ABSTRACT
Chlamydia trachomatis is one of the most common sexually transmitted pathogens and the development of an effective vaccine is highly desirable. The Major Outer Membrane Protein (MOMP) is one of the most abundant and immunogenic chlamydial proteins. Here we investigated the effects of phosphate substitution on the physicochemical and immunochemical properties of an experimental vaccine composed of serovar E recombinant MOMP (rMOMP) and a proprietary adjuvant system SPA08, consisting of aluminum oxyhydroxide (AlOOH) containing the TLR4 agonist E6020.
An increase in phosphate substitution in the AlOOH component of the adjuvant markedly decreased the adsorptive coefficient and adsorptive capacity for both Ser E rMOMP and E6020. In vaccine formulations used for immunizations, phosphate substitution induced a decrease in the % adsorption of Ser E rMOMP without affecting the % adsorption of E6020.
Immunogenicity studies in CD1 mice showed that an increase in phosphate substitution of the SPA08 adjuvant resulted in an increase in Ser E rMOMP-specific serum total IgG and IgG1 but not IgG2a titers. The degree of phosphate substitution in SPA08 also significantly increased in vitro neutralization concomitant with a decrease in proinflammatory cytokines secreted by Ser E rMOMP-restimulated splenocytes.
Taken together, the results of these studies suggest that the degree of phosphate substitution in AlOOH greatly affects the adsorption of E6020 and Ser E rMOMP to AlOOH resulting in significant effects on vaccine-induced cellular and humoral responses.
Introduction
Chlamydia trachomatis is an obligate intracellular Gram-negative bacterium that causes infections of the eye (trachoma) and the genital tract. Genital chlamydiosis is one of the most common sexually transmitted diseases worldwide, with 100 million new cases each year. Citation1 While C. trachomatis infections can be treated with antibiotics, most people are not aware of their infections as up to 90% of women and 50% of men do not experience clinical symptoms, and therefore do not seek medical attention. Although there is currently no vaccine available against C. trachomatis, prophylactic vaccination could have a dramatic impact on the health of sexually active people.Citation2
Studies in mice have shown that pathogenesis resulting from a chlamydia infection on the oviduct and mesosalpinx is dependent on signaling through Toll-like receptor 2 (TLR2) and not TLR4.Citation3 However, both TLR2 and TLR4 deficient mice were able to clear the infection similarly to wild-type mice. Both human and mouse data Citation4-6 indicate that TLR2 is the primary pathogen-recognition receptor in the upper genital tract able to alert the immune system and to drive the immune-pathogenic mechanisms associated with chlamydia infection. A chlamydia subunit vaccine adjuvanted with a TLR4 agonist may reduce or eliminate immunopathogenesis associated with C. trachomatis signaling through TLR2.
One of the preferred vaccination strategies against infection is the induction of specific neutralizing/blocking antibodies against chlamydial proteins to prevent the entry of elementary bodies (EB) into cells. Major outer membrane protein (MOMP) is the most abundant chlamydial protein constituting up to 60% of the EB dry weight. As well as being an immunodominant antigen,Citation7 it has been shown to induce protective immunity similar to that of live bacteria.Citation8
In recent years, aluminum salt adjuvants have been used as carriers for TLR agonists and immunomodulatory molecules.Citation9 Prophylactic vaccines containing TLR4 agonists adsorbed to aluminum salts are currently in various stages of clinical development, with one product, Cervarix® currently licensed in the US for the prevention of human papilloma virus infection.Citation10 There are several reports in the literature on the interaction of antigens with aluminum salt adjuvants and its impact on immunogenicity.Citation11-14 However, little is known about the effect of the degree and strength of adsorption of antigens formulated in combination with a TLR agonist and an aluminum salt adjuvant. Phosphate substitution of the aluminum salt would affect not only antigen adsorption but also TLR agonist adsorption and bioavailability, which could impact the immunogenicity of the vaccine. Here, we report the effect of phosphate substitution on the physicochemical properties of the vaccine as well as the immunogenicity of recombinant C. trachomatis serovar E MOMP (Ser E rMOMP) formulated with SPA08, a proprietary adjuvant based on aluminum oxyhydroxide (AlOOH) carrying a TLR4 agonist. The results suggest that the degree of Ser E rMOMP adsorption, as well as the strength of TLR4 agonist adsorption, significantly modulate Ser E rMOMP-specific immune response.
Results
Phosphate substitution significantly influences the adsorption of E6020 and Ser E rMOMP onto AlOOH
To investigate the impact of phosphate substitution on the adsorption properties of Ser E rMOMP and the TLR4 agonist E6020, different formulations of SPA08 were prepared in the presence of increasing concentrations of phosphate ions. The P/Al molar ratio of the AlOOH component was varied from 0 to 2 to prepare four different formulations: SPA08-1 (P/Al = 0), SPA08-2 (P/Al = 0.1), SPA08-3 (P/Al = 1) and SPA08-4 (P/Al = 2).
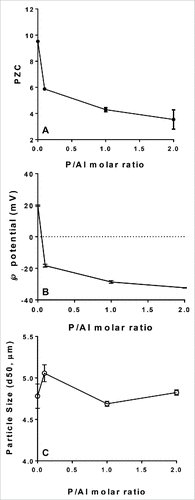
A significant decrease in the point of zero charge and the zeta potential at neutral pH for the SPA08 adjuvant was observed when the P/Al molar ratio was increased () suggesting a high degree of phosphate substitution at the surface of the AlOOH component of the adjuvant. The degree of phosphate substitution, however, did not significantly alter the particle size as judged by laser diffraction analysis (). Since E6020 is a synthetic phospholipid dimer,Citation15 binding to AlOOH likely occurs through a ligand exchange mechanism between the two phosphate moieties in E6020 and the surface hydroxyls of AlOOH. Therefore, increased phosphate substitution could decrease the degree of E6020 adsorption to AlOOH.
