inactivated RSV vaccine failed to mount neutralizing protection against subsequent RSV challenge and instead, exacerbated Th2-associated vaccine-enhanced immunopathology. In this study, while an RSV vaccine, formulated with AdvaxTM adjuvants, elicited high titer neutralizing antibodies and a Th1 phenotype, there was no reduction lung immunopathology associated with RSV vaccination. Interestingly, using the Advax-1 adjuvant alone induced minor pathology in the lung, but was more severe when combined with the RSV vaccine. In contrast, this pathology was not induced using Synagis,Citation3,31,74-76 an antibody directed against F protein, but does occur with F protein immunization with alum or live RSV priming. It is possible that treatment with anti-F antibody, then RSV exposure, may prime the host for exacerbated RSV lung disease should a second infection occur. Future studies will need to be performed to determine these effects.
Disclosure of potential conflicts of interest
The authors reported no conflicts of interest.
References
- Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, et al. The burden of respiratory syncytial virus infection in young children. N Eng J Med 2009; 360(6):588-98; PMID:19196675; http://dx.doi.org/10.1056/NEJMoa0804877
- American Academy of Pediatrics Committee on Infectious D, American Academy of Pediatrics Bronchiolitis Guidelines C. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014; 134(2):e620-38; PMID:25070304; http://dx.doi.org/10.1542/peds.2014-1666
- The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102(3):531-7; http://dx.doi.org/10.1542/peds.102.3.531
- Hampp C, Kauf TL, Saidi AS, Winterstein AG. Cost-effectiveness of respiratory syncytial virus prophylaxis in various indications. Arch Pediatr Adolescent Med 2011; 165(6):498-505. Epub 2011/02/09; PMID:21300647
- Lofland JH, Touch SM, O'Connor JP, Chatterton ML, Moxey ED, Paddock LE, Nash DB, Desai SA. Palivizumab for respiratory syncytial virus prophylaxis in high-risk infants: a cost-effectiveness analysis. Clin Therapeutics 2000; 22(11):1357-69; PMID:11117660; http://dx.doi.org/10.1016/S0149-2918(00)83032-5
- Nuijten MJC, Wittenberg W, Lebmeier M. Cost Effectiveness of Palivizumab for Respiratory Syncytial Virus Prophylaxis in High-Risk Children: A UK Analysis. Pharmaco Economics 2007; 25(1):55-71; PMID:17192118; http://dx.doi.org/10.2165/00019053-200725010-00006
- Wang D, Cummins C, Bayliss S, Sandercock J, Burls A. Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation. Health Technol Assessment (Winchester, England) 2008; 12(36): iii, ix-x:1-86. Epub 2008/12/04
- Mahadevia PJ, Masaquel AS, Polak MJ, Weiner LB. Cost utility of palivizumab prophylaxis among pre-term infants in the United States: a national policy perspective. J Medical Economics 2012; 15(5):987-96. Epub 2012/05/12; PMID:22574798; http://dx.doi.org/10.3111/13696998.2012.690013
- Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC, 3rd, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol 1994; 68(8):5321-5. Epub 1994/08/01; PMID:8035532
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89(4):422-34; PMID:4305198
- Olson MR, Varga SM. Pulmonary immunity and immunopathology: lessons from respiratory syncytial virus. Expert Rev Vaccines 2008; 7(8):1239-55; PMID:18844597; http://dx.doi.org/10.1586/14760584.7.8.1239
