ABSTRACT
Hepatitis B (HepB) infection remains a global public health problem, particularly in China. Vaccination for children and adult who are unvaccinated is an effective method for preventing the disease. In this study, we aimed to compare the effects of increased dosage of HepB vaccine on immunogenicity in healthy children and adults. A phase III, controlled, double-blinded clinical trial was performed. The subjects were assigned into groups I (age 5–14 y), II (age 15–24 y), and III (age ≥ 25 y). Subjects were randomly administered either 10 or 5 μg recombinant HepB vaccine; blood samples were collected before and after vaccination to estimate immunogenicity. The results showed that the seroconversion rate and geometric mean concentration of antibody to hepB surface antigen (anti-HBs) did not differ significantly between the dosages in each age group. Anti-HBs levels were reduced with age, and this effect was more obvious in adults administered 5 μg HepB vaccine. In conclusion, both vaccine dosages could be used to immunize children, and the 10 μg vaccine could be used for adults ages 15–24 y, whereas a higher dosage of the HepB vaccine may be required for adults ages 25 y and older.
Introduction
Hepatitis B (HepB) infection remains a serious public health concern in China. According to the China National Epidemiological Serosurvey of HepB conducted in 2006, the HepB surface antigen (HBsAg) carrier rate in general population was 7.18%, with rates of 2.42% and 8.57% for individuals ages 5–14 and 15–59 y, respectively.Citation1 HepB vaccination is believed to be the best economical and effective method for prevention and control of HepB infection.Citation2 In China, HepB vaccination was integrated into the routine National Expanded Program on Immunization (EPI) in 2002; since then, many efforts have been made to improve coverage rates of HepB vaccination in infants.Citation3 However, the coverage rate remains low in rural and developing areas in young children.Citation4,5 Therefore, children who did not receive the HepB vaccination during infancy and adults with a high risk of HepB infection should undergo a “catch-up” HepB vaccination.Citation6,7 Nevertheless, HepB vaccination has not been performed widely in noninfant populations, and there is still no standard vaccination dosage for adults.
In China, 5 μg of yeast-derived recombinant HepB vaccine is commonly used, 10 μg yeast-derived recombinant HepB vaccines are currently being produced and used as well.Citation8 Recent studies have suggested that 10 μg yeast-derived recombinant HepB vaccines can achieve better immunogenicity than the 5 μg dosage in infants.Citation9 However, it is not clear whether an alternative low dosage of the HepB vaccine can provide better immunogenicity in different age groups. Hence, in this study, the immunogenicity of these 2 dosages of HepB vaccine was evaluated in healthy children and adults.
Results
Study subjects
A total of 791 blood samples obtained from subjects were used to assess the presence of HBsAg and antibody to HepB surface (anti-HBs) by radioimmunoassay at the Jarud Banner Center of Disease Control and Prevention (CDC), Inner Mongolia Autonomous Region, China. 37 subjects with HBsAg or anti-HBs positive were excluded. In total, 411, 203, and 140 subjects were allocated to groups I, II, and III, respectively. Of these, 344 were male, and the age of the included individuals ranged from 5 to 70 y.
Following the analysis of all prevaccination blood samples at National Institute of Food and Drug Control, China(NIFDCC) by chemiluminescence immunoassays (CLIAs), individuals found to be HBsAg positive, antibody to HepB core antigen (anti-HBc) positive, or anti-HBs positive (n = 135) were excluded. Blood samples were collected 1 month after the third dosage of HepB vaccine; 43 subjects who did not complete the full-course of HepB vaccination or failed to provide postvaccination blood sample were excluded from the trial. Finally, group I included 337 subjects (176 receiving the 10 μg HepB vaccine; 161 receiving the 5 μg HepB), and groups II and III comprised 149 (73 receiving the 10 μg dose; 76 receiving the 5 μg dose) and 90 (48 receiving the 10 μg dose; 42 receiving the 5 μg dose) individuals, respectively (). No significant differences in age or sex ratio were found between the subjects of the 2 HepB vaccine dosages in each groups ().
Figure 1. Flow chart of the subjects enrolled in the study. Abbreviations: HepB, hepatitis B; HBsAg, HepB surface antigen; anti-HBc, HepB core antibody; anti-HBs, HB surface antibody; Group I, subjects age 5–14 y; Group II, subjects ages 15–24 y old; Group III, subjects age ≥ 25 y.