Figure 1. Effect of phosphate substitution of AlOOH on physicochemical characteristics of SPA08. The point of zero charge (PZC) (A), zeta potential at pH 7.4 (B), and the particle size (C) were measured as a function of the P/Al molar ratio. Error bars represent the standard deviation from the mean (n = 3).
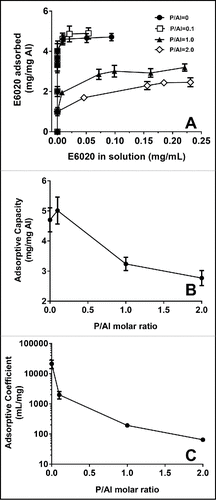
To investigate the effect of phosphate substitution on the % adsorption of E6020 to AlOOH, the concentration of unbound E6020 was quantified after centrifugation of SPA08 formulations containing different P/Al molar ratios and final concentration of 40 μg/mL E6020 and 2.4 mg/mL of elemental Al. In all formulations the amount of unbound E6020 in the supernatants was undetectable by RP-HPLC (data not shown), suggesting that E6020 remained bound to AlOOH despite significant phosphate substitution. When phosphate was added to AlOOH prior to the addition of E6020 the amount of unbound E6020 was also undetectable (data not shown) suggesting that the order of mixing of ingredients did not impacted the binding of E6020 to AlOOH. To investigate the effects of phosphate substitution on the adsorption characteristics of E6020 to AlOOH, Langmuir adsorption isotherms were constructed for E6020 onto AlOOH which was previously treated with increasing concentrations of phosphate ions. The adsorption isotherms for E6020 onto AlOOH with different degrees of phosphate substitution followed the Langmuir equation, which allowed us to determine the adsorptive coefficient and the adsorptive capacity (). As shown in , an increase in the phosphate substitution had a significant effect on both the adsorptive capacity () and the adsorptive coefficient (). These results suggest that the strength of adsorption of E6020 to AlOOH, as measured by the Langmuir adsorptive coefficient, is inversely related to the degree of phosphate substitution for surface hydroxyls in AlOOH.
Figure 2. Effect of phosphate substitution of AlOOH on adsorptive capacity. (A) Langmuir adsorption isotherms of E6020 on AlOOH with increasing P/Al ratio were obtained at 25°C and pH 7.4 Effect of P/AL ratio on the adsorption capacity (B) and adsorptive coefficient (C) obtained for the adsorption of E6020 onto AlOOH. Error bars represent the standard deviation from the mean (n = 2).
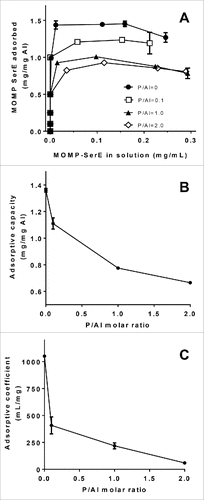
After characterizing the adsorption parameters of E6020 to AlOOH with different degrees of phosphate substitution it was of interest to study the adsorption characteristics of Ser E rMOMP to each phosphate treated SPA08 formulation by constructing Langmuir adsorption isotherms. The theoretical isoelectric point of Ser E rMOMP is 4.8 and therefore at neutral pH Ser E rMOMP is expected to bind to SPA08 primarily by electrostatic interaction. The adsorption isotherms for Ser E rMOMP to the SPA08 formulations with different degrees of phosphate substitution followed the Langmuir equation (). The linear form of the Langmuir equation was used to calculate the adsorptive capacity and the adsorptive coefficient (). As expected, Ser E rMOMP adsorption to SPA08-1 (P/Al = 0) had relatively high adsorptive coefficient and adsorptive capacity indicating the strong electrostatic interaction between protein and adjuvant at neutral pH. Both the adsorptive capacity and adsorptive coefficient decreased significantly as the degree of phosphate substitution increased from a P/Al molar ratio of 0 to 2. These results indicate that the amount of Ser E rMOMP that is adsorbed at the surface of the adjuvant and the strength of adsorption are significantly decreased by phosphate substitution likely due to the reduction of PZC which decreases the electrostatic interaction between Ser E rMOMP and SPA08 at neutral pH.
Figure 3. Effect of phosphate substitution of SPA08 on Ser E rMOMP adsorption isotherms. (A) Langmuir adsorption isotherms of Ser E rMOMP on SPA08 with increasing P/Al ratio were obtained at 25°C and pH 7.4. (B) The adsorptive capacity and (C) adsorptive coefficient values were calculated from the linearized form of the Langmuir equation and plotted as a function of P/Al molar ratio. Error bars represent the standard deviation from the mean (n = 2).
