ABSTRACT
The Antigenic Domain 2 (AD-2) is a short region near the N-terminus of glycoprotein B of human cytomegalovirus (HCMV). AD-2 has been shown to contain linear epitopes that are targets for neutralizing monoclonal antibodies from human subjects with natural HCMV infection. However, AD-2 appears to be masked by the adjacent immunodominant AD-1 region. We assessed a serum panel from HCMV-seropositive individuals and found a wide range of antibody titers to AD-2; these did not correlate to serum neutralization. To expose potential epitopes in AD-2, we constructed a series of AD-2 peptide-conjugate vaccines. Mice were immunized 3 times and produced high and sustained antibody titers to AD-2 peptides, but neutralization was weak even after a single boost with whole HCMV virions. Rabbits were likewise immunized with AD-2 peptide vaccines, and produced a robust antibody response, but neutralization was inferior to a recombinant gB vaccine with an oil-in-water adjuvant. These results highlight the challenges of developing a peptide-based vaccine specific to the HCMV gB AD-2 region.
Introduction
Human cytomegalovirus (HCMV) is an important pathogen which can cause in utero infection, leading to childhood neurodevelopmental disability.Citation1 To prevent congenital virus infection, a prophylactic vaccine could be developed, targeting the populations at risk of HCMV infection including HCMV seronegative women of child-bearing ages or female adolescents.Citation2,3 However, vaccines developed in previous years have failed to produce a robust protective response. Two vaccine candidates, a live attenuated virus Towne strain and a recombinant glycoprotein B (gB) formulated with MF59 adjuvant, have advanced to Phase II efficacy evaluation, with some indications of efficacy.Citation4-6 However, no vaccine candidate has yet been able to achieve broad and sustained protection against HCMV acquisition in HCMV seronegative subjects. At least 2 lines of investigation have been proposed to improve upon these vaccines. The first follows the discovery of the missing pentameric gH/gL/pUL128-131 complex not found in laboratory isolates, such as Towne or AD169, due to extended passages in fibroblast cells.Citation7 The second is based upon potential immunological misdirection toward non-neutralizing epitopes in the gB.
The HCMV gB is a logical target for vaccine design for 2 reasons. First, it is essential for viral infectivity.Citation8 Specifically, gB is critical for viral entry to cells, and has been suggested as a fusogen based on its homology to the HSV-1 gB structure and understanding of its role in the fusion mechanism.Citation9-11 Peptides including the heptad repeat in the coiled-coil structure inhibit membrane fusion.Citation12 Second, gB is a dominant antigen in HCMV-infected humans and experimental animals exposed to HCMV.Citation13 The dominant antibodies binding to HCMV recognize gB, and gB-specific CD4 and CD8 T-cell responses are found in HCMV seropositive individuals.Citation14
The antigenic domain 2 (AD-2) contains protective epitopes and spans a short region near the N-terminus of the HCMV gB (approximately amino acids 68–81). It was identified more than 2 decades agoCitation15 using a human monoclonal antibody, C23,Citation16 and is considered to be a linear epitope region that is highly conserved among clinical isolates of the virus and can neutralize viral infection in both fibroblasts and epithelial cells.Citation17 Interestingly, an AD-2-specific antibody, ITC88,Citation18 has also been shown to mediate other functions as well, as it is able to prevent early stages of the anti-apoptotic effect that allows HCMV-infected cells to survive after infection.Citation19 Most recently, 2 human mAbs targeting AD-2 have been reported to be under preclinical development as therapeutic agents: TRL345Citation20 and TCN-202.Citation21
In contrast, in natural infection, immunity to AD-2 does not usually develop effectively; only a fraction of naturally HCMV-seropositive individuals develop such antibodies and often at low titers.Citation22 It has been hypothesized that AD-2 is somewhat immunogenic but is often masked by the immunodominant antigenic domain 1 (AD-1), spatially located near AD-2. Because AD-2-specific neutralizing Abs appear to recognize continuous, so-called linear, epitopes, one could design synthetic peptide-conjugate vaccines to purposely induce the antibodies of the AD-2 specificity. In this study, we explore this vaccination concept by designing a series of synthetic carrier-conjugated peptides covering AD-2 and its flanking regions. Here we demonstrated that such a vaccine could produce strong binding titers specific to AD-2 but was unable to generate significant levels of neutralization in a well-characterized assay. The results were benchmarked against an epidemiological survey presented here.
