ABSTRACT
Repulsive guidance molecule a (RGMa) is an axonal guidance molecule that has recently found to exert function in immune system. This study evaluated the function of RGMa in modulation of dendritic cells (DCs) function stimulated with Achyranthes bidentata polysaccharide (ABP) and lipopolysaccharide (LPS) using a RGMa-neutralizing antibody. Compared with the Control-IgG/ABP and Control-IgG/LPS groups, DCs in the Anti-RGMa/ABP and Anti-RGMa/LPS groups 1) showed small, round cells with a few cell processes and organelles, and many pinocytotic vesicles; 2) had decreased MHC II, CD86, CD80, and CD40 expression; 3) displayed the decreased IL-12p70, IL-1β and TNF-α levels and increased IL-10 secretion; 4) had a high percentage of FITC-dextran uptake; and 5) displayed a reduced ability to drive T cell proliferation and reinforced T cell polarization toward a Th2 cytokine pattern. We conclude that DCs treated with RGMa-neutralizing antibodies present with tolerogenic and immunoregulatory characteristics, which provides new insights into further understanding of the function of RGMa.
Introduction
Anchored to the cell membrane by covalent linkage to glycosylphosphatidylinositol, repulsive guidance molecule a (RGMa) plays an important role in nervous system development, including neuronal survival, cell adhesion, and endochondral bone formation.Citation1-3 Thought to be exclusively expressed in the central nervous system (CNS), RGMa has been recently found to be expressed in spleen, monocytes, and lymphocytes, which suggests a role within the immune system.Citation4 Futhermore, a study on multiple sclerosis (MS) and a mouse model of myelin oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis (EAE), in which dendritic cells (DCs) play an irreplaceable role, demonstrates that application of a RGMa-neutralizing antibody can reduce the clinical signs and immune responses of EAE by DCs.Citation5 Consistent with the study, we found that the expression of RGMa was induced in mature DCs. These studies all suggest that RGMa may function in immune regulation.
DCs harbor an extraordinary capacity to stimulate naive T cells, generate primary and secondary immune responses against foreign antigens, and serve as a bridge connecting the innate and adaptive immune systems.Citation6,7 Mature DCs express high levels of MHC II and costimulatory molecules and therefore incline to induce T cell responses.Citation8,9 Immature DCs with a deficiency in co-stimulatory molecules and inflammatory cytokines induce T cell anergy and Treg cell generation, and play the vital role in immune tolerance.Citation10,11 Recent studies have also revealed a certain DCs subset which is involved in the immune tolerance; such cells are called tolerogenic dendrtic cells (tolDCs). TolDCs can modulate the immune system by several mechanisms, including expressing low levels of proinflammatory cytokines and co-stimulatory molecules, secreting immunosuppressive cytokines, conversion of naive T cells into Treg cells, and inhibiting the functions of effector T cells.Citation12,13
In addition to lipopolysaccharide (LPS), many other stimuli can induce DCs maturation, like Poly (I:C) (Tlr-3 agonist) and chinese medicine polysaccharide.Citation14,15 Achyranthes bidentata polysaccharide (ABP), which is isolated from A.bidentata, has the immunoregulatory function and can be used in restoring immune system of cancer patients. In addition, our research group found ABP could induce DCs maturation.Citation16 Given the immunoregulatory characteristics of RGMa in other immune responses, we speculate that this molecule may play an important role in DCs function. Therefore, we added the RGMa-neutralizing antibody into cell culture medium to inhibit the expression of this molecule and explored the influences of RGMa on DCs function.
Results
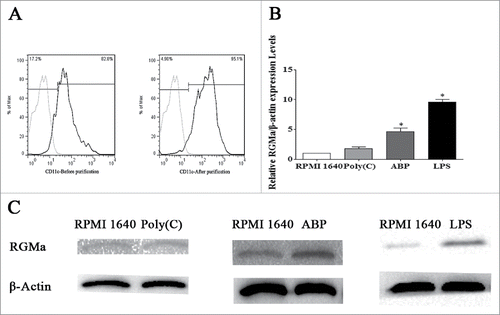
Sustained induction of RGMa in DCs exposed to LPS and ABP
DCs were purified using a CD11c positive selection kit and MACS columns. Purity, as detected by flow cytometry, was up to 95% (). To determine whether RGMa was involved in DCs maturation, we analyzed the changes in RGMa expression during the process of DCs maturation induced by Poly (I:C), ABP and LPS. As shown in , low RGMa mRNA expression was noted in immature DCs, whereas a significant increase in expression was observed in mature DCs after ABP and LPS stimulation (P < 0.05), and no obvious differences were found after DCs were stimulated with Poly (I:C) (P > 0.05). In addition, we also analyzed the cell membrane expression by Western blot. As shown in , the cell membrane expression of RGMa was induced in DCs stimulated with ABP and LPS, and no obvious differences were found after DCs were stimulated with Poly (I:C) (P > 0.05). These results suggested that the maturation status of DCs could influence RGMa expression.
Figure 1. RGMa expression in DCs treated with ABP and LPS. (A) DCs were purified using MACS columns and characterized by flow cytometry. Light-colored lines represent background stained with isotype control mAbs, and dark-colored lines represent CD11c staining. (B) Real-time PCR indicated relative RGMa mRNA expression in DCs after Poly (I:C), ABP and LPS treatment for 24 h. (C) Western blotting showed the relative cell membrane expression of RGMa/β-actin in DCs after Poly (I:C), ABP and LPS treatment for 24 h. Data were expressed as the mean ± SEM of three independent experiments. *P< 0.05 vs. RPMI 1640 DCs
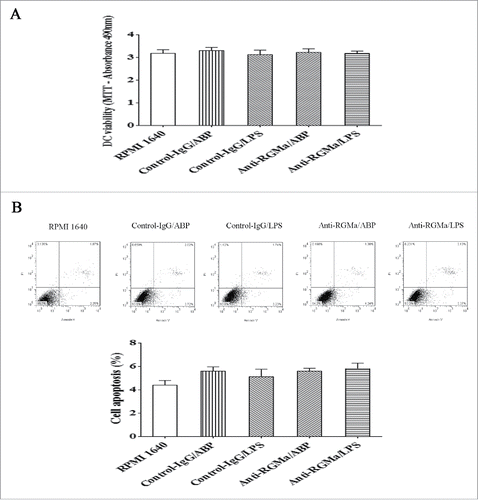
Inhibition of RGMa by neutralizing antibody does not affect DCs survival or apoptosis
We sought to evaluate the toxicity of the neutralizing antibody by measuring the viability and apoptosis of DCs. As shown in , there were no obvious differences among the treatment groups (P > 0.05). Cell apoptosis was measured using annexin V and propidium iodine (PI) staining. As shown in , there were also no obvious differences among the treatment groups (P > 0.05).