Phosphate substitution in SPA08 modulates immunogenicity of Ser E rMOMP from C trachomatis
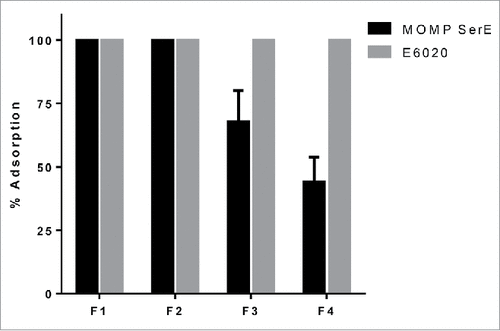
To study the effect of phosphate substitution, its impact on antigen and TLR agonist adsorption and the vaccine's immunogenicity, groups of ten CD1 mice were immunized i.m. three times, three weeks apart with either 10 µg or 34 µg of Ser E rMOMP formulated with the four SPA08 preparations which differed in the degree of phosphate substitution. The formulations were prepared by mixing a stock solution of Ser E rMOMP with the different SPA08 preparations, which were then immediately injected. Upon mixing, the % adsorption of both E6020 and Ser E rMOMP was measured (). In all four formulations, E6020 was 100% adsorbed to AlOOH, whereas a progressive decrease in the % adsorption was observed for Ser E rMOMP with increasing phosphate substitution ().
Figure 4. Adsorption of Ser E rMOMP and E6020 to SPA08 in the experimental vaccine. Experimental vaccine formulations were prepared by mixing one volume of a stock solution of Ser E rMOMP with one volume of SPA08-1 (F1), SPA08-2 (F2), SPA08-3 (F3) or SPA08-4 (F4). Error bars represent the standard deviation from the mean (n = 2).
After immunization, Ser E rMOMP-specific immune responses were quantified for total IgG, IgG1 and IgG2a subclasses, in vitro neutralizing capacity against EBs of Ser E infection, and cytokine secretion upon in vitro restimulation of splenocytes with Ser E rMOMP.
The degree of phosphate substitution significantly increases Ser E rMOMP-specific serum total IgG and IgG1 but not IgG2a titers
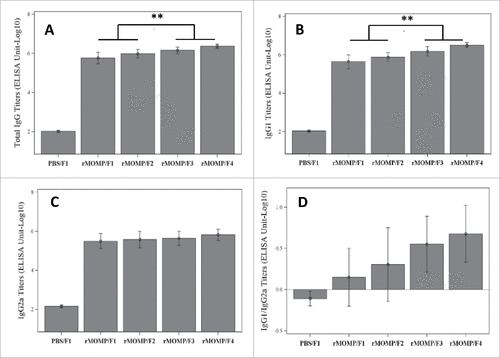
The serum antibody response induced by Ser E rMOMP in the presence of the SPA08 adjuvant with or without added phosphate was strongly elevated over the negative control (PBS/SPA08-1). The degree of phosphate substitution in the SPA08 was associated with increased total IgG () and IgG1 () titers in a dose dependent manner. The total IgG titer increased 4-fold from a P/Al ratio of 0 to 2. Phosphate substitution also qualitatively impacted the IgG response, with an increasingly predominant and significant IgG1 subclass response for the Ser E rMOMP/SPA08-3 and Ser E rMOMP/SPA08-4 formulations when compared to Ser E rMOMP/SPA08-1 and Ser E rMOMP/SPA08-2 formulations, which had a more balanced IgG1/IgG2a response (). There was no apparent impact of phosphate substitution on the IgG2a titers (). Similar results were observed for both doses of Ser E rMOMP used for immunization (only results for Ser E rMOMP at 10 µg shown).
Figure 5. IgG serum titers in mice immunized twice with 10 µg of Ser E rMOMP/SPA08 formulations (F1, SPA08-1; F2, SPA08-2; F3, SPA08-3; F4, SPA08-4) or control. (A) Total IgG titers; (B) IgG1 titers; (C) IgG2a titers; (D) IgG1/IgG2a ratio. Connecting lines representing the statistical comparison indicate that either of the selected 2 groups is significantly different to either of the other 2 groups. ** p<0.01.
The degree of phosphate substitution in SPA08 significantly increases in vitro neutralization response
The in vitro neutralization assay has been long used to measure the functionality of MOMP-specific antibody responses Citation16 and the generation of neutralizing antibodies against EBs is one of the preferred mechanisms of protection of chlamydia vaccines. The ability of sera to neutralize infection of Hela cells by chlamydial Ser E EBs was tested in vitro.
The serum from Ser E rMOMP/SPA08-3 and Ser E rMOMP/SPA08-4 immunized mice elicited significantly higher neutralizing titers (70 and 321, respectively) than the serum from Ser E rMOMP/SPA08-1 and Ser E rMOMP/SPA08-2 immunized mice (19 and 23, respectively) (p < 0.01) (). The groups immunized with 34 µg of Ser E rMOMP showed a similar increasing trend in the neutralization titers, however the results were not statistically significant (data not shown).
Figure 6. Neutralizing effects of sera from mice immunized with 10 µg of Ser E rMOMP/SPA08 formulations (F1, SPA08-1; F2, SPA08-2; F3, SPA08-3; F4, SPA08-4). (A) Neutralizing titers as the inverse dilution at which 50% of cells are not infected, (B) percent of mice with neutralizing serum response. Similar results were obtained in mice immunized with 34 µg of Ser E rMOMP/SPA08 formulations. Connecting lines representing the statistical comparison indicate that either of the selected 2 groups is significantly different to either of the other 2 groups. ** p < 0.01.