- Openshaw PJM, Tregoning JS. Immune Responses and Disease Enhancement during Respiratory Syncytial Virus Infection. Clin Microbiol Rev 2005; 18(3):541-55; PMID:16020689; http://dx.doi.org/10.1128/CMR.18.3.541-555.2005
- Prince GA, Jenson AB, Hemming VG, Murphy BR, Walsh EE, Horswood RL, Chanock RM. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactiva ted virus. J Virol 1986; 57(3):721-8. Epub 1986/03/01; PMID:2419587
- Vaux-Peretz F, Chapsal J, Meignier B. Comparison of the ability of formalin-inactivated respiratory syncytial virus, immunopurified F, G and N proteins and cell lysate to enhance pulmonary changes in Balb/c mice. Vaccine 1992; 10(2):113-8; PMID:1539464; http://dx.doi.org/10.1016/0264-410X(92)90027-H
- De Swart RL, Kuiken T, Timmerman HH, van Amerongen G, Van Den Hoogen BG, Vos HW, Neijens HJ, Andeweg AC, Osterhaus AD. Immunization of macaques with formalin-inactivated respiratory syncytial virus (RSV) induces interleukin-13-associated hypersensitivity to subsequent RSV infection. J Virol 2002; 76(22):11561-9. Epub 2002/10/22; PMID:12388717; http://dx.doi.org/10.1128/JVI.76.22.11561-11569.2002
- Lindell DM, Morris SB, White MP, Kallal LE, Lundy PK, Hamouda T, Baker JR Jr, Lukacs NW. A Novel Inactivated Intranasal Respiratory Syncytial Virus Vaccine Promotes Viral Clearance without Th2 Associated Vaccine-Enhanced Disease. PLoS One 2011; 6(7):e21823; PMID:21789184; http://dx.doi.org/10.1371/journal.pone.0021823
- Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 Is Responsible for Acute Lung Immunopathology but Increases Survival of Respiratory Influenza Virus Infection. J Virol 2005; 79(10):6441-8; PMID:15858027; http://dx.doi.org/10.1128/JVI.79.10.6441-6448.2005
- Prince GA, Potash L, Horswood RL, Camargo E, Suffin SC, Johnson RA, Chanock RM. Intramuscular inoculation of live respiratory syncytial virus induces immunity in cotton rats. Infect Immun 1979; 23(3):723-8.; PMID:572342
- Delgado M, Coviello S, Monsalvo A, Melendi G, Hernandez J, Batalle J. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med 2009; 15:34-41; PMID:19079256; http://dx.doi.org/10.1038/nm.1894
- Moghaddam A, Olszewska W, Wang B, Tregoning JS, Helson R, Sattentau QJ, Openshaw PJ. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med 2006; 12(8):905-7. Epub 2006/07/25; PMID:16862151; http://dx.doi.org/10.1038/nm1456
- Gauger PC, Vincent AL, Loving CL, Henningson JN, Lager KM, Janke BH, Kehrli ME, Jr, Roth JA. Kinetics of lung lesion development and pro-inflammatory cytokine response in pigs with vaccine-associated enhanced respiratory disease induced by challenge with pandemic (2009) A/H1N1 influenza virus. Veterinary Pathol 2012; 49(6):900-12. Epub 2012/03/31; PMID:22461226; http://dx.doi.org/10.1177/0300985812439724
- Khurana S, Loving CL, Manischewitz J, King LR, Gauger PC, Henningson J, Vincent AL, Golding H. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Translational Med 2013; 5(200):200ra114. Epub 2013/08/30; PMID:23986398; http://dx.doi.org/10.1126/scitranslmed.3006366
- Vincent AL, Lager KM, Janke BH, Gramer MR, Richt JA. Failure of protection and enhanced pneumonia with a US H1N2 swine influenza virus in pigs vaccinated with an inactivated classical swine H1N1 vaccine. Veterinary Microbiol 2008; 126(4):310-23. Epub 2007/08/28; PMID:17719188; http://dx.doi.org/10.1016/j.vetmic.2007.07.011