Results
To explore the antigenic potential of AD-2 of HCMV gB in detail, we synthesized overlapping 21-mer peptides that spanned and flanked the AD-2 region, as shown in . Two peptides flank AD-2 (peptides 1 and 11), while peptides 3 and 9 overlap and extend beyond AD-2. In the central region, we tested a higher density of overlapping peptides (peptides 5, 6, and 7). This set of AD-2 peptides was evaluated in parallel with a recombinant gB vaccine designed to mimic a vaccine clinical candidate,Citation5 and the repaired intact AD169rev virion.Citation23,24
Figure 1. Scheme of peptides that flank and span the CMV Antigenic Determinant 2 (AD-2) region based on a series of overlapping peptides. The peptide labels as well as the amino acid spanned by each within the AD169 gB are shown. The minimal linear epitope recognized by AD-2 specific mAbs is underlined, and flanking regions identified in some literature reports as included in the AD-2 epitope are in boldface.

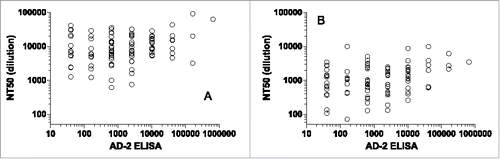
To understand the response rate to the AD-2 region in HCMV seropositive subjects, we have assembled a serum panel from HCMV seropositive and seronegative women of age 18–39 as described in the section of Materials and Methods. From this panel, we selected 100 seropositive subjects and tested their sera for neutralization activity in epithelial (ARPE-19) and fibroblast (MRC-5) cells, and for binding by ELISA to a pool of AD-2 peptides, recombinant gB antigen, or purified HCMV AD169rev virions. To test if neutralization could be predicted by ELISA titers to these antigens, we calculated the linear correlation of log-transformed neutralization and log-transformed ELISA titers. The neutralization and ELISA data appear to be log-distributed. While all correlations were highly statistically significant (p < 0.01), the R2 values were low, demonstrating that the variation in the neutralization data could not be explained from ELISA titers alone. ELISA titers to the whole AD169rev virion antigen predicted approximately 20% of variation in neutralization (in ARPE-19 R2= 0.19, in MRC-5 R2= 0.21), and ELISA titers to gB somewhat less (in ARPE-19 R2= 0.13, in MRC-5 R2= 0.22). In particular, the AD-2 ELISA titer was not predictive of neutralization (in ARPE-19 R2= 0.06, in MRC-5 R2= 0.07). Neutralization titers vs. AD-2 ELISA titers are plotted in for ARPE-19 and MRC-5 cells. There is a trend of increasing neutralization with AD-2-specific binding, but the variation in the neutralization at each endpoint titer is high. Thus, the data suggest that the majority of neutralizing activity in naturally infected immune sera is not predicted by antibody specificity and titers to AD-2.
Figure 2. Serum neutralizing titers (NT50) in (A) epithelial (ARPE-19) and (B) fibroblast (MRC-5) cells vs ELISA titers (AD-2 peptides 5, 6, and 7) from the epidemiological study. Correlations were highly significant (p ≤ 0.01), but most of the variability in neutralization is not predicted by AD-2 peptide ELISA (R2 = 0.06, 0.07).
While AD-2 ELISA titers do not appear to be highly predictive of neutralization in persons naturally exposed to HCMV, we hypothesized that AD-2-specific vaccination could elicit strong serological neutralization. This would follow from the idea that AD-2 contains potent epitopes for neutralization, but that its immunogenicity is ranked low in the immune recognition hierarchy. The most straightforward method to test this hypothesis is to develop an AD-2-centric vaccine and evaluate the immune responses in animal models. Accordingly, we prepared synthetic peptides corresponding to AD-2, conjugated to CRM carriers, as described in the Materials and Methods. The AD-2 peptide-conjugate vaccines were then tested in mice and rabbits.
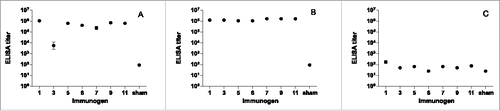
Mice of 10 per group were immunized with AD-2 peptide-conjugate vaccines at days 0, 21, 48, and 110, and then further boosted with AD169 virions at day 138. A control group was vaccinated with saline buffer with alum but no peptide or virions. The ELISA binding titers after 3 priming immunizations and after the boost are shown in . All AD-2 peptide-conjugate vaccines were immunogenic and high titers were achieved (), approximately 1:106 for most vaccines. These titers were sustained and enhanced after boosting with AD169 virions (). After the single boost injection with AD169 virions, antibody titers to AD169 were detectable but comparatively low (); group geometric means ranged from 25 to 170.