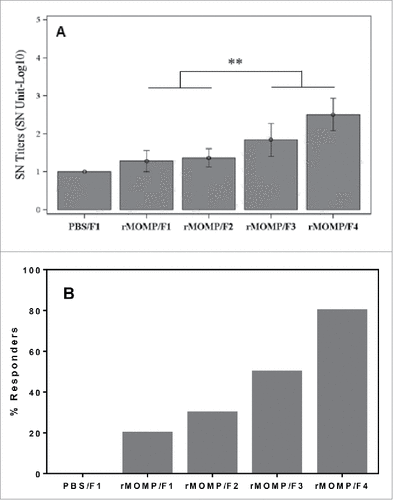
With regards to the percentage of mice with neutralizing sera, an increase was observed from SPA08-1 (20%) to SPA08-4 (80%), for mice immunized with 10 µg Ser E rMOMP (), following a similar trend observed for the titers of total IgG and IgG1. Intermediate percentages were found for the SPA08-2 (30%) and SPA08-3 (50%) formulations. In the groups of mice immunized with 34 µg Ser E rMOMP, the percentage of animals with neutralizing response was as follows: 30% for SPA08-1, 40% for SPA08-2, 60% for SPA08-3 and 100% for SPA08-4. No significant difference was observed between the two doses of antigen (data not shown). No neutralization response was detected upon sham immunization.
Phosphate substitution in SPA08 modulates cytokine responses
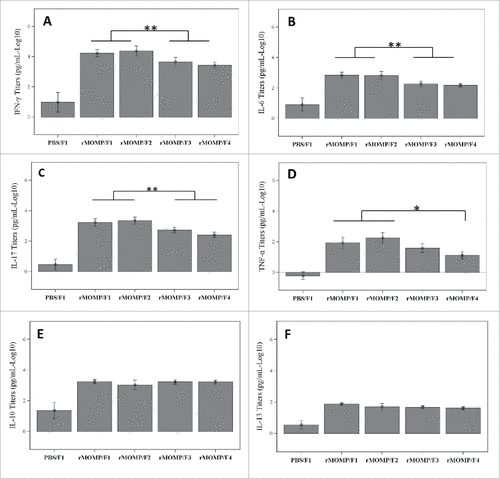
Protection against C trachomatis infection requires IFN-γ production by CD4 Th1 cells, which have been implicated in long-term protection after infection.Citation17 To investigate the effect of phosphate substitution on cytokine production, the secretion of Th1, Th2 and Th17 cytokines by restimulated splenocytes was measured. All study groups mounted significantly higher cytokine responses compared to the sham immunized control animals (). Two trends of cytokine secretion were identified. For Th1/Th17 cytokines, the Ser E rMOMP-specific IFN-γ, IL-6 and IL-17 secretions were significantly lower for SPA08-3 and SPA08-4 formulations versus SPA08-1 and SPA08-2 formulations (p-value < 0.01), while the Ser E rMOMP-specific TNF-α secretion was significantly lower only for the SPA08-4 formulation. The decrease in IFNγ and IL-17 production from SPA08-1 and SPA08-2 to SPA08-3 and SPA08-4 was substantial (up to an 8.8-fold decrease). These results suggest that phosphate substitution lowers the proinflammatory and Th1/Th17 character of the Ser E rMOMP response upon immunization (). Th2 cytokines such as IL-10 and IL-13 were not significantly affected by phosphate substitution ().
Figure 7. Cytokine secretion by cultured splenocytes from mice immunized with 34 µg of Ser E rMOMP/SPA08 formulations (F1, SPA08-1; F2, SPA08-2; F3, SPA08-3; F4, SPA08-4). (A) IFN-y, (B) IL-6, (C) IL-17, (D) TNF-α, (E) IL-10, (F) IL-13. Similar results were obtained with 10 µg Ser E rMOMP dose. Connecting lines representing the statistical comparison indicate that either of the selected 2 groups is significantly different to either of the other 2 groups (panels A, B and C), or to the singly selected group (panel D). * p < 0.05, **p < 0.01.
Discussion
The goal of this study was to investigate the effect of phosphate substitution on the physicochemical and immunological properties of Ser E rMOMP vaccine formulated with an adjuvant complex composed of AlOOH and a the synthetic TLR4 agonist E6020. Adjuvant systems comprised of aluminum salts and a TLR4 agonist are a new generation of adjuvants now licensed in human vaccines.Citation18,19 Although the effects of TLR4 agonists with aluminum salts on the production of pro-inflammatory cytokines has been studied,Citation20,21 there is limited information related to the interactions between the antigen and aluminum salt and between TLR4 and aluminum salt and their impact on immunogenicity. Previous work by Hansen et al. using the hepatitis B surface antigen demonstrated that the strength of adsorption of the antigen to AlOOH adjuvant can affect the vaccine's immunogenicity and showed that antibody production was reduced when the antigen was adsorbed too strongly.Citation12 This highlighted the importance of controlling the adsorption of antigens to aluminum salt adjuvants during the formulation to optimize vaccine immunogenicity. For the case of TLR agonists formulated with aluminum salts, attention should be taken to optimize not only the antigen-aluminum salt interaction but also interactions between the TLR agonist and the aluminum salt.
Here, we demonstrated that increasing the phosphate substitution of the AlOOH induces a progressive decrease in the strength of adsorption of both E6020 and SerE rMOMP. Additionally, significant phosphate substitution (P/Al > 1) induces a decrease in the % adsorption of SerE rMOMP in the final vaccine formulation without significantly affecting the % adsorption of E6020. The reasons for such different adsorption behavior may likely be due to the different mechanisms of adsorption to AlOOH by E6020 and SerE rMOMP. A ligand-exchange interaction is the mechanism of adsorption of E6020 to AlOOH, which is stronger than electrostatic interaction proposed for the adsorption of SerE rMOMP to AlOOH.