- Bendelja K, Gagro A, Bace A, Lokar-Kolbas R, Krsulovic-Hresic V, Drazenovic V. Predominant type-2 response in infants with respiratory syncytial virus (RSV) infection demonstrated by cytokine flow cytometry. Clin Exp Immunol 2000; 121:332-8; PMID:10931150; http://dx.doi.org/10.1046/j.1365-2249.2000.01297.x
- Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JLL, Bont L. Respiratory Syncytial Virus and Recurrent Wheeze in Healthy Preterm Infants. N Eng J Med 2013; 368(19):1791-9; PMID:23656644; http://dx.doi.org/10.1056/NEJMoa1211917
- Carbonell-Estrany X, Figueras-Aloy J, Law BJ, Group IRIpVRSS. Identifying Risk Factors for Severe Respiratory Syncytial Virus Among Infants Born After 33 Through 35 Completed Weeks of Gestation: Different Methodologies Yield Consistent Findings. Pediatr Infect Dis J 2004; 23(11):S193-S201.; PMID:15577573; http://dx.doi.org/10.1097/01.inf.0000144664.31888.53
- Christiaansen AF, Knudson CJ, Weiss KA, Varga SM. The CD4 T cell response to respiratory syncytial virus infection. Immunol Res 2014; 59(1-3):109-17. Epub 2014/05/20; PMID:24838148; http://dx.doi.org/10.1007/s12026-014-8540-1
- Research CfBEa. Vaccine Safety & Availability - Common Ingredients in US. Licensed Vaccines. 2015
- Flach TL, Ng G, Hari A, Desrosiers M, Zhang P, Ward SM, Seamone ME, Vilaysane A, Mucsi AD, Fong Y, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med 2011; 17(4):479-87; PMID:21399646; http://dx.doi.org/10.1038/nm.2306
- Neuzil K, Johnson J, Tang Y, Prieels J, Slaoui M, Gar N, Graham BS. Adjuvants influence the quantitative and qualitative immune response in BALB/c mice immunized with respiratory syncytial virus FG subunit vaccine. Vaccine 1997; 15(5):525-32; PMID:9160520; http://dx.doi.org/10.1016/S0264-410X(97)00218-1
- Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014; 134(2):e620-38. Epub 2014/07/30; PMID:25070304; http://dx.doi.org/10.1542/peds.2014-1666
- Keitel W, Groth N, Lattanzi M, Praus M, Hilbert AK, Borkowski A, Tsai TF. Dose ranging of adjuvant and antigen in a cell culture H5N1 influenza vaccine: safety and immunogenicity of a phase 1/2 clinical trial. Vaccine 2010; 28(3):840-8. Epub 2009/10/20; PMID:19835829; http://dx.doi.org/10.1016/j.vaccine.2009.10.019
- Falsey A, Walsh E, Capellan J, Gravenstein S, Zambon M, Yau E, Gorse GJ, Edelman R, Hayden FG, McElhaney JE, et al. Comparison of the safety and immunogenicity of 2 respiratory syncytial virus (rsv) vaccines-nonadjuvanted vaccine or vaccine adjuvanted with alum-given concomitantly with influenza vaccine to high-risk elderly individuals. J Infect Dis 2008; 198(9):1317-26; PMID:18855558; http://dx.doi.org/10.1086/592168
- Cherukuri A, Stokes K, Patton K, Kuo H, Sakamoto K, Lambert S, Stillman E, Moore ML, Lee S. An adjuvanted respiratory syncytial virus fusion protein induces protection in aged BALB/c mice. Immunity Ageing 2012; 9(1):21; PMID:23031690; http://dx.doi.org/10.1186/1742-4933-9-21
- Knudson CJ, Hartwig SM, Meyerholz DK, Varga SM. RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS Pathog 2015; 11(3):e1004757. Epub 2015/03/15; PMID:25769044; http://dx.doi.org/10.1371/journal.ppat.1004757
- Derscheid RJ, Gallup JM, Knudson CJ, Varga SM, Grosz DD, van Geelen A, Hostetter SJ, Ackermann MR. Effects of Formalin-Inactivated Respiratory Syncytial Virus (FI-RSV) in the Perinatal Lamb Model of RSV. PLoS One 2013; 8(12):e81472; PMID:24324695; http://dx.doi.org/10.1371/journal.pone.0081472