Figure 2. Analysis of DCs viability and apoptosis after neutralization of RGMa. (A) Cell viability was assessed by MTS assay. (B) Cell apoptosis was measured using annexin V and PI staining. The bar graph represents cell apoptosis. Data were expressed as the mean ± SEM of three independent experiments.
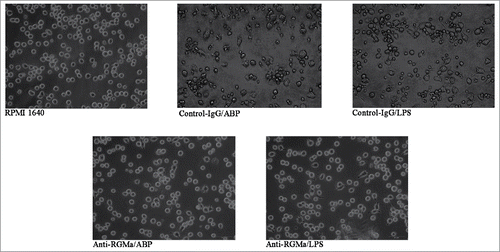
Morphology of DCs treated with RGMa-neutralizing antibodies
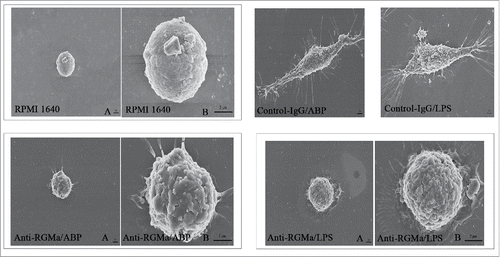
To evaluate the role of RGMa in DCs phenotypes, we added the RGMa-neutralizing antibody into cell culture medium to examine DCs morphology by SEM, TEM, and light microscope. As shown in , DCs in the RPMI 1640, Anti-RGMa/ABP and Anti-RGMa/LPS groups displayed more pinocytotic vesicles, lysosomes and a few organelles, indicating the important characteristic of phagocytosis. In contrast, DCs in the Control-IgG/ABP and Control-IgG/LPS groups displayed multiple mitochondria, endoplasmic reticulum, and Golgi apparatus organelles. As shown in , DCs in the RPMI 1640, Anti-RGMa/ABP and Anti-RGMa/LPS groups showed small, round cells with a few body extensions. However, DCs in the Control-IgG/ABP and Control-IgG/LPS groups showed an irregular shape with long, regular and wide cellular processes. As shown in , DCs in the RPMI 1640, Anti-RGMa/ABP and Anti-RGMa/LPS groups showed small, round cells with smooth boundaries. However, DCs in the Control-IgG/ABP and Control-IgG/LPS groups showed large cells with irregular shape and blunt dendritic processes.
Figure 3. Morphology of DCs treated with RGMa-neutralizing antibodies under TEM. Morphology was determined after 10 μg/ml antibody treatment for 48 h. In the RPMI 1640, Anti-RGMa/ABP and Anti-RGMa/LPS groups, DCs displayed more pinocytotic vesicles (thick solid arrow) and lysosomes (fine line arrow); in the Control-IgG/ABP and Control-IgG/LPS groups, DCs showed multiple organelles, such as mitochondria (single-dashed line arrow) and Golgi apparatus (double-dashed line arrow). Magnification ×10,000
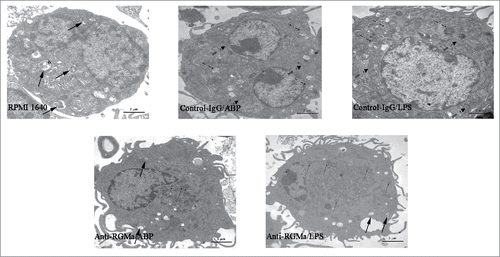
Figure 4. Morphology of DCs treated with RGMa-neutralizing antibodies under SEM. Morphology was determined after 10 μg/ml antibody treatment for 48 h (RPMI 1640 (B), Anti-RGMa/ABP (B) and Anti-RGMa/LPS groups (B) : magnification × 10,000; RPMI 1640 (A), Anti-RGMa/ABP (A), Anti-RGMa/LPS (A), Control-IgG/ABP and Control-IgG/LPS groups: magnification × 3,000.)
Reduced expression of surface markers in DCs treated with RGMa-neutralizing antibodies
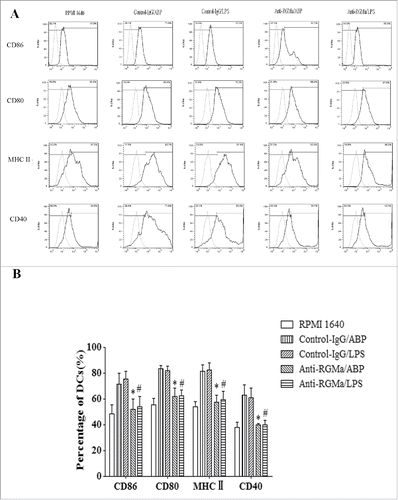
To examine the effect of RGMa on DCs maturation, expression of surface markers were analyzed. As shown in , DCs in the Anti-RGMa/ABP and Anti-RGMa/LPS groups displayed low levels of MHC II, CD86, CD80, and CD40 via flow cytometry compared with Control-IgG/ABP and Control-IgG/LPS groups, respectively (P < 0.05 for all). These results suggested that RGMa-neutralizing antibody could restrict the DCs maturation induced by LPS and ABP.