The changes induced by phosphate substitution on the interaction between vaccine components were found to generate marked effects in immunogenicity, which were characterized by an increase in the neutralizing antibody titers concomitant with a decrease in proinflammatory cytokines. The total IgG increase seen with formulations Ser E rMOMP/SPA08-3 and Ser E rMOMP/SPA08-4 is due to an increase of the IgG1 and not the IgG2a response. This is clearly shown in where the IgG1/IgG2a ratio steadily increases from the base formulation, Ser E rMOMP/SPA08-1 to the Ser E rMOMP/SPA08-4 formulation. It is likely that the diminished Th1/Th17 character of the Ser E rMOMP response upon phosphate substitution fostered an environment more suitable for the IgG1 class switch. The increased antibody production was also associated by an elevated neutralization response. This increase in neutralization is surprising since IgG1 is a poor complement activator and the in vitro neutralization assay is complement-dependent. In the hierarchical functional activity of mouse IgG subclasses, IgG2b stands beside or just under IgG2a and is also an efficient complement activator.Citation22 An increasing amount of IgG2b in the Ser E rMOMP/SPA08-3 and Ser E rMOMP/SPA08-4 groups, may explain the higher neutralizing capacity of sera from these groups. IL-21, which is a cytokine secreted by Th2 and Th17 cells,Citation23,24 was shown to induce in vitro class switching to IgG1 and IgG2b, whereas IFN-γ and Th17 create an environment suitable for IgG2a switch. While the IgG2a titers were not decreased despite diminishing IFN-γ and Th17 responses, the increased neutralization response seen upon phosphate substitution (as in formulations F3 and F4) could be explained if there were an increase in IL-21 production which would favor a switch to IgG2b.Citation25 Future investigations should focus on the associations between IL-21 production and IgG2b, along with the contribution of IgG2b to neutralization. Alternatively, the increase in IgG1 titer may have been sufficient to overcome its poor activator properties to induce a neutralization response.
Why phosphate substitution impacts the IgG production may be related to how the antigen processing and presentation unfolds after immunization with the various formulations. In the formulations with no or low phosphate substitution (F1 and F2), SerE rMOMP is fully absorbed onto AlOOH, while in those with higher phosphate substitution (F3 and F4) the antigen is partly in solution. B cells are particularly adapted to take up soluble antigens via their immunoglobulin receptors and they may further contribute to processing and presentation of soluble SerE rMOMP favoring stronger antibody responses.Citation26,27 The antigen processing via B cells may not happen for formulations F1 and F2 where Ser rMOMP is fully absorbed.
It is perhaps surprising that a decrease in the degree and strength of adsorption of Ser E rMOMP and E6020 were able to induce a significant suppression of the Th1/Th17 response. One possibility is that the decrease in the strength of adsorption of E6020 may be responsible for the observed switch. This is supported by the fact that TLR agonists have been shown to enhance their pro-inflammatory adjuvant effects when bound to the aluminum salt.Citation28,29 Although E6020 remained adsorbed, the significant decrease in the strength of adsorption observed upon phosphate substitution may be responsible for the change in the cytokine profile. Upon vaccine injection, it is likely that E6020 in formulations with high phosphate substitution and thus a reduced strength of adsorption may become desorbed from the AlOOH due to the relatively high concentration of competing ions in the interstitial fluid. In contrast, formulations with low phosphate substitution may better retain and concentrate E6020 and SerE rMOMP within the AlOOH particles resulting in an enhanced uptake by APC, an efficient maturation of dendritic cells and improved presentation of antigens to T cells.Citation30
The Th1 response is essential in the resolution of chlamydia infection. The phosphate-treated vaccine, while inducing an increased neutralization response (seen as a desired effect), also diminished the Th1 and Th17 cytokine production. Bacterial challenge studies in animals may help to understand how phosphate substitution, neutralization titers and cytokine profiles impact the vaccine efficacy against C trachomatis infection.
In the experiments described here, formulations were prepared extemporaneously by mixing adjuvant and antigen just prior the immunizations. It is currently unknown whether the binding strength of both SerE rMOMP antigen and TLR4 agonist to AlOOH may increase or decrease over a longer period of time and how such changes would impact immunogenicity. Given the significant influence of adsorption on vaccine immunogenicity observed in our study, understanding the long term stability of binding will be essential for the development of an effective ready to use vaccine formulation.
Altogether we report a significant effect of phosphate substitution on the immunogenicity of a Ser E rMOMP subunit vaccine adjuvanted with AlOOH carrying a synthetic TLR4 agonist. The significant differences in the antibody and cytokine responses to the vaccine with varying degrees of phosphate substitution of the AlOOH component highlight the importance of formulation optimization in the development of effective vaccines.
Materials and methods
Vaccine formulations
Recombinant C. trachomatis Serovar E/Bour MOMP (Ser E rMOMP) was expressed in E. coli as inclusion bodies, then refolded and purified by sequential column chromatography. Final purity was greater than 90% as evaluated by SDS-PAGE.
SPA08 adjuvant is composed of the TLR4 agonist E6020 (ESAI) adsorbed to aluminum oxyhydroxide (AlOOH) (Brenntag). SPA08 was prepared by dissolving E6020 in 100 % ethanol followed by dilution in Tris buffered saline (TBS) (50 mM Tris, 100 mM NaCl, pH 7.4) with continuous stirring. The E6020 suspension was added to AlOOH with continuous mixing to a final concentration of 40 μg/mL E6020 and 2.4 mg/mL of elemental Al.
Phosphate substitution was conducted by addition of the desired amount of 0.8 M phosphate buffer pH 7.4 to achieve a final concentration of 8.8 mM phosphate (phosphorous/ aluminum (P/A) = 0.1), 88 mM phosphate (P/Al = 1.0) and 176 mM phosphate (P/Al = 2.0). A total of four adjuvant formulations were prepared: SPA08-1 or F1 (no phosphate treatment), SPA08-2 or F2 (8.8 mM phosphate), SPA08-3 or F3 (88 mM phosphate) and SPA08-4 or F4 (176 mM phosphate).