Figure 3. Binding ELISA responses to AD-2 peptides at day 69 (A), AD-2 peptides at day 146 (B), and AD169 virions at day 146 (C). Mice (n=10) were immunized with CRM-conjugated AD-2 peptides at days 0, 21, 48, and 110, and boosted with AD169 virions at day 138. Sham vaccine is a saline buffer with alum but no peptide. Sham vaccinated animals were tested against a pool of all AD-2 peptides. All AD-2 peptides were immunogenic and this was sustained and enhanced after boosting with AD169 virions. Antibody titers to AD169 were comparatively low. Error bars represent the SEM.
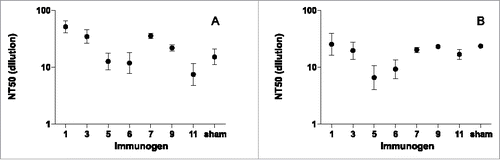
We tested for neutralization with the sera collected at the same time point of highest antibody titer, after the boost. Neutralization was tested in the same manner as the serological survey (), in ARPE-19 cells () and MRC-5 cells (). In both epithelial and fibroblast cells, neutralizing titers were comparatively modest; geometric mean neutralizing titers ranged from 7 to 52. This contrasts with the robust ELISA binding titers shown in , and the strong neutralization seen in the human subjects with natural HCMV infection (). Neutralizing titers of AD-2-vaccinated and sham-vaccinated mice were generally similar; group geometric means were no more than 3.5-fold higher and less than 2-fold lower than sham-vaccinated groups. This modest potency and lack of differentiation from sham-vaccinated controls demonstrated no significant neutralization even after multiple immunizations with CRM-conjugated AD-2 peptides.
Figure 4. Neutralizing titers (NT50) in (A) ARPE-19 cells, (B) MRC-5 cells at day 146 from sera of mice immunized with CRM-conjugated AD-2 peptides at days 0, 21, 48, and 110, and boosted with AD169 virions at day 138. Even with the robust binding titers to AD-2 peptides (), no significant neutralization due to immunization with AD-2 was observed (i.e., neutralizing titers of AD2-vaccined and sham-vaccinated mice were generally similar). Error bars represent the SEM.
To evaluate the AD-2 peptide concept in another benchmark animal model, we vaccinated rabbits with the same AD-2 peptide-conjugate vaccines. To focus on the core AD-2 region, rabbits of 4 per group were vaccinated with the vaccines of peptide 5, peptide 6, or peptide 7. These three peptides focused on the core AD-2 region. The recombinant gB antigen with an oil-in-water adjuvantCitation25 was included as a positive control. Vaccinations occurred on weeks 0, 3, and 8, with intervening blood collections, as described in the section of Materials and Methods.
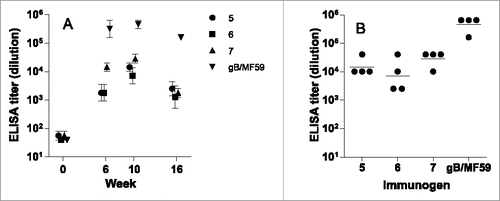
ELISA titers were robust across multiple time points, as shown in . For each vaccinated group, titers rose at week 6, peaked at week 10, and subsided to a lower level at week 16. At the maxima time point (week 10), the binding titers from the 4 individual rabbits were narrowly distributed within each group (). The group geometric means were very similar between AD-2 peptides 5, 6, and 7. This is not unexpected, due to the high degree of overlap between these peptides. Rabbits immunized with adjuvanted recombinant gB vaccine had higher binding titers as can be determined by inspection of data in . Because the maximum titers were reached at week 10, and the intra-group titers were narrowly distributed, this time point was selected for neutralization assays.
Figure 5. ELISA from rabbits immunized with CRM-conjugated AD-2 peptides, or gB with an oil-in-water adjuvant. (A) Time course of immune response, group geometric mean and standard error; and (B) individual rabbits' titers at the peak response (week 10). The lower limit of detection is 1:40. None of the AD-2 peptide vaccines were superior to gB/MF59 at any time after immunization.