Figure 6. Markers expression on the surface of DCs treated with RGMa-neutralizing antibodies. (A) Expression of surface markers in DCs, including CD86, CD80, MHC-II and CD40, was determined by flow cytometry after 10 μg/ml antibody treatment for 48 h. Light-colored lines represent background stained with isotype control mAbs, and dark-colored lines represent CD86, CD80, MHC-II and CD40 staining. (B) The bar graph represents markers expression of CD86, CD80, MHC-II and CD40. Data were expressed as the mean ± SEM of three independent experiments. *P< 0.05 vs. Control-IgG/ABP group.#P < 0.05 vs. Control-IgG/LPS group
Reduced secretion of proinflammatory cytokines in DCs treated with RGMa-neutralizing antibodies
To estimate the effects of RGMa blockade on cytokines, IL-10, IL-12p70, IL-1β and TNF-α levels were measured by ELISA. As shown in , IL-12p70, IL-1β and TNF-α levels in the Anti-RGMa/ABP and Anti-RGMa/LPS groups were significantly lower than those in the Control-IgG/ABP and Control-IgG/LPS groups, respectively (P < 0.05 for all). In contrast, IL-10 levels in the Anti-RGMa/ABP and Anti-RGMa/LPS groups were significantly higher than those in the Control-IgG/ABP and Control-IgG/LPS groups, respectively (P < 0.05 for all).
Figure 7. Pro-inflammatory cytokines and CCR7 expression in DCs treated with RGMa-neutralizing antibodies. (A) Cytokine secretion, including IL-12p70, IL-1β, TNF-α, and IL-10, was determined by ELISA after 10 μg/ml antibody treatment for 48 h. (B) Real-time PCR indicated relative CCR-7 mRNA expression in DCs after 10 μg/ml antibody treatment for 48 h. Data were shown as the mean ± SEM of three independent experiments. *P< 0.05 vs. Control-IgG/ABP group.#P < 0.05 vs. Control-IgG/LPS group
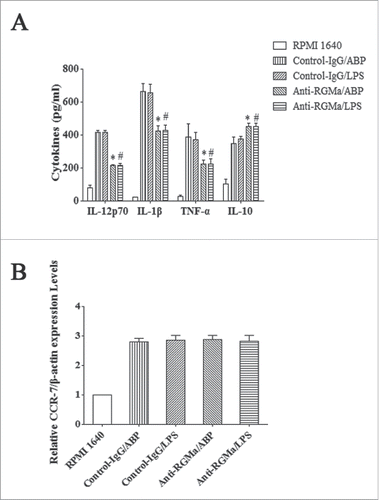
The expression of CCR7 in DCs was analysed by real-time PCR. As shown in , after induced by ABP and LPS, there were also no obvious differences among the treatment groups (P > 0.05).
Reduced capacity of phagocytosis in DCs treated with RGMa-neutralizing antibodies
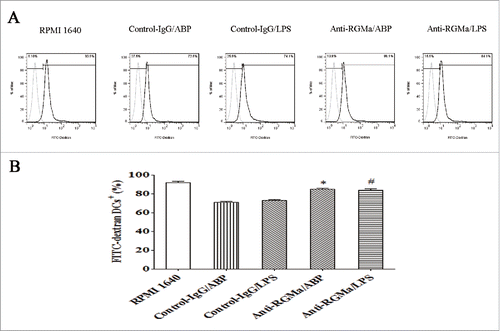
To assess the phagocytic ability of DCs treated with RGMa-neutralizing antibody, FITC-dextran uptake was measured by flow cytometry. As shown in , DCs in the Anti-RGMa/ABP and Anti-RGMa/LPS groups had a high percentage of FITC-dextran uptake compared with those in the Control-IgG/ABP and Control-IgG/LPS groups, respectively (P < 0.05 for all).
Figure 8. Phagocytosis in DCs treated with RGMa-neutralizing antibodies. FITC-dextran uptake was measured by flow cytometry and used as a measure of phagocytosis after 10 μg/ml antibody treatment for 48 h. (A) Light-colored lines represent background stained with isotype control mAbs, and dark-colored lines represent FITC-dextran staining. (B) The bar graph represents FITC-dextran uptake. Data were expressed as the mean ± SEM of three independent experiments. *P< 0.05 vs. Control-IgG/ABP group.#P < 0.05 vs. Control-IgG/LPS group
Reduced capacity to induce T cell proliferation and differentiation in DCs treated with RGMa-neutralizing antibodies
Absorbance values in a co-culture environment represented the ability of DCs to induce T cell proliferation. As shown in , DCs in the Anti-RGMa/ABP and Anti-RGMa/LPS groups displayed a reduced ability to drive T cell proliferation at the DCs to T cells ratios of 1:1 and 1:10, compared with those in the Control-IgG/ABP and Control-IgG/LPS groups, respectively (P < 0.05 for all). However, after induced by ABP and LPS, there were no obvious differences for the treatment groups when ratios of DCs to T cells were 1:50 and 1:100 (P > 0.05 for all).
Figure 9. Induction of T cell proliferation and differentiation by DCs treated with RGMa-neutralizing antibodies. (A) Absorbance values represent T cell proliferation. CD4+T lymphocytes were co-cultured at various ratios with DCs for 72 h. (B) Cytokine secretion into the supernatant of a co-culture environment represens T cell differentiation. CD4+T lymphocytes were co-cultured with DCs at a ratio of 1:1 for 72 h. Cytokine secretion, including IL-2, IFN-γ, IL-10, and IL-13, was determined by ELISA. Data represented the mean ± SEM of three independent experiments. *P< 0.05 vs. Control-IgG/ABP group.#P < 0.05 vs. Control-IgG/LPS group
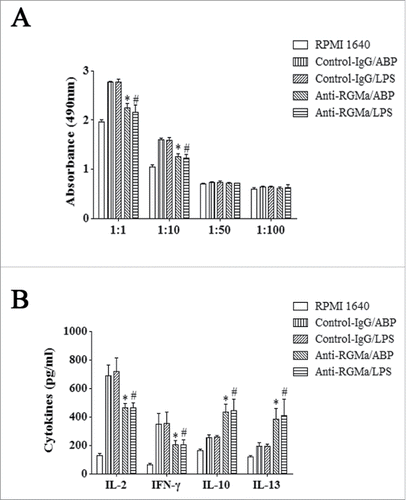
Because the ability to induce T cell differentiation was an important characteristic of DCs, IL-2, IFN-γ, IL-10, and IL-13 secreted by T cells in a co-culture environment, were measured at the DCs to T cells ratio of 1:1. As shown in , T cells co-cultured with DCs in the Anti-RGMa/ABP and Anti-RGMa/LPS groups showed the decreased IL-2 and IFN-γ levels and increased IL-10 and IL-13 secretion compared with those in the Control-IgG/ABP and Control-IgG/LPS groups (P < 0.05 for all).