Adsorption isotherms
Adsorption isotherms to evaluate the adsorption of E6020 to AlOOH were conducted by titration of AlOOH with different degrees of phosphate substitution (P/A, 0.0, 0.1, 1.0 and 2.0) with increasing concentrations of E6020 in TBS. Samples were mixed on an orbital mixer for 30 min at room temperature. The samples were then centrifuged for 5 min at 4000 × g. The supernatants were collected and the E6020 concentration was measured by reversed-phase high performance liquid chromatography (RP-HPLC) in an Agilent 1200 HPLC system equipped with a diode array UV detector. Separation was conducted using an Waters Xterra RP8 column (Waters Corporation) and a mobile-phase gradient of buffer A [50/50 % Water/Ethanol, 2% H3PO4] and buffer B [100% ethanol with 2% H3PO4], with a gradient of 2.8% of buffer B per min over 25 min at a flow rate of 0.8 mL/min. Limit of quantification was 3 µg/mL E6020.
Adsorptive capacity and adsorptive coefficient were calculated using the linearized Langmuir equation as previously reported.Citation31
Adsorption isotherms to evaluate the adsorption of Ser E rMOMP to the different phosphate-treated SPA08 were conducted by titration of the desired adjuvant with increasing concentrations of Ser E rMOMP in Tris buffer saline pH 7.4. SPA08 adjuvants with different degrees of phosphate substitution were prepared by addition of E6020 to AlOOH followed by addition of phosphate to reach the desired P/Al ratio. The adjuvants were diluted to 200 μg/mL of Al in 1.5 mL conical polypropylene tubes and mixed with increasing concentration of Ser E rMOMP. The tubes were mixed in an orbital mixer for 30 min at room temperature. The samples were then centrifuged 5 min at 4000 × g. The supernatants were collected and the protein concentration was measured by UV absorption spectroscopy at 280 nm in an Agilent 8453 UV/VIS spectrophotometer. Adsorptive capacity and adsorptive coefficient were calculated using the linearized Langmuir equation as previously reported.Citation31
Particle size determination
The particle size distribution of different adjuvant formulations was measured by laser diffraction using a Mastersizer 2000 linked to a Hydro 2000S sample dispersion unit (Malvern Instruments Ltd.). The results were processed by volume and the data compared by the volume median diameter d(0.5) which correspond to the diameter below which 50% of the particles are distributed by volume.
Point of zero charge
The point of zero charge (PZC) was measured by phase analysis light scattering using the Zeta PALS instrument (Brookhaven Instruments Corporation). Samples were diluted 1:20 in ultra-pure water and the pH was adjusted from 3 to 10 using 0.1 N HCl or 0.1 N NaOH at intervals of one pH unit. The PZC was determined graphically using linear fitting of the data as the pH where the zeta potential was zero.
Immunization procedures
Nine-week-old female CD1 mice (Charles River Laboratories) were housed at the Sanofi Pasteur, (Marcy l'Etoile, France). The Animal Care and Use Committee approved all animal protocols.
A total of ten mice per group received a 50 µL intramuscular (i.m.) injection of the primary immunizing dose consisting of either 10 μg or 34 μg of Ser E rMOMP and formulated with one of the four adjuvants, SPA08-1 to SPA08-4, in 1:1 v/v ratio. The order of addition of vaccine components was the essentially the same as the one used for obtaining SerE rMOMP adsorption isotherms. Mice were injected immediately after doses were prepared on days 0, 21 and 42. Control mice were sham immunized with the same volume of inoculum containing SPA08-1 in phosphate buffered saline (PBS). Mice were euthanized on day 56, their serum was collected, heat-inactivated at 56°C for 30 min and stored at −20 °C until further use. Spleens were removed, processed and used to monitor antigen specific T cell responses as described below.
Cell mediated immune responses
To assess the T cell responses, an in vitro multiplexed cytokine assay (Meso Scale Discovery (MSD)) was performed 15 days after the last immunization. Cytokine profiles were determined using restimulated splenocytes. Briefly, 100 μL of freshly processed splenocytes were co-cultured at 1 × 106 cells/mL in 96-well plates for 3 days with 100 μL of RPMI 1640 medium (supplemented with 20 mM glutamine, 1 mg/mL streptomycin, 1000 U/mL penicillin, 0.1% β-mercaptoethanol and 10% fetal bovine serum (FBS)) (Life Sciences) and 100 μL of 10 µg/mL Ser E rMOMP or control stimulus. After 3 days of incubation, the cell supernatants were collected and frozen at −80°C until the MSD assay was performed.
Custom-made MSD plates were used for the measuring levels of IFN-γ, IL-17, IL-6, IL-10, IL-13 and TNF-α. These multiplex plates were used for the analysis of cytokine secretion in culture supernatants from Ser E rMOMP-restimulated splenocytes from mice immunized with Ser E rMOMP. Assays were performed according to the manufacturer's instructions. All standards and samples were measured in single replicates. MSD plates were analyzed on a SECTOR imager (MSD). Results were expressed as cytokine concentration in pg/mL (geometric mean per group).