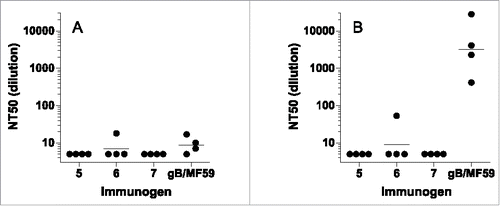
Neutralization assays for individual rabbit sera were performed similarly to the mouse protocol in ARPE-19 cells. Neutralization titers were modest for all groups (). To amplify weak neutralization and detect complement-dependent neutralization, the neutralization assays were performed with and without the addition of rabbit complement as described in the Materials and Methods. For the AD-2 peptide-conjugate vaccine groups, without complement, all titers were below the limit of detection (1:5) with the exception of one rabbit with a modest 1:18 titer. With the addition of complement, that rabbit reached a 1:53 titer. In contrast, the 4 animals in the gB/MF59 group had modest neutralizing titers without complement (< 1:5, 1:7, 1:10, 1:17) but significantly higher neutralization titers with complement (410, 28000, 2230, 4100, respectively). Similarly to the mouse experiment, the AD-2 peptide-conjugate vaccine generated only weakly neutralizing serological responses despite robust antibody binding titers.
Figure 6. Neutralizing titers (NT50) from rabbits immunized with CRM-conjugated AD-2 peptides or gB with MF59 adjuvant. (A) sera alone, (B) with added rabbit complement. The lower limit of detection is a 1:5. Horizontal bars represent the geometric mean of each group. None of the AD-2 peptide vaccines were superior to gB/MF59.
Discussion
From our survey of HCMV seropositive individuals, antibodies to the linear peptides in AD-2 are generated in natural infection, but these are only weakly correlated with neutralization. Given that HCMV-neutralizing AD-2-specific human antibodies have been discovered, there are several possible explanations. First, the majority of AD-2-directed antibodies may be non-neutralizing, potentially with a minute minority of strongly neutralizing antibodies. This may explain the fact that only a few of AD-2 neutralizing mAbs have been reported in the past 20 y. Second, the neutralizing antibodies may be predominantly directed against conformational non-continuous epitopes within AD-2 that are not faithfully captured by linear peptides. By this account, any epitope-focused vaccine approach, including peptide conjugate vaccines as described in this study, or the chimeric virus-like particle approach by displaying a linear non-conformational epitope on hepatitis B virus core antigen,Citation26 would unlikely be effective for induction of functional antibodies. In contrast, if a chimeric virus-like particle could present the AD-2 epitope in its native confirmation, this could be a viable approach. Third, different antigenic domains on distinct regions of the gB antigen may act synergistically,Citation27 and neutralizing responses to AD-2 may require this cooperative effect.
Here we tested the hypothesis that a CRM-conjugated AD-2 peptide vaccine would be able to elicit AD-2-directed antibodies that could exhibit neutralization. We did consistently observe a strong and specific binding response by ELISA, but were unable to detect significant neutralization in either of 2 species, mouse and rabbit (even in the presence of complement). Our experiment to prime the mice with the AD-2 peptide-conjugate vaccines followed with whole AD169 virion boost also failed to elicit neutralizing activity. We did not identify any clear immunological hot spots in the AD-2 region by peptide mapping; all peptide vaccines generated sufficient antibody titers in animals. For this reason, a more focused vaccine comprising a subset of these peptides would not likely be effective.
The combination of the serological survey and negative neutralization results from the AD-2 peptide-conjugate vaccine study point to the limitation of some avenues but also point to future approaches for vaccines targeting AD-2. The highly immunogenic but non-neutralizing responses, and lack of correlation in human sera, indicate that conjugated peptide vaccines are unlikely to ever be effective. A more complex structured protein construct may be required to generate antibodies that are similar to the previously identified AD-2 neutralizing antibodies. One approach would be to generate constructs that demonstrate binding to the few identified AD-2 neutralizing monoclonal antibodies either by screening of a structured random peptide library or synthetic peptide mimetics with rationales of structural knowledge. Furthermore, because monoclonal antibodies specific to AD-2 exhibit considerable somatic hypermutation,Citation28,29 and the general observation that HCMV seropositive persons are chronically infected, significant maturation may be required for neutralization. This would require an extensive scheme of repeated immunizations perhaps including boosting with heterologous constructs. Further experiments along these lines would definitively test if the AD-2 region can provide the basis for a durable vaccine for the prevention of HCMV infection.