Discussion
Nowadays, accumulating evidences have showed that RGMa exerts function in immune system. Kong et al. indicated that after cerebral ischemia/reperfusion, in which inflammation plays an important role in pathogenesis, RGMa expression was increased together with high secretion of the inflammatory cytokines IL-1β and IL-6.Citation17 Ischemic post-conditioning can significantly alleviate ischemic immune injury, accompanied by a decrease in RGMa, IL-1β and IL-6. RGMa-neutralizing antibody application reduces neurite outgrowth inhibition and growth cone collapse in activated microglia, and inhibition of activation decreases RGMa expression, suggesting that RGMa is involved in the immune regulation of microglia.Citation18 Additionally, RGMa has been considered a potential candidate gene in multiple sclerosis.Citation5,19 It is also found that inhibiting RGMa expression attenuates clinical signs and reduces neuronal damage in a specific Th17-induced EAE.Citation20 These lines of evidence all suggest a role for RGMa in immune regulation. Additionally, our data also suggested that RGMa expression was upregulated after DCs were stimulated with ABP and LPS, which suggests a role in DCs function.
In our study, RGMa-neutralizing antibody could keep DCs in a steady immature status with low levels of MHC and costimulatory molecules and stronger phagocytosis activity even stimulated with ABP and LPS. However, the neutralizing antibody did not have effect on CCR7, perhaps because the most important function for the chemokine is organizing DCs migration, not regulating DCs maturation or tolerance. Additionally, CCR7 can modulate DCs maturation only in activated DCs, not in immature DCs,Citation21 which is consistent with our study. Interestingly, we also noticed that DCs treated with RGMa-neutralizing antibody could secrete the medium levels of immunostimulatory cytokines and high levels of IL-10, unlike immature DCs. Moreover, recent studies have revealed the pivotal role of IL-10 in the differentiation process of tolDCs.Citation22 Therefore, the phenotype and cytokine secretion properties of these cells are similar to those of tolDCs. Moreover, accumulating evidences have demonstrated that tolDCs can be induced from iDCs by specific stimuli.Citation23,24 For example, DCs transduced with suppressor of cytokine signaling 1 (SOCS1) gene restrict maturation induced by LPS, induce T-cell hyporesponsiveness, and promote the generation of tolDCs.Citation25 To further investigate, we examined the ability of DCs to induce T cell responses. Our data suggested that DCs in the Anti-RGMa/ABP and Anti-RGMa/LPS groups displayed a reduced ability to induce T cell proliferation and reinforced T cell polarization toward a Th2 cytokine pattern, as evidenced by decreased IL-2 and IFN-γ expression and increased IL-10 and IL-13 expression, confirming the tolerogenic function of these cells in vitro. Taken together, the results suggested that the RGMa-neutralizing antibody could arrest maturation of DCs, and such cells presented with tolerogenic and immunoregulatory characteristics, which means RGMa may be involved in the immune regulation.
A study on MS and EAE, in which DCs play a vital role, confirmed that RGMa can modulate T cell activation and attenuate the immune response of EAE by DCs.Citation5 RGMa expression is increased after induction of mouse EAE, and mature DCs obtained from autopsied MS samples are immunoreactive for RGMa. The adoptive transfer of RGMa small interfering RNA-transfected DCs in a MOG-induced EAE model results in moderately reduced clinical disease scores. These studies indicate that RGMa plays an important role in DCs function, which is in agreement with the findings in the present study. Muramatsu et al. also reported that RGMa-neutralizing antibody application decreased T cell proliferation and modulated related cytokine secretion, such as reduced IL-2, IL-4, IFN-γ, and increased IL-10 secretion after induction of mouse EAE.Citation5 However, the exact mechanism(s) involved were not identified by the author. Our study explains how RGMa influences the proliferation and differentiation of T cells by DCs in the peripheral immune system. These lines of evidence together with our in vitro results highlight the notion that RGMa plays a significant role in immune regulation, particularly in DCs function.
DCs function can be induced by Toll-like receptor 4 (TLR4) pathways, including the myeloid differentiation factor 88 (MyD88)-dependent pathway and Toll/interleukin 1 receptor (TIR) domain-containing adapter-inducing IFN β (TRIF)-dependent pathway. By interactions between TLR4 and TIR domain-containing adaptor protein (TIRAP), MyD88 is recruited to activate interleukin receptor-associated kinases (IRAKs) and TNF receptor-associated factor 6 (TRAF6), which triggers nuclear factor kappa-B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways.Citation26-28 In the TRIF-dependent pathway, the TLR4 myeloid differentiation protein 2 (MD2)-LPS complex is internalized and transferred to early endosomes via TRIF-related adaptor molecule (TRAM), where TRAM, TRIF, TRAF3, TRAF6, and other adaptor proteins are recruited to activate NF-κB and interferon responses.Citation29-31 Activation of NF-κB, MAPK, and interferon results in enhanced inflammatory cytokine responses and induction of DCs function. However, accumulating evidences have confirmed that other transcription factors can also make important contributions to the inflammatory responses. Our study shows that RGMa acts as an immune regulator in DCs and hints that the molecule may make important contributions to the TLR4 pathway or downstream signaling pathways.
Binding to the receptor Neogenin, RGMa mediates nervous system function by BMP signaling and RhoA/Rho-kinase signaling pathways, which have been characterized. More and more evidence show that RGMa plays a negative role in axon outgrowth by activating the RhoA-Rho kinase signaling pathway in vitro.Citation32-34 Furthermore, in primary cortical neurons, RGMa exerts the suppressive function by phosphorylating collapsin response mediator protein-2 (CRMP-2) via both Rho-kinase and GSK-3β signaling pathways.Citation35 Also, in cerebellar granule neurons, RGMa inhibits neurite outgrowth via myosin IIA, a downstream effector of Rho kinase.Citation36 However, RGMa also functions as a novel co-receptor of bone morphogenetic proteins (BMPs), which belong to the superfamily of transforming growth factor beta (TGF-β). By increasing the binding of BMP ligands to ActRIIA, a type II receptor, RGMa activates the BMP2 or BMP4 signaling.Citation37 Moreover, RGMa can mediate BMP signaling through the classical BMP signaling pathway, which enhances Smad proteins and inhibitor of differentiation (Id1) protein, but not by TGF-β.Citation38 However, there is no definitive evidence as to whether and how RGMa participates in TLR4 or downstream signaling pathways, which will require further study.