- Hem SL, Johnston CT, HogenEsch H. Imject® Alum is not aluminum hydroxide adjuvant or aluminum phosphate adjuvant. Vaccine 2007; 25(27):4985-6; PMID:17543429; http://dx.doi.org/10.1016/j.vaccine.2007.04.078
- Grun JL, Maurer PH. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: The role of endogenous interleukin 1 in proliferative responses. Cellular Immunol 1989; 121(1):134-45; http://dx.doi.org/10.1016/0008-8749(89)90011-7
- Ghimire TR. The mechanisms of action of vaccines containing aluminum adjuvants: an in vitro vs in vivo paradigm. SpringerPlus 2015; 4:181. Epub 2015/05/02; PMID:25932368; http://dx.doi.org/10.1186/s40064-015-0972-0
- Honda-Okubo Y, Saade F, Petrovsky N. Advax, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine. 2012; 30(36):5373-81. Epub 2012/06/26; PMID:22728225; http://dx.doi.org/10.1016/j.vaccine.2012.06.021
- Gordon DL, Sajkov D, Woodman RJ, Honda-Okubo Y, Cox MM, Heinzel S, Petrovsky N. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax polysaccharide adjuvant. Vaccine 2012; 30(36):5407-16. Epub 2012/06/22; PMID:22717330; http://dx.doi.org/10.1016/j.vaccine.2012.06.009
- Bielefeldt-Ohmann H, Prow NA, Wang W, Tan CS, Coyle M, Douma A, Hobson-Peters J, Kidd L, Hall RA, Petrovsky N. Safety and immunogenicity of a delta inulin-adjuvanted inactivated Japanese encephalitis virus vaccine in pregnant mares and foals. Veterinary Res 2014; 45:130. Epub 2014/12/18; PMID:25516480; http://dx.doi.org/10.1186/s13567-014-0130-7
- Honda-Okubo Y, Barnard D, Ong CH, Peng BH, Tseng CT, Petrovsky N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol 2015; 89(6):2995-3007. Epub 2014/12/19; PMID:25520500; http://dx.doi.org/10.1128/JVI.02980-14
- Honda-Okubo Y, Barnard D, Ong CH, Peng B-H, Tseng C-TK, Petrovsky N. Severe Acute Respiratory Syndrome-Associated Coronavirus Vaccines Formulated with Delta Inulin Adjuvants Provide Enhanced Protection while Ameliorating Lung Eosinophilic Immunopathology. J Virol 2015; 89(6):2995-3007; PMID:25520500; http://dx.doi.org/10.1128/JVI.02980-14
- Harris K, Ream R, Gao J, Eichelberger M. Intramuscular immunization of mice with live influenza virus is more immunogenic and offers greater protection than immunization with inactivated virus. Virol J 2011; 8(1):251; PMID:21600020; http://dx.doi.org/10.1186/1743-422X-8-251
- San-Juan-Vergara H, Sampayo-Escobar V, Reyes N, Cha B, Pacheco-Lugo L, Wong T, Peeples ME, Collins PL, Castaño ME, Mohapatra SS. Cholesterol-Rich microdomains as docking platforms for respiratory syncytial virus in normal human bronchial epithelial cells. J Virol 2012; 86(3):1832-43; PMID:22090136; http://dx.doi.org/10.1128/JVI.06274-11
- Wong TM, Boyapalle S, Sampayo V, Nguyen HD, Bedi R, Kamath SG, Moore ML, Mohapatra S, Mohapatra SS. Respiratory syncytial virus (rsv) infection in elderly mice results in altered antiviral gene expression and enhanced pathology. PLoS One 2014; 9(2):e88764
- Boyapalle S, Wong T, Garay J, Teng M, San Juan-Vergara H, Mohapatra S, Mohapatra S. Respiratory syncytial virus NS1 protein colocalizes with mitochondrial antiviral signaling protein MAVS following infection. PLoS One 2012; 7(2):e29386; PMID:22383950; http://dx.doi.org/10.1371/journal.pone.0029386
- Cooper PD, Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising β-D-[2 -> 1] poly(fructo-furanosyl) α-D-glucose polymers. Glycobiology 2011; 21(5):595-606. Epub 2010/12/15; PMID:21147758; http://dx.doi.org/10.1093/glycob/cwq201