One of the limitations to our vaccine design was lack of structural knowledge of the AD-2 epitope in the context of native pre-fusion form of the gB protein. Without such information, we would have to assume a simple continuous epitope which was demonstrated by our data incapable of driving functional antibodies as peptide-conjugate vaccines. Unfortunately, AD-2 is located in N-terminal region that was not present in the structure recently solved for a post-fusion form HCMV gB.Citation30 Co-crystallization of a neutralizing antibody with its cognate antigen has tremendous values in rational design of synthetic vaccine, and such endeavor has been reported for peptide mimetics vaccines for HIV-1 gp41 fusion intermediate epitope.Citation31 Thus, a structure of an AD-2 neutralizing antibody with a native gB protein containing the AD-2 region would provide molecular basis for refining the design for an AD-2 based vaccine.
Materials and methods
Antigens and vaccines
Overlapping linear 21-mer peptides from the AD169 strain of HCMV gB (UL55) were chosen to map serological responses across the AD-2 region. The definition of each peptide is shown in . Peptides 1 and 11 lie outside the defined AD-2 region, and peptides were most closely overlapping near the center of the AD-2 region to maximize the possibility of detecting an optimal AD-2 immunogen. The peptides were conjugated to a recombinant CRM197 carrier,Citation32 via a linker of aminohexanoic acid at the C-terminus, and glutamic acids (E or EEE) to balance pI as described previously.Citation33,34 Conjugation of peptides was confirmed by the shift of the molecular weight of CRM197 protein on SDS-PAGE (data not shown). In addition, vaccines were characterized by amino acid analysis for purity, peptide loading efficiency and peptide-conjugate concentration (Supplemental Table 1). For immunization of mice and rabbits, vaccines were buffered in 150 mM NaCl, 25 mM HEPES with 2X amorphous aluminum hydroxylphosphate sulfate (AAHS) adjuvant (450 μg/mL). The dilution to 1X AAHS adjuvant was performed within 3 hours of vaccination.
Reagents, cells and viruses
Recombinant protein HCMV gB, based upon the Towne strain, was constructed and expressed as reported previously,Citation35 and sourced from Sino Biological, Inc. (Beijing, China). An oil-in-water emulsion similar in composition to MF59 adjuvant was constructed at Merck for comparison.Citation25 ARPE-19 and MRC-5 cells were cultured as previously described.Citation36 AD169 was obtained from ATCC, and propagated in MRC-5 cells.Citation37 HCMV AD169 revertant virions (AD169rev) have been described elsewhere.Citation38 The HCMV virions were purified from the supernatant by ultracentrifugation at 64000xg at 20oC through a 20% Sorbitol cushion in PBS as previously reported.Citation38 Purified virion pellets were resuspended in Hank's balanced salt solution and stored at −70oC.
Vaccinations
Mice and rabbits were maintained in the animal facility of Merck, West Point, PA. All animal studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by Merck's Institutional Animal Care and Use committee (IACUC). Specific pathogen-free Balb/c female mice of 4–6 weeks age were purchased from Taconic Farm. Mice were immunized by i.m. injection of both quadriceps in 50 μL volume per site without anesthesia. Female New Zealand White (NZW) rabbits of 3–4 months of ages were purchased from a specific pathogen-free colony at Covance (Denver, PA). The animals were housed individually and were immunized by i.m. injection of quadriceps in 0.5 mL volume without anesthesia.
Groups of 10 mice were vaccinated with each peptide at 6 μg per dose. An additional group of 10 mice was sham-vaccinated with alum in saline for use as a negative control. Mice were vaccinated on days 0, 21, 48, and 110. Mice were boosted on day 138 with 3 μg of AD169rev virions.Citation38 Mice were bled on days 55, 69, 132, and 146 (8 d after the boost). Groups of 4 rabbits were vaccinated with AD-2 peptides 5, 6, or 7, (10 μg dose) or a positive control (gB with oil-in-water adjuvant, 30 μg dose). Rabbits were vaccinated at weeks 0, 3, and 8. Rabbits were bled at weeks 0, 6, 10, and 16.
Epidemiological survey
We have previously assembled and characterized a panel of serum samples from 200 healthy female subjects (18–39 y old) from a local blood bank with donor consent.Citation39 Serum aliquots were treated at 56oC for 30 min to inactivate complement. Serum samples were tested for HCMV reactivity using a commercial CMV IgG Enzyme Immunoassay Kit (BioCheck, Inc.), and grouped into seropositive and seronegative cohorts based on the cut-off value recommended by the manufacturer. For this report, a set of 100 HCMV seropositive subjects' sera were tested in ELISAs and viral neutralization assays. Seven HCMV seronegative subjects were included to provide an estimate of assay sensitivity and serve as negative controls.