Sero-neutralization assay
All serum samples were tested for their ability to neutralize the elementary bodies (EBs) of the C. trachomatis serovar E/Bour in an in vitro neutralization assay. Briefly, 6 replicates of 2-fold serial dilutions of the serum were made using DMEM with 5% baby rabbit complement (BRC) and incubated for 45 min at 37°C in 5% CO2 with EBs of serovar E/Bour (produced in-house) at 3 × 104 infectious units (IFU)/mL. The serum/bacteria mixtures were added to HeLa cell monolayers and centrifuged for 1 hour. Supernatants were removed and 1 µg/mL of cycloheximide (Sigma-Aldrich) was added and incubated for 48 h at 37°C with 5% CO2. Then, the monolayers were fixed with methanol (Sigma-Aldrich). The inclusions were stained using an anti-MOMP variable domain antibody (produced in our laboratory). A HRP-donkey anti-rabbit IgG (Jackson ImmunoResearch) was added and developed with a DAB substrate kit (Thermo Scientific). Plates were dried before analysis in the plate reader to count the infected cells in each well. Neutralizing end-point titers were defined as the reciprocal of the last dilution that fell below the calculated 50% specific-signal value.
ELISA
Total IgG, IgG2a and IgG1 were measured by ELISA. Briefly, 96-well plates were coated overnight at 4°C with 2 µg/well of Ser E rMOMP, in 0.05 M carbonate/bicarbonate buffer (pH 9.6). Plates were then blocked for 1 hour at 37°C with PBS-Tween-milk (0.05% Tween 20, 1% (w/v) powdered skim milk). All following incubations were carried out in a final volume of 100 μL, followed by 3 washes with PBS-Tween. Serial 2-fold dilutions of serum samples diluted in PBS-Tween-milk, starting from 1/1000 or 1/10000, were added to the wells and incubated for 90 min at 37°C. After washing, anti-mouse IgG- (Jackson ImmunoResearch), IgG1- or IgG2a-peroxidase conjugate (Southern Biotech) diluted in PBS-Tween-milk at 1/10000 (IgG) or 1/2000 (IgG1 and IgG2a) was added and plates were incubated for 90 min at 37°C. Plates were further washed, and then incubated in the dark for 30 min at 20°C after addition of 100 μL of a ready-to-use tetramethylbenzidine (TMB) substrate solution (Tebu-Bio). The reaction was stopped with 100 μL of 1 M HCl.
The optical density (OD) was measured at 450 nm to 650 nm in a SpectraMax plate reader (Molecular Devices). The IgG antibody titers were calculated using the CodUnit software, for the OD range of 0.2 to 3.0, from the titration curve (hyperimmune mouse serum standards were prepared on each plate). The IgG titer of this reference, expressed in arbitrary ELISA units (EU) corresponds to the log10 of the reciprocal dilution giving an OD of 1.0. The threshold of antibody detection was 10 ELISA units (1.0 log10). All final titers were expressed in log10.
Statistical analysis
Statistical analyses of comparisons between groups were based on a model of analysis of variances with 2 factors (rMOMP doses and adjuvants) as well as the interaction between these 2 factors. The p-values of these statistical tests were used to conclude whether or not differences were significant. For multiple comparisons, in order to take into account the increase of the α risk, comparisons were adjusted using Tukey's method.
All analyzes were performed using SAS® v9.2 software, at an α risk of 5% for the main effects and 10% for the interaction effects.
Disclosure of potential conflicts of interest
LV, VS, AM and SA are employees of Sanofi Pasteur. All other authors have no conflict of interest.
Acknowledgments
We thank Christelle Serraille for excellent technical assistance; Ausra Mancevsky, Catherine Hessler and Catherine Caillet for supervision and support; and Fred To and Fred Vogel for proofreading and raising the clarity and quality of the manuscript.
References
- Vodstrcil LA, McIver R, Huston WM, Tabrizi SN, Timms P, Hocking JS. The Epidemiology of Chlamydia trachomatis Organism Load During Genital Infection: A Systematic Review. J Infect Dis 2015; 211(10):1628-45; PMID:25492913; http://dx.doi.org/10.1093/infdis/jiu670
- Hafner LM, Wilson DP, Timms P. Development status and future prospects for a vaccine against Chlamydia trachomatis infection. Vaccine 2014; 32(14):1563-71; PMID:23973245; http://dx.doi.org/10.1016/j.vaccine.2013.08.020
- Darville T, O'Neill JM, Andrews CW, Jr., Nagarajan UM, Stahl L, Ojcius DM. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol 2003; 171(11):6187-97; PMID:14634135; http://dx.doi.org/10.4049/jimmunol.171.11.6187
- Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling. J Immunol 2002; 168(5):2424-32; PMID:11859134; http://dx.doi.org/10.4049/jimmunol.168.5.2424
- Massari P, Toussi DN, Tifrea DF, de la Maza LM. Toll-like receptor 2-dependent activity of native major outer membrane protein proteosomes of Chlamydia trachomatis. Infect Immun 2013; 81(1):303-10; PMID:23132491; http://dx.doi.org/10.1128/IAI.01062-12
- Pioli PA, Amiel E, Schaefer TM, Connolly JE, Wira CR, Guyre PM. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect Immun 2004; 72(10):5799-806; PMID:15385480; http://dx.doi.org/10.1128/IAI.72.10.5799-5806.2004