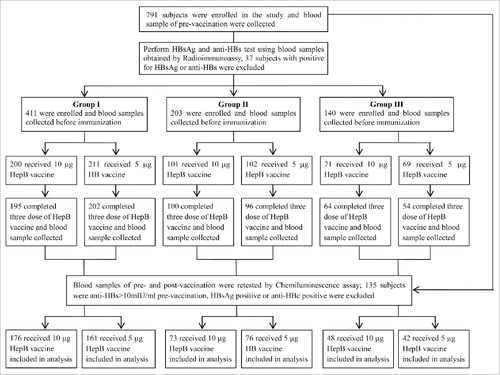
Table 1. Distribution of age and sex of the study subjects.
Immunogenicity
summarizes the anti-HBs response of the individuals in the 3 age groups 1 month after completion of HepB vaccination (full-term). When comparing the 10 and 5 μg HepB vaccines in same age group, the seroconversion rates and geometric mean concentrations (GMCs) did not differ significantly. Furthermore, when comparing the seroconversion rates and GMCs at the same vaccine dosage in different age groups, the seroconversion rate of the 5 μg HepB vaccine was lower in group III than it was in group I. Additionally, the GMCs for 10 μg HepB vaccine were lower in group II and III than in groups I, and GMCs for 5 μg HepB vaccine were different between any 2 of groups.
Table 2. Seroconversion rates and GMCs for anti-HBs 1 month post-third vaccination
The correlation coefficient between the anti-HBs titers of different dosages of the HepB vaccine and the age of the individuals (range per group) was also calculated. The results indicated a lack of association between the anti-HBs titer and age in the 10 μg HepB subgroup for both groups I and II. Nevertheless, the anti-HBs titer was negatively correlated with age in the 5 μg HepB vaccine subgroup of group II and in both the 10 and 5 μg HepB subgroups of group III ().
Table 3. Correlation between GMCs for anti-HBs and age at 1 month post-third vaccination.
Discussion
The 5 μg recombinant HepB vaccine has been widely used for EPI in China over the past 2 decades; however, the EPI has recently recommended the production and usage of the 10 μg recombinant HepB vaccine in infants.Citation8 Consequently, 10 μg will be the primary recombinant HepB vaccine dosage in China. The 20 μg recombinant HepB vaccine has been recommended for adults in developed country for many years;Citation10 however, there are no specific guidelines for the HepB vaccine dosage for children and adults in China. Therefore, immunogenicity toward different antigen dosages in individuals of different ages must be determined. Many studies have attempted to determine the associations among rates of seroconversion, GMCs, and different antigen dosages; these studies have obtained different results because of the variations in research design, category of the vaccine, study population, region, age, and vaccination procedure.Citation11,12 In this study, we have attempted to determine the seroconversion rates and GMCs of enhanced HepB vaccination in healthy children and adults. We observed no significant differences in the rates of seroconversion or GMCs as a result of the increased HepB antigen dosage in the different groups. That is, 10 μg HepB vaccine antigen dosage induced the same level of immunogenicity in individuals of the same age group as the low dosage of 5 μg HepB vaccine. This result was similar to those observed in other studies.Citation13,14
Previous study found that seroconversion rates will be decreased with age for adult.Citation15 In this study, the seroconversion rates of 5 μg HepB vaccine was lower in group III than it was in group I. However, no significant difference of seroconversion rates was found in subject received 10μg HepB vaccine among 3 age groups. Hence, administration 10 μg HepB vaccine would improve seroconversion rates for adult. This result was consistent with other studies' conclusion that enhance vaccine antigen dosages can yield better seroconversion rates for adults.Citation16,17
Age appeared to play a major role in influencing immunogenicity.Citation18 Although adult subjects displayed lower GMCs of anti-HBs than children at either 10 or 5 μg HepB vaccine, simplified comparison of the GMCs may not clarify the whole picture; therefore, we further evaluated the association between age and the anti-HBs titer in each group. The results indicated that these factors were not significantly correlated in children administered either of the vaccine dosages and young adult aged 15-24 y old administrated 10μg HepB vaccine. Similar results were also found in a recent study.Citation19 However, the anti-HBs titer induced by administration of the 5 μg HepB vaccine decreased with age in the young adult group. Moreover, the anti-HBs titer induced by the 10 or 5 μg HepB vaccine also decreased with age in group III. In other words, the age-dependent of anti-HBs titer decrease was more obvious in the adult group after administration of the 5 μg HepB vaccine. Thus, adults aged 15-24 y old can exhibit identical anti-HBs immune responses after immunized with 10 μg HepB vaccine, as compared with 5 μg HepB vaccine.