ELISA
The method was reported previously.Citation39 Briefly, 96-well plates were coated with either individual peptides at 2 μg /mL or AD169 virions at 1 μg /mL in PBS and incubated overnight at 4oC. The next day, plates were washed and blocked with 3% milk in PBS for 2 hours at room temperature. After washing, sera were added in titration with 4-fold dilutions and incubated for 90 minutes at room temperature. After washing, HRP conjugated goat anti-mouse IgG was added at 1:2000 and plates were incubated for one hour at room temperature. Plates were then washed and developed with 3,3′,5,5′ – tetramethylbenzidine (TMB) for 3–5 minutes, stopped with H2SO4, and read on a standard plate reader. Endpoint titers were calculated as the highest dilution that was greater than twice the geometric mean of the negative controls.
Viral neutralization assay
An immunostaining (IMS) neutralization assay was performed as previously described.Citation36 Mouse sera were tested at a lowest dilution of 1:40, and rabbit sera at a lowest dilution of 1:5, in serial 2-fold dilutions. On each microtiter plate, media with virus were used as positive controls, and naïve sera were used as negative controls. Rabbit sera were tested with and without complement. Rabbit complement (Cedarlane Labs) was titrated to determine the dilution (1:32) at which the complement itself does not affect the viral antigen staining. Then, titrated immune sera in the presence or absence of complement were incubated with HCMV virus at room temperature for one hour and the mixture was transferred into cell coated plates. Assays were then performed as reported.Citation36
Statistical analysis
Statistical calculations were performed in JMP Pro 10.0 and GraphPad Prism 5.04.
Disclosure of potential conflicts of interest
No conflicts of interest were reported by the authors.
Supplementary Table
Download MS Word (13.2 KB)Acknowledgments
We thank Liz Ottinger, Robert Hepler and Maya Salnikova for technical advice and assistance. We also thank the staff of the Laboratory of Animal Research group at Merck for their assistance with animal studies.
References
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007; 17:253-76; PMID:17579921; http://dx.doi.org/10.1002/rmv.535
- Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. Vaccine Development to Prevent Cytomegalovirus Disease: Report from the National Vaccine Advisory Committee. Clin Infect Dis 2004; 39:233-9; PMID:15307033; http://dx.doi.org/10.1086/421999
- Griffiths PD. Burden of disease associated with human cytomegalovirus and prospects for elimination by universal immunisation. Lancet Infect Dis 2012; 12:790-8; PMID:23017365; http://dx.doi.org/10.1016/S1473-3099(12)70197-4
- Adler SP, Starr SE, Plotkin SA, Hempfling SH, Buis J, Manning ML, Best AM. Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age. J Infect Dis 1995; 171:26-32; PMID:7798679; http://dx.doi.org/10.1093/infdis/171.1.26
- Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang M-L, Corey L, Hill J, Davis E, Flanigan C, et al. Vaccine Prevention of Maternal Cytomegalovirus Infection. N Engl J Med 2009; 360:1191-9; PMID:19297572; http://dx.doi.org/10.1056/NEJMoa0804749
- Griffiths PD, Stanton A, McCarrell E, Smith C, Osman M, Harber M, Davenport A, Jones G, Wheeler DC, O'Beirne J, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. The Lancet 2011; 377:1256-63; http://dx.doi.org/10.1016/S0140-6736(11)60136-0
- Wang D, Shenk T. Human Cytomegalovirus UL131 Open Reading Frame Is Required for Epithelial Cell Tropism. J Virol 2005; 79:10330-8; PMID:16051825; http://dx.doi.org/10.1128/JVI.79.16.10330-10338.2005
- Isaacson MK, Compton T. Human Cytomegalovirus Glycoprotein B Is Required for Virus Entry and Cell-to-Cell Spread but Not for Virion Attachment, Assembly, or Egress. J Virol 2009; 83:3891-903; PMID:19193805; http://dx.doi.org/10.1128/JVI.01251-08
- Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. Crystal Structure of Glycoprotein B from Herpes Simplex Virus 1. Science 2006; 313:217-20; PMID:16840698; http://dx.doi.org/10.1126/science.1126548
- Heldwein EE, Krummenacher C. Entry of herpesviruses into mammalian cells. Cell Mol Life Sci 2008; 65:1653-68; PMID:18351291; http://dx.doi.org/10.1007/s00018-008-7570-z
- Stampfer SD, Lou H, Cohen GH, Eisenberg RJ, Heldwein EE. Structural Basis of Local, pH-Dependent Conformational Changes in Glycoprotein B from Herpes Simplex Virus Type 1. J Virol 2010; 84:12924-33; PMID:20943984; http://dx.doi.org/10.1128/JVI.01750-10
- Lopper M, Compton T. Coiled-coil domains in glycoproteins B and H are involved in human cytomegalovirus membrane fusion. J Virol 2004; 78:8333-41; PMID:15254205; http://dx.doi.org/10.1128/JVI.78.15.8333-8341.2004
- Britt WJ. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology 1984; 135:369-78; PMID:6330979; http://dx.doi.org/10.1016/0042-6822(84)90193-4
- Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 2005; 202:673-85; PMID:16147978; http://dx.doi.org/10.1084/jem.20050882
- Meyer H, Sundqvist V-A, Pereira L, Mach M. Glycoprotein gp116 of human cytomegalovirus contains epitopes for strain-common and strain-specific antibodies. J Gen Virol 1992; 73:2375-83; PMID:1383409; http://dx.doi.org/10.1099/0022-1317-73-9-2375
- Matsumoto Y, Sugano T, Miyamoto C, Masuho Y. Generation of hybridomas producing human monoclonal antibodies against human cytomegalovirus. Biochem Biophys Res Commun 1986; 137:273-80; PMID:3013182; http://dx.doi.org/10.1016/0006-291X(86)91206-4
- Macagno A, Bernasconi NL, Vanzetta F, Dander E, Sarasini A, Revello MG, Gerna G, Sallusto F, Lanzavecchia A. Isolation of Human Monoclonal Antibodies That Potently Neutralize Human Cytomegalovirus Infection by Targeting Different Epitopes on the gH/gL/UL128-131A Complex. J Virol 2010; 84:1005-13; PMID:19889756; http://dx.doi.org/10.1128/JVI.01809-09
- Ohlin M, Sundqvist VA, Mach M, Wahren B, Borrebaeck CA. Fine specificity of the human immune response to the major neutralization epitopes expressed on cytomegalovirus gp58/116 (gB), as determined with human monoclonal antibodies. J Virol 1993; 67:703-10; PMID:7678304
- Reeves MB, Breidenstein A, Compton T. Human cytomegalovirus activation of ERK and myeloid cell leukemia-1 protein correlates with survival of latently infected cells. Proc Natl Acad Sci 2012; 109:588-93; PMID:22203987; http://dx.doi.org/10.1073/pnas.1114966108
- Kauvar LM, Liu K, Park M, DeChene N, Stephenson R, Tenorio E, Ellsworth SL, Tabata T, Petitt M, Tsuge M, et al. A high-affinity native human antibody neutralizes human cytomegalovirus infection of diverse cell types. Antimicrob Agents Chemother 2015; 59:1558-68; PMID:25534746; http://dx.doi.org/10.1128/AAC.04295-14
- Theraclone Sciences, Inc. Safety Study of Human Anti-Cytomegalovirus Monoclonal Antibody [Internet]. In: ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US); 2014. Available from: http://clinicaltrials.gov/ct2/show/NCT01594437
- Ohlin M, Silvestri M, Sundqvist VA, Borrebaeck CA. Cytomegalovirus glycoprotein B-specific antibody analysis using electrochemiluminescence detection-based techniques. Clin Diagn Lab Immunol 1997; 4:107-11; PMID:9008292
- Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A 2005; 102:18153-8; PMID:16319222; http://dx.doi.org/10.1073/pnas.0509201102
- Freed DC, Tang Q, Tang A, Li F, He X, Huang Z, Meng W, Xia L, Finnefrock AC, Durr E, et al. Pentameric complex of viral glycoprotein H is the primary target for potent neutralization by a human cytomegalovirus vaccine. Proc Natl Acad Sci 2013; 110:E4997-5005; PMID:24297878; http://dx.doi.org/10.1073/pnas.1316517110