In summary, our results indicate that even with LPS and ABP stimulation, DCs treated with RGMa-neutralizing antibodies present with tolerogenic and immunoregulatory characteristics, which suggests a novel role of RGMa in immune system. Consequently, we speculate that RGMa may offer potential therapeutic applications, and future clinical studies treating patients with RGMa-neutralizing antibodies should be developed.
Conclusion
We found that DCs treated with RGMa-neutralizing antibodies present with tolerogenic and immunoregulatory characteristics. Even with ABP and LPS stimulation, RGMa-neutralizing antibody application decreased expression of IL-12p70, TNF-α, IL-1β and key surface molecules, including CD80, CD86, CD40, and MHC II, and increased IL-10 secretion in DCs. In addition, DCs in the Anti-RGMa/ABP and Anti-RGMa/LPS groups displayed a reduced ability to drive T cell proliferation and reinforced T cell polarization toward a Th2 cytokine pattern. The present study suggests a novel role for RGMa in the immune system.
Materials and methods
Mice
Six-week-old male C57BL/6 mice were purchased from the Laboratory Animal Center at China Medical University. All mice were housed under controlled lighting (12 h light/dark cycle), temperature (22 ± 2°C), and given access to food and water ad libitum.
Key chemicals
Recombinant GM-CSF (Catalog number: 315–03) and IL-4 (Catalog number: 214-14) were purchased from PeproTech Inc. LPS (Catalog number: L-2880), Poly (I:C) (Catalog number: P1530), and control IgG antibody (Catalog number: I5006) were from Sigma Aldrich, Inc. ABP (98% purity) was obtained from the Chinese Academy of Sciences. A Naive T Cell CD4+/CD62L+/CD44low Column Kit (Catalog number: MCD45) was obtained from R&D Systems Inc. FITC Annexin V Apoptosis Detection Kit II (Catalog number: 556570) was from BD Pharmingen. RGMa-neutralizing antibody was from IBL (Catalog number: 28045F), and anti-RGMa antibody (Catalog number: ab26287) was purchased from Abcam PLC. Mem-PER® Eukaryotic Membrane Protein Extraction Reagent Kit (Catalog number: 89826) was from Thermo Fisher Scientific Inc. PrimeScript™ RT reagent (Catalog number: RR047A) and SYBR® Premix Ex Taq™ II kits (Catalog number: RR820A) were from Takara. ELISA kits for murine IL-12p70 (Catalog number: 88-7121), TNF-α (Catalog number: 88-7324), IL-10 (Catalog number: 88-7314), IL-2 (Catalog number: 88-7024), IL-1β (Catalog number: 88-7013), IL-13 (Catalog number: 88-7137), and IFN-γ (Catalog number: 88-7314) were obtained from eBioscience Inc. The following mAbs were used: anti-CD11c APC (Catalog number: 17-0114), anti-CD80 APC (Catalog number: 17-0801), anti-MHCII PE (Catalog number: 12-5321), anti-CD86 FITC (Catalog number: 11-0862), and anti-CD40 PE (Catalog number: 12-0401), all from eBioscience Inc. CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay Kit (Catalog number: G1112) was purchased from Promega. The remaining chemicals were purchased from Sigma Aldrich or BD Pharmingen.
Incubation of DCs
DCs were generated from bone marrow cells of male C57BL/6 mice, as previously described.Citation39 In brief, the marrow cells were washed out from femurs and tibias with RPMI1640 medium. After depleting red blood cells (RBCs) with RBCs lysing buffer, cells were placed in bone-marrow derived dendritic cell (BMDC) medium (RPMI 1640 with 10 ng/ml recombinant GM-CSF and IL-4). At 3 d, the old medium containing floating cells was gently removed and fresh BMDC medium was added. Half of the medium was replaced every other day. At 5 d, non-adherent and non-proliferating aggregates of immature DCs were harvested, and CD11c positive selection kits and magnetic activated cell sorting (MACS) columns were used to purify DCs. DCs purity was evaluated by fluorescence-activated cell sorting (FACS), with a target purity of greater than 95%. Purified DCs were used in the following experiments.
DCs treatment with RGMa-neutralizing antibodies
The study consisted of the RPMI 1640 group, Control-IgG antibodies plus ABP group (Control-IgG/ABP group), Control-IgG antibodies plus LPS group (Control-IgG/LPS group), RGMa-neutralizing antibodies plus ABP group (Anti-RGMa/ABP group), and the RGMa-neutralizing antibodies plus LPS group (Anti-RGMa/LPS group).To find the optimum concentration, we detected the IL-12p70 expression by altering the concentration of RGMa-neutralizing antibody from 2.5 μg/ml to 15 μg/ml. As shown in Supplementary Fig, DCs were treated with a certain concentration of RGMa-neutralizing antibody and LPS or ABP. At concentrations from 2.5 μg/ml to 10 μg/ml, the IL-12p70 expression was decreased along with increase of the dose. However, when the concentrations were from 10μg/ml to 15 μg/ml, no significant differences were found. Obviously, the most suitable concentration of RGMa-neutralizing antibody was 10 μg/ml. In our study, 10 μg/ml RGMa-neutralizing antibody, or 10 μg/ml control-IgG antibody were added to the cultures on day 5. To induce DCs maturation, ABP at 10 μg/ml, Poly (I:C) at 20 μg/ml or LPS at 1 μg/ml were added to the cultures on day 6 and cells were collected after 24 h stimulation.