A low dosage of HepB vaccine has been recommended to reduce the cost of vaccination in endemic areas of low-income countries.Citation20 Despite the economic development and expansion of the self-production capacity of China, high dosages of HepB vaccine remain unavailable to the masses. In addition to ensuring comparable immunogenicity, the immunization strategy of administering a low dosage of HepB vaccine enhances cost effectiveness.Citation21 The results of our study implied that children can be administered 10 or 5 μg of the HepB vaccine and that young adults display better immunogenicity when administered 10 μg of the HepB vaccine. However, the immunogenicity induced by both 10 and 5 μg of the HepB vaccine was weaker in adults ages 25 y and older. Therefore, these data suggested that better immunogenicity may be obtained in elderly adults by administering 20 μg or more of the vaccine.Citation22
There were some limitations to our study. First, the number of female subjects in group III was higher than the number of male subjects. This may because the prevalence of HBsAg is significantly higher in men than women in China.Citation1 Additionally, we failed to analyze confounding factors, such body mass index and smoking status, which may affect immunity. However, because this study was designed as a randomized control trial, confounding factors should be balanced in both the 10 and 5 μg HepB vaccine group. Moreover, the levels of anti-HBs would decrease after a full HepB vaccination schedule,Citation23 we did not observe long-term changes in anti-HBs after vaccination at 2 dosages in different age groups. Future studies are needed to evaluate the persistence of immune responses in children and adults generated by 10 and 5 μg HepB vaccines.
In conclusion, the results of our study suggested that both of the HepB vaccine dosages could be used in children, and 10 μg HepB vaccine could be used in young adult aged 15-24 y old. However, adults ages 25 y and older may require higher dosages of the vaccine.
Materials and methods
Study design
A phase III, controlled, randomized, double-blinded (the subject, investigator, and laboratory staff did not know which vaccine was administered to any particular person) clinical trial was conducted to assess the immunogenicity of 2 dosages of recombinant HepB vaccine in healthy children and adults. The study was approved by the China State Food and Drug Administration and was conducted by investigators from the Jiangsu Provincial CDC, China. Institutional Review Board approval was obtained from the Ethics Committee of the Jiangsu Provincial CDC, China. Written informed consent was obtained from all trial subjects or their guardians. This trial is registered with ClinicalTrials.gov (number NCT02152709).
Subjects
This study was conducted in Jarud Banner, Inner Mongolia Autonomous Region, China in November of 2008. This region lies in the western part of China and had low HepB vaccine coverage rates at beginning of the clinical trial.Citation24
The following inclusion criteria were employed to recruit study subjects: healthy children (age 5–14 y) and adults (age ≥ 15 y), without a documented history of HepB infection; no record of HepB vaccination on the vaccination certificate booklet, as reported by guardians of children or adults themselves if vaccination certificate document was lost; and an axillary temperature of 37.0°C or less. The exclusion criteria included subjects with acute infectious diseases, nervous system damage, congenital malformations, developmental disorders or serious chronic illnesses, and thrombocytopenia or other coagulation disorders. Subjects administered immunoglobulin during the trial period were also excluded. Prevaccination venous blood samples were collected from all subjects; HBsAg and anti-HBs expression in these samples was tested by radioimmunoassay at the local CDC. Individuals positive for HBsAg or anti-HBs were excluded from the study.
The enrolled subjects were assigned to different age groups. According to the results of the China National Epidemiological Serosurvey of HepB in 2006, the prevalence of HBsAg positivity in adults is high after 25 y of age.Citation1 A recent survey by the China National HepB Demonstration Project was also showed that high proportion of new HepB infection occurs from individuals 25 y old or older.Citation25 Therefore, adult subjects in this study were divided into 2 groups. The groups were defined as follows: group I, consisting of subjects ages 5–14 y; group II, consisting of subjects ages 15–24 y; and group III, consisting of subjects ages 25 y and older. Individuals in each group were randomly administered 3 doses of 10 or 5 µg HepB vaccine (1:1 ratio) at 0, 1, and 6 months.
Vaccine
Single dose of (10 or 5 µg/0.5 mL) HepB vaccine were produced by recombinant Saccharomyces cerevisiae at Beijing Tiantan Biological Products (Beijing, China). All vaccines were administered via the intramuscular route in the deltoid region by qualified nurses.
Laboratory testing
Pre- and postvaccination venous blood samples were collected before vaccination and at month 7 (1 month after the third dose) from individuals in each age group. After collection, blood samples were left standing for 2–3 h, and serum was separated by centrifugation. The serum samples were then stored at −40°C. The frozen separated serum samples were sent to the NIFDCC. HBsAg, anti-HBs, and anti-HBc were evaluated by CLIAs. HBsAg sample over mean cpm of negative controls 0.05 mIU/mL was set as the cutoff indicating the presence of HBsAg; an anti-HBc antibody level of 1 mIU/mL was considered positive; and samples with an anti-HBs level of 1000 mIU/mL were diluted for further testing.