- Nest GV, Ott G, Barchfeld G. Adjuvant formulation comprising a submicron oil droplet emulsion [Internet]. 2001 [cited 2015 Jul 14]; Available from: http://www.google.com/patents/US6299884
- Fu T-M, Grimm KM, Citron MP, Freed DC, Fan J, Keller PM, Shiver JW, Liang X, Joyce JG. Comparative immunogenicity evaluations of influenza A virus M2 peptide as recombinant virus like particle or conjugate vaccines in mice and monkeys. Vaccine 2009; 27:1440-7; PMID:19146898; http://dx.doi.org/10.1016/j.vaccine.2008.12.034
- Ohlin M, Söderberg-Nauclér C. Human antibody technology and the development of antibodies against cytomegalovirus. Mol Immunol 2015; 67:153-70; PMID:25802091; http://dx.doi.org/10.1016/j.molimm.2015.02.026; Available from: http://www.sciencedirect.com/science/article/pii/S0161589015000759
- McLean GR, Olsen OA, Watt IN, Rathanaswami P, Leslie KB, Babcook JS, Schrader JW. Recognition of Human Cytomegalovirus by Human Primary Immunoglobulins Identifies an Innate Foundation to an Adaptive Immune Response. J Immunol 2005; 174:4768-78; PMID:15814702; http://dx.doi.org/10.4049/jimmunol.174.8.4768
- Ohlin M. A new look at a poorly immunogenic neutralization epitope on cytomegalovirus glycoprotein B. Is there cause for antigen redesign? Mol Immunol 2014; 60:95-102; PMID:24802891; http://dx.doi.org/10.1016/j.molimm.2014.03.015
- Burke HG, Heldwein EE. Crystal Structure of the Human Cytomegalovirus Glycoprotein B. PLoS Pathog 2015; 11:e1005227; PMID:26484870; http://dx.doi.org/10.1371/journal.ppat.1005227
- Bianchi E, Joyce JG, Miller MD, Finnefrock AC, Liang X, Finotto M, Ingallinella P, McKenna P, Citron M, Ottinger E, et al. Vaccination with peptide mimetics of the gp41 prehairpin fusion intermediate yields neutralizing antisera against HIV-1 isolates. Proc Natl Acad Sci U S A 2010; 107:10655-60; PMID:20483992; http://dx.doi.org/10.1073/pnas.1004261107
- Shinefield HR. Overview of the development and current use of CRM(197) conjugate vaccines for pediatric use. Vaccine 2010; 28:4335-9; PMID:20452430; http://dx.doi.org/10.1016/j.vaccine.2010.04.072
- Nahas DD, Palladino JS, Joyce JG, Hepler RW. Amino acid analysis of peptide loading ratios in conjugate vaccines: a comparison of direct electrochemical detection and 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate pre-column derivatization methods. Bioconjug Chem 2008; 19:322-6; PMID:18072716; http://dx.doi.org/10.1021/bc700232z
- Caro-Aguilar I, Ottinger E, Hepler RW, Nahas DD, Wu C, Good MF, Batzloff M, Joyce JG, Heinrichs JH, Skinner JM. Immunogenicity in mice and non-human primates of the Group A Streptococcal J8 peptide vaccine candidate conjugated to CRM197. Hum Vaccines Immunother 2013; 9:488-96; http://dx.doi.org/10.4161/hv.23224
- Spaete RR, Pachl CA. Polynucleotides encoding CMV neutralizing proteins [Internet]. 1998 [cited 2015 Jul 14]; Available from: http://www.google.com/patents/US5834307
- Tang A, Li F, Freed DC, Finnefrock AC, Casimiro DR, Wang D, Fu T-M. A novel high-throughput neutralization assay for supporting clinical evaluations of human cytomegalovirus vaccines. Vaccine 2011; 29:8350-6; PMID:21888937; http://dx.doi.org/10.1016/j.vaccine.2011.08.086
- Britt WJ. Human cytomegalovirus: propagation, quantification, and storage. Curr Protoc Microbiol 2010; 18:E:14E.3:14E.3.1–14E.3.17; PMID:20812216.
- Fu T-M, Wang D, Freed DC, Tang A, Li F, He X, Cole S, Dubey S, Finnefrock AC, ter Meulen J, et al. Restoration of viral epithelial tropism improves immunogenicity in rabbits and rhesus macaques for a whole virion vaccine of human cytomegalovirus. Vaccine 2012; 30:7469-74; PMID:23107592; http://dx.doi.org/10.1016/j.vaccine.2012.10.053
- Wang D, Li F, Freed DC, Finnefrock AC, Tang A, Grimes SN, Casimiro DR, Fu T-M. Quantitative analysis of neutralizing antibody response to human cytomegalovirus in natural infection. Vaccine 2011; 29:9075-80; PMID:21945962; http://dx.doi.org/10.1016/j.vaccine.2011.09.056