Preparation and purification of T lymphocytes
T lymphocytes were purified from spleen cells, following the protocols of the Mouse Naive T Cell CD4+/CD62L+/CD44low Column Kit provided by the manufacturer, with minor modifications. Briefly, pre-washed, pre-packaged enrichment columns were prepared and washed with column buffer. Spleen cells were generated from C57BL/6 mice, depleted of RBCs with RBC lysing buffer, mixed with a monoclonal antibody cocktail, and washed twice with column buffer. Remaining cells were resuspended in column buffer and applied to the enrichment column. Purified T lymphocytes were eluted from the column, with a purity over 90%.
Western blotting
Membrane Protein was extracted using the Mem-PER® Eukaryotic Membrane Protein Extraction Reagent Kit according to the manufacturer's protocol. Proteins were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 10% acrylamide gels and transferred onto a polyvinylidene difluoride (PVDF) membranes, which were then blocked by 5% skimmed milk for 2 h at room temperature. After washing, PVDF membranes were incubated with anti-RGMa (1:1000) and anti-β-actin antibodies (1:5000) overnight at 4°C. Following washing in TBST, membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:1000) for 2 h at room temperature. Chemi-luminescence was visualized by Electrophoresis Gel Imaging Analysis System (Alpha Innotech, Santa Clara, CA, USA), and the relative expression of β-actin was used as standard control.
Real-Time PCR
Real-time PCR was performed to analyze the expression of RGMa and CCR7 mRNA. RNA extraction from cells was performed using TRIzol reagent, and reverse transcription optimized for real-time PCR was performed using the Prime Script RT reagent kit per manufacturer's instruction. PCR amplification was performed using SYBR PremixExTaq II, and reactions were as follows: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, and 63°C for 34 s. Primer sequences were designed as follows: β-actin, sense 5′-TCAACGAGCGGTTCCGATG-3′, antisense 5′-GCCACAGGATTCCATACCCA-3′; and RGMa sense 5′-CGTGCAAGTCACCAATACACCTG-3′, antisense 5′-T C TGGTCCACACACTCTTGGAA-3′; CCR7 sense 5′-A C A G C GGCCTCCAGAGAGACAGCGG-3′, antisense 5′-TGACGTCATAGGCAATGTTGAGCTG-3′. Results were quantified by a Light-Cycler1480 (Roche, Nutley, NJ, USA), and each sample was normalized to β-actin mRNA content.
MTS assay for DCs viability
After treatment, DCs viability was assessed using MTS assay, and the optical density(OD) was assayed using an iMark microplate reader (Bio-Rad, Hercules, CA, USA) at a wavelength of 490 nm.
FACS analysis of DCs apoptosis
Cultured DCs were collected and suspended in binding buffer, stained with FITC Annexin V and PI for 15 min at room temperature in the dark. Next, samples were detected by FAC Scan (BD Biosciences, Mountain View, CA, USA) and analyzed by FlowJo (Treestar Inc., Ashland, OR, USA).
Observation under scanning and transmission electron microscopy, and light microscopy
Cultured DCs were collected and resuspended in BMDC medium. The cell suspension was dropped onto a cover slip and incubated for 24 h. Sample preparation included glutaraldehyde fixation, cleaning, ethanol dehydration, replacement of critical point drying, and spraying gold. Following these steps, samples were observed under SEM (Hitachi S4800 field-emission scanning electron microscope).
Cultured DCs were collected, washed with PBS, and fixed in 2.5% glutaraldehyde. Cell preparation steps included cleaning, osmium teroxide fixation, and ethanol dehydration. Samples were then embedded in resin and observed under TEM (Hitachi H-7650 transmission electron microscope).
The morphology of cultured DCs were also observed under Inverted Phase Contrast Microscope (CMS GmbH Light Microscopes, Leica Microsystems).
Cytokine production determined by ELISA
The supernatant was analyzed for the production of cytokines IL-12p70, TNF-α, IL-10, IL-2, IL-1β, IL-13, and IFN-γ using ELISA kits according to the manufacturer's protocol. Results were quantified at 450nm using an iMark microplate reader (Bio-Rad, Hercules, CA, USA), and results were expressed in pg/ml.
FACS analysis of key cell surface markers
Cells were suspended in PBS containing 2% FBS, stained with anti-CD80 APC, anti-MHCII PE, anti-CD86 FITC, anti-CD40 PE and isotype-matched Abs for 30 min at 4°C, and then washed with PBS containing 2% FBS. Next, samples were resuspended in PBS containing 2% FBS, detected by FAC Scan (BD Biosciences, Mountain View, CA, USA) and analyzed by FlowJo (Treestar Inc., Ashland, OR, USA).
Quantitation of endocytosis by FACS
To assess endocytosis by DCs, FITC-dextran acting as an extrinsic antigen, was used to feed DCs and the endocytosis was measured by flow cytometry. Cells were suspended in PBS containing 2% FBS, incubated with FITC-labeled dextran for 1 h at 4°C, and resuspended in PBS containing 2% FBS. FITC-dextran uptaking was detected by FACScan (BD Biosciences, Mountain View, CA, USA) and analyzed by FlowJo (Treestar Inc., Ashland, OR, USA).
Mixed lymphocyte reaction (MLR)
CD4+T lymphocytes (3 × 105 cells/well) as responding cells were incubated in 100 µL medium in flat-bottomed 96-well plates. DCs from each group were treated with 25 μg/ml mitomycin C for 45 min and resuspended in 100 µL medium in graded numbers, which ensured the ratios of DCs to T cells were 1:1, 1:10, 1:50, 1:100. Cells were then cultured for 72 h under normal growth conditions. After that, the solution in the Cell Proliferation Assay Kit was added in each well with 20 µL, and the 96 well assay plates were continued to be cultured for 2 h under normal growth conditions. The OD was assayed using an iMark microplate reader (Bio-Rad, Hercules, CA, USA) at a wavelength of 490 nm. When the ratio of DCs to T cells was 1:1, culture supernatants were measured by ELISA to examine the ability of DCs to induce T cell differentiation.
Statistical analysis
Data were expressed as the mean ± SEM. Experiments were independently repeated at least three times for accurate results. Results were analyzed by Student's t-test, and P < 0.05 was regarded as significant.