Immunogenicity
The seroconversion rates and GMCs of anti-HBs were analyzed to determine the immunogenicity. The seroconversion rates were defined as the number of individuals with anti-HBs titers of 10 mIU/mL or more at 1 month postvaccination. After serum sample of prevaccination were retested by CLIAs, subjects having anti-HBs levels of more than 10 mIU/mL pre-vaccination or those who were positive for HBsAg or anti-HBc were excluded for the analysis.
Statistical analysis
Immunogenicity was assessed with respect to the anti-HBs seroconversion rate and GMCs, which was compared between the 10 and 5 μg dosage for individuals in each age group or the same dosage in different age group. Seroconversion rates, GMCs, and the corresponding 95% confidence intervals (95% CIs) were calculated. The sex ratio and the seroconversion rates were compared between groups and for each dosage by chi-squared or Fisher's exact tests. Age and GMCs were compared by Student's t tests, analysis of variance, SNK tests after anti-HBs titer logarithmic transformation or rank test for skewed distributions. Spearman correlation analysis was used to evaluate the association between anti-HBs titers and the age of the individuals in each group. Statistical significance was defined at p < 0.05. All data analyses were performed in using the SPSS 18.0 software package (IBM, Armonk, NY, USA).
Abbreviations
| Anti-HBc | = | antibody to hepatitis B core antigen |
| Anti-HBs | = | antibody to hepatitis B surface antigen |
| CDC | = | center of disease control and prevention |
| CI | = | confidence interval |
| CLIAs | = | chemiluminescence immunoassays |
| EPI | = | expanded program on immunization |
| GMCs | = | geometric mean concentrations |
| HepB | = | hepatitis B |
| HBsAg | = | hepatitis B surface antigen |
| NIFDCC | = | National Institute of Food and Drug Control, China |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was supported by the Jarud Banner CDC. The authors thank all study participants, staffs of Jarud Banner CDC, the local general practitioners, study nurses, and personnel who contributed to this study.
Funding
This study was supported by the National Scientific & Technological Major Project of China (grant no. 2008ZX10002-001).
References
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Reprint of: Epidemiological serosurvey of Hepatitis B in China–declining HBV prevalence due to Hepatitis B vaccination. Vaccine 2013; 31 Suppl 9:J21-8; PMID:23948229; http://dx.doi.org/10.1016/j.vaccine.2013.08.012
- Hepatitis B vaccines. Wkly Epidemiol Rec 2009; 84:405-19; PMID:19817017
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992–2005 in China. J Infect Dis 2009; 200:39-47; PMID:19469708; http://dx.doi.org/10.1086/599332
- Hutin Y, Hennessey K, Cairns L, Zhang Y, Li H, Zhao L. et al. Improving hepatitis B vaccine timely birth dose coverage: lessons from five demonstration projects in China, 2005-2009. Vaccine 2013; 31 Suppl 9:J49-55
- Zhou Y, Xing Y, Liang X, Yue C, Zhu X, Hipgrave D. Household survey analysis of the impact of comprehensive strategies to improve the expanded programme on immunisation at the county level in western China, 2006-2010. BMJ Open 2016; 6:e008663.