- Empey KM, Orend JG, Peebles RS, Jr, Egaña L, Norris KA, Oury TD, Kolls JK. Stimulation of Immature Lung Macrophages with Intranasal Interferon Gamma in a Novel Neonatal Mouse Model of Respiratory Syncytial Virus Infection. PLoS One 2012; 7(7):e40499; PMID:22792355; http://dx.doi.org/10.1371/journal.pone.0040499
- Fuentes S, Crim RL, Beeler J, Teng MN, Golding H, Khurana S. Development of a simple, rapid, sensitive, high-throughput luciferase reporter based microneutralization test for measurement of virus neutralizing antibodies following Respiratory Syncytial Virus vaccination and infection. Vaccine 2013; 31(37):3987-94. Epub 2013/06/08; PMID:23742994; http://dx.doi.org/10.1016/j.vaccine.2013.05.088
- McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, et al. Structure of RSV Fusion Glycoprotein Trimer Bound to a Prefusion-Specific Neutralizing Antibody. Science 2013; 340(6136):1113-7; PMID:23618766; http://dx.doi.org/10.1126/science.1234914
- Connors M, Kulkarni AB, Firestone CY, Holmes KL, Morse HC, 3rd, Sotnikov AV, Murphy BR. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol 1992; 66(12):7444-51. Epub 1992/12/01; PMID:1433525
- Walsh E, Falsey AR. Humoral and Mucosal Immunity in Protection from Natural Respiratory Syncytial Virus Infection in Adults. J Infect Dis 2004; 190(2):373-8; PMID:15216475; http://dx.doi.org/10.1086/421524
- Walsh EE, Peterson DR, Kalkanoglu AE, Lee FE-H, Falsey AR. Viral Shedding and Immune Responses to Respiratory Syncytial Virus Infection in Older Adults. J Infect Dis 2013; 207(9):1424-32; PMID:23382572; http://dx.doi.org/10.1093/infdis/jit038
- Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 1991; 163(4):693-8. Epub 1991/04/01; PMID:2010624; http://dx.doi.org/10.1093/infdis/163.4.693
- Hall CB, Walsh EE, Schnabel KC, Long CE, McConnochie KM, Hildreth SW, Anderson LJ. Occurrence of groups A and B of respiratory syncytial virus over 15 years: associated epidemiologic and clinical characteristics in hospitalized and ambulatory children. J Infect Dis 1990; 162(6):1283-90. Epub 1990/12/01; PMID:2230258; http://dx.doi.org/10.1093/infdis/162.6.1283
- Matsuoka M, Yoshida K, Maeda T, Usuda S, Sakano H. Switch circular DNA formed in cytokine-treated mouse splenocytes: evidence for intramolecular DNA deletion in immunoglobulin class switching. Cell 1990; 62(1):135-42; PMID:2114219; http://dx.doi.org/10.1016/0092-8674(90)90247-C
- Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med 1994; 179(4):1285-95. Epub 1994/04/01; PMID:7908323; http://dx.doi.org/10.1084/jem.179.4.1285
- Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol 2007; 178(5):2706-13. Epub 2007/02/22; PMID:17312112; http://dx.doi.org/10.4049/jimmunol.178.5.2706
- Brown DM, Roman E, Swain SL. CD4 T cell responses to influenza infection. Semin Immunol 2004; 16(3):171-7; PMID:15130501; http://dx.doi.org/10.1016/j.smim.2004.02.004
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature 1996; 383(6603):787-93; PMID:8893001; http://dx.doi.org/10.1038/383787a0
- Parker DC. T cell-dependent B cell activation. Annu Rev Immunol 1993; 11:331-60; PMID:8476565; http://dx.doi.org/10.1146/annurev.iy.11.040193.001555
- Lobigs M, Pavy M, Hall RA, Lobigs P, Cooper P, Komiya T, Toriniwa H, Petrovsky N. An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. J General Virol 2010; 91(Pt 6):1407-17; PMID:20130134; http://dx.doi.org/10.1099/vir.0.019190-0