- Farris CM, Morrison RP. Vaccination against Chlamydia genital infection utilizing the murine C. muridarum model. Infect Immun 2011; 79(3):986-96; PMID:21078844; http://dx.doi.org/10.1128/IAI.00881-10
- Pal S, Peterson EM, de la Maza LM. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect Immun 2005; 73(12):8153-60; PMID:16299310; http://dx.doi.org/10.1128/IAI.73.12.8153-8160.2005
- Tagliabue A, Rappuoli R. Vaccine adjuvants: the dream becomes real. Hum Vaccin 2008; 4(5):347-9; PMID:18682690; http://dx.doi.org/10.4161/hv.4.5.6438
- Fox CB, Friede M, Reed SG, Ireton GC. Synthetic and natural TLR4 agonists as safe and effective vaccine adjuvants. Subcell Biochem 2010; 53:303-21; PMID:20593273; http://dx.doi.org/10.1007/978-90-481-9078-2_14
- Hansen B, Sokolovska A, Hogenesch H, Hem SL. Relationship between the strength of antigen adsorption to an aluminum-containing adjuvant and the immune response. Vaccine 2007; 25(36):6618-24; PMID:17681647; http://dx.doi.org/10.1016/j.vaccine.2007.06.049
- Hansen B, Belfast M, Soung G, Song L, Egan PM, Capen R, Hogenesch H, Mancinelli R, Hem SL. Effect of the strength of adsorption of hepatitis B surface antigen to aluminum hydroxide adjuvant on the immune response. Vaccine 2009; 27(6):888-92; PMID:19071182; http://dx.doi.org/10.1016/j.vaccine.2008.11.078
- Hem SL, Hogenesch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Rev Vaccines 2007; 6(5):685-98; PMID:17931150; http://dx.doi.org/10.1586/14760584.6.5.685
- Hogenesch H, Dunham A, Hansen B, Anderson K, Maisonneuve JF, Hem SL. Formulation of a killed whole cell pneumococcus vaccine - effect of aluminum adjuvants on the antibody and IL-17 response. J Immune Based Ther Vaccines 2011; 9:5; PMID:21801401; http://dx.doi.org/10.1186/1476-8518-9-5
- Ishizaka ST, Hawkins LD. E6020: a synthetic Toll-like receptor 4 agonist as a vaccine adjuvant. Expert Rev Vaccines 2007; 6(5):773-84; PMID:17931157; http://dx.doi.org/10.1586/14760584.6.5.773
- Byrne GI, Stephens RS, Ada G, Caldwell HD, Su H, Morrison RP, Van der Pol B, Bavoil P, Bobo L, Everson S. Workshop on in vitro neutralization of Chlamydia trachomatis: summary of proceedings. J Infect Dis 1993; 168(2):415-20; PMID:8335979; http://dx.doi.org/10.1093/infdis/168.2.415
- Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, Perro M, Vrbanac VD, Tager AM, Shi J, et al. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 2015; 348(6241):aaa8205; PMID:26089520; http://dx.doi.org/10.1126/science.aaa8205
- Garcon N, Morel S, Didierlaurent A, Descamps D, Wettendorff M, Van MM. Development of an AS04-adjuvanted HPV vaccine with the adjuvant system approach. BioDrugs 2011; 25(4):217-226; PMID:21815697; http://dx.doi.org/10.2165/11591760-000000000-00000
- O'Hagan DT, Fox CB. New generation adjuvants–from empiricism to rational design. Vaccine 2015; 33(Suppl 2):B14-20; PMID:26022561; http://dx.doi.org/10.1016/j.vaccine.2015.01.088
- Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol 2009; 183(10):6186-97; PMID:19864596; http://dx.doi.org/10.4049/jimmunol.0901474
- Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev 2011; 239(1):178-96; PMID:21198672; http://dx.doi.org/10.1111/j.1600-065X.2010.00978.x
- Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 2005; 310(5753):1510-2; PMID:16322460; http://dx.doi.org/10.1126/science.1118948
- Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem 2007; 282(48):34605-10; PMID:17884812; http://dx.doi.org/10.1074/jbc.M705100200
- Wurster AL, Rodgers VL, Satoskar AR, Whitters MJ, Young DA, Collins M, Grusby MJ. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med 2002; 196(7):969-77; PMID:12370258; http://dx.doi.org/10.1084/jem.20020620
- Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A 2010; 107(32):14292-7; PMID:20660725; http://dx.doi.org/10.1073/pnas.1009234107
- Murphy K, Travers P, Walport P. Janeway's Immunobiology; 7 ed.; Garland Science: New York, 2008.pp. 865
- Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity 2007; 26(4):491-502; PMID:17379546; http://dx.doi.org/10.1016/j.immuni.2007.02.011
- Brookes RH, Hakimi J, Ha Y, Aboutorabian S, Ausar SF, Hasija M, Smith SG, Todryk SM, Dockrell HM, Rahman N. Screening vaccine formulations for biological activity using fresh human whole blood. Hum Vaccin Immunother 2014; 10(4):1129-35; PMID:24401565; http://dx.doi.org/10.4161/hv.27657
- Garcon N. Preclinical development of AS04. Methods Mol Biol 2010; 626:15-27; PMID:20099118; http://dx.doi.org/10.1007/978-1-60761-585-9_2
- Morefield GL, Hawkins LD, Ishizaka ST, Kissner TL, Ulrich RG. Synthetic Toll-like receptor 4 agonist enhances vaccine efficacy in an experimental model of toxic shock syndrome. Clin Vaccine Immunol 2007; 14(11):1499-504; PMID:17715328; http://dx.doi.org/10.1128/CVI.00153-07
- Ljutic B, Ochs M, Messham B, Ming M, Dookie A, Harper K, Ausar SF. Formulation, stability and immunogenicity of a trivalent pneumococcal protein vaccine formulated with aluminum salt adjuvants. Vaccine 2012; 30(19):2981-8; PMID:22381074; http://dx.doi.org/10.1016/j.vaccine.2012.02.038