Abbreviations
| BMDC | = | bone-marrow derived dendritic cell |
| CNS | = | central nervous system |
| DCs | = | dendritic cells |
| EAE | = | experimental autoimmune encephalomyelitis |
| ELISA | = | enzyme-linked immunosorbent assay |
| FACS | = | fluorescence activated cell sorting |
| GM-CSF | = | granulocyte macrophage-colony stimulating factor |
| HRP | = | horseradish peroxidase |
| IRAKs | = | interleukin receptor-associated kinases |
| LPS | = | lipopolysaccharide |
| MACS | = | magnetic activated cell sorting |
| MAPK | = | mitogen-activated protein kinase |
| MD2 | = | myeloid differentiation protein 2 |
| MHC | = | major histocompatibility complex |
| MLR | = | Mixed lymphocyte reaction |
| MOG | = | myelin oligodendrocyte glycoprotein |
| MS | = | multiple sclerosis |
| MyD88 | = | myeloid differentiation factor 88 |
| NF-κB | = | nuclear factor kappa-B |
| OD | = | optical density |
| PBS | = | phosphate balanced solution |
| PI | = | propidium iodine; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt |
| PVDF | = | polyvinylidene difluoride |
| RBCs | = | red blood cells |
| RGMa | = | repulsive guidance molecule a |
| RIPA | = | radio-immunoprecipitation assay |
| SDS-PAGE | = | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SEM | = | scanning electron microscopy |
| SOCS1 | = | suppressor of cytokine signaling 1 |
| TEM | = | transmission electron microscopy |
| TIR | = | interleukin 1 receptor |
| TIRAP | = | TIR domain-containing adaptor protein |
| TLR4 | = | Toll-like receptor 4 |
| TRAF6 | = | TNF receptor-associated factor 6 |
| TRAM | = | TRIF-related adaptor molecule |
| TRIF | = | TIR domain-containing adapter-inducing IFN β |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KHVI_A_1164361_Supplement.docx
Download MS Word (2.2 MB)Acknowledgments
This work was supported financially by Liaoning Province Science and Technology Project-Animal Scientific Research and Clinical Application for Major Disease of Liaoning Province (2012225021) and Technology Projects of Liaoning Province (2009225010-2) to Juan Feng.
References
- Koeberle PD, Tura A, Tassew NG, Schlichter LC, Monnier PP. The repulsive guidance molecule, RGMa, promotes retinal ganglion cell survival in vitro and in vivo. Neuroscience 2010; 169:495-504; PMID:20457227; http://dx.doi.org/10.1093/10.1016/j.neuroscience.2010.04.079
- Severyn CJ, Shinde U, Rotwein P. Molecular biology, genetics and biochemistry of the repulsive guidance molecule family. Biochem J 2009; 422:393-403; PMID:19698085; http://dx.doi.org/10.1042/BJ20090978
- Matsunaga E, Nakamura H, Chedotal A. Repulsive guidance molecule plays multiple roles in neuronal differentiation and axon guidance. J Neurosci 2006; 26:6082-8; PMID:16738252.
- Mirakaj V, Brown S, Laucher S, Steinl C, Klein G, Kohler D, Skutella T, Meisel C, Brommer B, Rosenberger P, et al. Repulsive guidance molecule-A (RGM-A) inhibits leukocyte migration and mitigates inflammation. Proc Natl Acad Sci U S A 2011; 108:6555-60;PMID:21467223; http://dx.doi.org/10.1073/pnas.1015605108
- Muramatsu R, Kubo T, Mori M, Nakamura Y, Fujita Y, Akutsu T, Okuno T, Taniguchi J, Kumanogoh A, Yoshida M, et al. RGMa modulates T cell responses and is involved in autoimmune encephalomyelitis. Nat Med 2011; 17:488-94; PMID:21423182; http://dx.doi.org/10.1038/nm.2321
- Mohammad MG, Hassanpour M, Tsai VW, Li H, Ruitenberg MJ, Booth DW, Serrats J, Hart PH, Symonds GP, Sawchenko PE, et al. Dendritic cells and multiple sclerosis: disease, tolerance and therapy. Int J Mol Sci 2012; 14:547-62; PMID:23271370; http://dx.doi.org/10.3390/ijms14010547
- Martin-Fontecha A, Lanzavecchia A, Sallusto F. Dendritic cell migration to peripheral lymph nodes. Handb Exp Pharmacol 2009; 188: 31-49; PMID:19031020.
- Dudek AM, Martin S, Garg AD, Agostinis P. Immature, semi-mature, and fully mature dendritic cells: Toward a DC-cancer cells interface that augments anticancer immunity. Front Immunol 2013; 4:438; PMID:24376443; http://dx.doi.org/10.3389/fimmu.2013.00438
- Jalili A. Dendritic cells and their role in cancer immunotherapy. Iran J Immunol 2007; 4:127-44; PMID:17767012.
- Strioga M, Schijns V, Powell DJ, Jr., Pasukoniene V, Dobrovolskiene N, Michalek J. Dendritic cells and their role in tumor immunosurveillance. Innate Immun 2013; 19:98-111; PMID:22732734; http://dx.doi.org/10.1177/1753425912449549
- van Duivenvoorde LM, van Mierlo GJ, Boonman ZF, Toes RE. Dendritic cells: vehicles for tolerance induction and prevention of autoimmune diseases. Immunobiology 2006; 211:627-32; PMID:16920501.
- Kim SJ, Diamond B. Modulation of tolerogenic dendritic cells and autoimmunity. Semin Cell Deve Biol 2015; 41:49-58; PMID:24747368.
- Hayashi T, Yao S, Crain B, Promessi VJ, Shyu L, Sheng C, Kang M, Cottam HB, Carson DA, Corr M. Induction of tolerogenic dendritic cells by a PEGylated TLR7 ligand for treatment of type 1 diabetes. PloS one 2015; 10:e0129867; PMID:26076454.
- Zhu M, Xu W, Su H, Huang Q, Wang B. Addition of CpG ODN and Poly (I:C) to a standard maturation cocktail generates monocyte-derived dendritic cells and induces a potent Th1 polarization with migratory capacity. Hum Vaccin Immunotherapeut 2015; 11:1596-605.