- Layton RC, Petrovsky N, Gigliotti AP, Pollock Z, Knight J, Donart N, Pyles J, Harrod KS, Gao P, Koster F. Delta inulin polysaccharide adjuvant enhances the ability of split-virion H5N1 vaccine to protect against lethal challenge in ferrets. Vaccine 2011; 29(37):6242-51. Epub 2011/07/09; PMID:21736913; http://dx.doi.org/10.1016/j.vaccine.2011.06.078
- Cristillo AD, Ferrari MG, Hudacik L, Lewis B, Galmin L, Bowen B, Thompson D, Petrovsky N, Markham P, Pal R. Induction of mucosal and systemic antibody and T-cell responses following prime-boost immunization with novel adjuvanted human immunodeficiency virus-1-vaccine formulations. J Gen Virol 2011; 92(Pt 1):128-40. Epub 2010/12/21; PMID:21169215; http://dx.doi.org/10.1099/vir.0.023242-0
- Eckersley A.M. PN, Kinne J, Wernery R, Wernery U. Improving the dromedary antibody response: The hunt for the ideal camel adjuvant. J Camel Practice Res 2011; 18(1):30-46
- Töpfer E, Boraschi D, Italiani P. Innate Immune Memory: The latest frontier of adjuvanticity. J Immunol Res 2015; 2015:7
- Rodriguez-Del Rio E, Marradi M, Calderon-Gonzalez R, Frande-Cabanes E, Penades S, Petrovsky N, Alvarez-Dominguez C. A gold glyco-nanoparticle carrying a Listeriolysin O peptide and formulated with Advax delta inulin adjuvant induces robust T-cell protection against listeria infection. Vaccine 2015; 33(12):1465-73. Epub 2015/02/11; PMID:25659269; http://dx.doi.org/10.1016/j.vaccine.2015.01.062
- Polack FP, Teng MN, L.Collins P, Prince GA, Exner M, Regele H, Lirman DD, Rabold R, Hoffman SJ, Karp CL, et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med 2002; 196(6):859-65; PMID:12235218; http://dx.doi.org/10.1084/jem.20020781
- Crabtree TD, Jin L, Raymond DP, Pelletier SJ, Houlgrave CW, Gleason TG, Pruett TL, Sawyer RG. Preexposure of Murine Macrophages to CpG oligonucleotide results in a biphasic tumor necrosis factor alpha response to subsequent lipopolysaccharide challenge. Infect Immun 2001; 69(4):2123-9; PMID:11254566; http://dx.doi.org/10.1128/IAI.69.4.2123-2129.2001
- Ito S, Pedras-Vasconcelos J, Klinman DM. CpG oligodeoxynucleotides increase the susceptibility of normal mice to infection by candida albicans. Infect Immun 2005; 73(9):6154-6; PMID:16113339; http://dx.doi.org/10.1128/IAI.73.9.6154-6156.2005
- Prince GA, Mond JJ, Porter DD, Yim KC, Lan SJ, Klinman DM. Immunoprotective activity and safety of a respiratory syncytial virus vaccine: mucosal delivery of fusion glycoprotein with a CpG oligodeoxynucleotide adjuvant. J Virol 2003; 77(24):13156-60; PMID:14645572; http://dx.doi.org/10.1128/JVI.77.24.13156-13160.2003
- Chang RK, Chen A. Impact of palivizumab on rsv hospitalizations for children with hemodynamically significant congenital heart disease. Pediatr Cardiol 2010; 31(1):90-5; PMID:19915892; http://dx.doi.org/10.1007/s00246-009-9577-0
- Clementi N, Mancini N, Solforosi L, Castelli M, Clementi M, Burioni R. Phage display-based strategies for cloning and optimization of monoclonal antibodies directed against human pathogens. Int J Mol Sci 2012; 13(7):8273-92; PMID:22942702; http://dx.doi.org/10.3390/ijms13078273
- Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH, Jr, Connor EM, Sondheimer HM. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003; 143(4):532-40; PMID:14571236; http://dx.doi.org/10.1067/S0022-3476(03)00454-2