- Chen Y, Meng Y, Cao Y, Wen H, Luo H, Gao X, Shan F. Novel analysis of maturation of murine bone-marrow-derived dendritic cells induced by Ginkgo Seed Polysaccharides. Hum Vaccin Immunother 2015; 11:1387-93; PMID:25806792; http://dx.doi.org/10.80/21645515.2015.1023971
- Zou Y, Meng J, Chen W, Liu J, Li X, Li W, Lu C, Shan F. Modulation of phenotypic and functional maturation of murine dendritic cells (DCs) by purified Achyranthes bidentata polysaccharide (ABP). Int Immunopharmacol 2011; 11:1103-8; PMID:21439398; http://dx.doi.org/10.1016/j.intimp.2011.03.006
- Kong Y, Rogers MR, Qin X. Effective neuroprotection by ischemic postconditioning is associated with a decreased expression of RGMa and inflammation mediators in ischemic rats. Neurochem Res 2013; 38:815-25; PMID:23389659; http://dx.doi.org/10.1007/s11064-013-0984-5
- Kitayama M, Ueno M, Itakura T, Yamashita T. Activated microglia inhibit axonal growth through RGMa. PloS one 2011; 6:e25234; PMID:21957482.
- Nohra R, Beyeen AD, Guo JP, Khademi M, Sundqvist E, Hedreul MT, Sellebjerg F, Smestad C, Oturai AB, Harbo HF, et al. RGMA and IL21R show association with experimental inflammation and multiple sclerosis. Gen Immun 2010; 11:279-93; PMID:20072140
- Tanabe S, Yamashita T. Repulsive guidance molecule-a is involved in Th17-cell-induced neurodegeneration in autoimmune encephalomyelitis. Cell Rep 2014; 9:1459-70; PMID:25456136; http://dx.doi.org/10.1016/j.celrep.2014.10.038
- Marsland BJ, Battig P, Bauer M, Ruedl C, Lassing U, Beerli RR, Dietmeier K, Ivanova L, Pfister T, Vogt L, et al. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity 2005; 22:493-505; PMID:15845453.
- Frick JS, Grunebach F, Autenrieth IB. Immunomodulation by semi-mature dendritic cells: a novel role of Toll-like receptors and interleukin-6. Int J Med Microbiol 2010; 300:19-24; PMID:19781988; http://dx.doi.org/10.1016/j.ijmm.2009.08.010
- Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Pozo D, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc Natl Acad Sci U S A 2005; 102:13562-7.
- Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity 2003; 18:605-17; PMID:12753738.
- Fu H, Song S, Liu F, Ni Z, Tang Y, Shen X, Xiao L, Ding G, Wang Q. Dendritic cells transduced with SOCS1 gene exhibit regulatory DC properties and prolong allograft survival. Cell Mol Immunol 2009; 6:87-95; PMID:19403057; http://dx.doi.org/10.1038/cmi.2009.12
- Narayanan KB, Park HH. Toll/interleukin-1 receptor (TIR) domain-mediated cellular signaling pathways. Apoptosis 2015; 20:196-209; PMID:25563856; http://dx.doi.org/10.1007/s10495-014-1073-1
- Pasterkamp G, Van Keulen JK, De Kleijn DP. Role of Toll-like receptor 4 in the initiation and progression of atherosclerotic disease. Eur J Clin Invest 2004; 34:328-34; PMID:15147329.
- O'Neill LA, Dinarello CA. The IL-1 receptor/toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today 2000; 21:206-9; PMID:10782049.
- Liaunardy-Jopeace A, Gay NJ. Molecular and cellular regulation of toll-like receptor-4 activity induced by lipopolysaccharide ligands. Front Immunol 2014; 5:473; PMID:25339952; http://dx.doi.org/10.3389/fimmu.2014.00473
- Buchanan MM, Hutchinson M, Watkins LR, Yin H. Toll-like receptor 4 in CNS pathologies. J Neurochem 2010; 114:13-27; PMID:20402965; http://dx.doi.org/10.1111/j.1471-4159.2010.06736.x
- Palsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunol 2004; 113:153-62; PMID:15379975.
- Kubo T, Hata K, Yamaguchi A, Yamashita T. Rho-ROCK inhibitors as emerging strategies to promote nerve regeneration. Curr Pharmaceut Des 2007; 13:2493-9; PMID:17692017.
- Yamashita T, Fujitani M, Yamagishi S, Hata K, Mimura F. Multiple signals regulate axon regeneration through the Nogo receptor complex. Mol Neurobiol 2005; 32:105-11; PMID:16215275.
- Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Dis 2005; 4:387-98; PMID:15864268.
- Wang T, Wu X, Yin C, Klebe D, Zhang JH, Qin X. CRMP-2 is involved in axon growth inhibition induced by RGMa in vitro and in vivo. Mol Neurobiol 2013; 47:903-13; PMID:23275173; http://dx.doi.org/10.1007/s12035-012-8385-3
- Kubo T, Endo M, Hata K, Taniguchi J, Kitajo K, Tomura S, Yamaguchi A, Mueller BK, Yamashita T. Myosin IIA is required for neurite outgrowth inhibition produced by repulsive guidance molecule. J Neurochem 2008; 105:113-26; PMID:18005226.
- Xia Y, Yu PB, Sidis Y, Beppu H, Bloch KD, Schneyer AL, Lin HY. Repulsive guidance molecule RGMa alters utilization of bone morphogenetic protein (BMP) type II receptors by BMP2 and BMP4. J Biol Chem 2007; 282:18129-40; PMID:17472960.
- Babitt JL, Zhang Y, Samad TA, Xia Y, Tang J, Campagna JA, Schneyer AL, Woolf CJ, Lin HY. Repulsive guidance molecule (RGMa), a DRAGON homologue, is a bone morphogenetic protein co-receptor. J Biol Chem 2005; 280:29820-7; PMID:15975920.
- Inaba K, Swiggard WJ, Steinman RM, Romani N, Schuler G, Brinster C. Isolation of dendritic cells. Current protocols in immunology / edited by John E Coligan [et al] 2009; Chapter 3:Unit 3.7-Unit 3.7; PMID:19653207; http://dx.doi.org/10.1002/0471142735.im0307s86