- Jia Y, Li L, Cui F, Zhang D, Zhang G, Wang F, Gong X, Zheng H, Wu Z, Miao N, et al. Cost-effectiveness analysis of a hepatitis B vaccination catch-up program among children in Shandong Province, China. Hum Vaccin Immunother 2014; 10:2983-91; PMID:25483678; http://dx.doi.org/10.4161/hv.29944
- Schillie S, Murphy TV, Sawyer M, Ly K, Hughes E, Jiles R, de Perio MA, Reilly M, Byrd K, Ward JW. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013; 62:1-19; PMID:24352112
- Yin J, Ji Z, Liang P, Wu Q, Cui F, Wang F, Liang X, Zhuang G. The doses of 10mug should replace the doses of 5mug in newborn hepatitis B vaccination in China: A cost-effectiveness analysis. Vaccine 2015; 33:3731-8; PMID:26057138; http://dx.doi.org/10.1016/j.vaccine.2015.05.082
- Li J, Hu J, Liang X, Wang F, Li Y, Yuan ZA. Predictors of poor response after primary immunization of hepatitis B vaccines for infants and antibody seroprotection of booster in a metropolis of China. Asia Pac J Public Health 2015; 27:NP1457-66; PMID:24097922; http://dx.doi.org/10.1177/1010539514524817
- Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep 1991; 40:1-25
- Tazhibi M, Hajivandi A, Tafti AD, Fallahzadeh H. The efficacy of hepatitis B vaccine in Iranian population: A systematic review and meta-analysis. J Educ Health Promot 2014; 3:53; PMID:25077146; http://dx.doi.org/10.4103/2277-9531.145917
- Jin H, Tan Z, Zhang X, Wang B, Zhao Y, Liu P. Comparison of Accelerated and Standard Hepatitis B Vaccination Schedules in High-Risk Healthy Adults: A Meta-Analysis of Randomized Controlled Trials. PLoS One 2015; 10:e0133464; PMID:26196903; http://dx.doi.org/10.1371/journal.pone.0133464
- Minervini G, McCarson BJ, Reisinger KS, Martin JC, Stek JE, Atkins BM, Nadig KB, Liska V, Schödel FP, Bhuyan PK. Safety and immunogenicity of a modified process hepatitis B vaccine in healthy neonates. Vaccine 2012; 30:1476-80; PMID:22227229; http://dx.doi.org/10.1016/j.vaccine.2011.12.095
- Gilca V, De Serres G, Boulianne N, De Wals P, Murphy D, Trudeau G, Deceuninck G, Massé R, Duval B. Antibody and immune memory persistence after vaccination of preadolescents with low doses of recombinant hepatitis B vaccine. Hum Vaccin 2010; 6:212-8; PMID:19946212; http://dx.doi.org/10.4161/hv.6.2.10299
- Van Der Meeren O, Crasta P, Cheuvart B, De Ridder M. Characterization of an age-response relationship to GSK's recombinant hepatitis B vaccine in healthy adults: An integrated analysis. Hum Vaccin Immunother 2015; 11:1726-9; PMID:25996260
- Li J, Yao J, Shan H, Chen Y, Jiang ZG, Ren JJ, Xu KJ, Ruan B, Yang SG, Wang B, et al. Comparison of the effect of two different doses of recombinant hepatitis B vaccine on immunogenicity in healthy adults. Hum Vaccin Immunother 2015; 11:1108-13; PMID:25607773
- Lin CS, Xie SB, Liu J, Zhao ZX, Chong YT, Gao ZL. Effect of revaccination using different schemes among adults with low or undetectable anti-HBs titers after hepatitis B virus vaccination. Clin Vaccine Immunol 2010; 17:1548-516; PMID:20719983; http://dx.doi.org/10.1128/CVI.00064-10
- Giefing-Kroll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 2015; 14:309-21; PMID:25720438; http://dx.doi.org/10.1111/acel.12326
- Yang L, Yao J, Li J, Chen Y, Jiang ZG, Ren JJ, Xu KJ, Ruan B, Yang SG, Wang B, et al. Suitable hepatitis B vaccine for adult immunization in China. Immunol Res 2016; 64:242-50; PMID:26645972; http://dx.doi.org/10.1007/s12026-015-8742-1
- Kim SY, Salomon JA, Goldie SJ. Economic evaluation of hepatitis B vaccination in low-income countries: using cost-effectiveness affordability curves. Bull World Health Organ 2007; 85:833-42; PMID:18038073
- Velu V, Nandakumar S, Shanmugam S, Shankar EM, Thangavel S, Kulkarni PS, Thyagarajan SP. Comparative efficacy of two dosages of recombinant hepatitis B vaccine in healthy adolescents in India. Pediatr Infect Dis J 2007; 26:1038-41; PMID:17984812; http://dx.doi.org/10.1097/INF.0b013e3181342887
- Gilbert CL, Klopfer SO, Martin JC, Schodel FP, Bhuyan PK. Safety and immunogenicity of a modified process hepatitis B vaccine in healthy adults >/=50 years. Hum Vaccin 2011; 7:1336-42; http://dx.doi.org/10.4161/hv.7.12.18333
- Keck JW, Bulkow LR, Raczniak GA, Negus SE, Zanis CL, Bruce MG, Spradling PR, Teshale EH, McMahon BJ. Hepatitis B virus antibody levels 7 to 9 years after booster vaccination in Alaska native persons. Clin Vaccine Immunol 2014; 21:1339-42; PMID:25056363; http://dx.doi.org/10.1128/CVI.00263-14
- Cui F, Liang X, Gong X, Chen Y, Wang F, Zheng H, Wu Z, Miao N, Hadler SC, Hutin YJ, et al. Preventing hepatitis B though universal vaccination: reduction of inequalities through the GAVI China project. Vaccine 2013; 31 Suppl 9:J29-35; PMID:24331017; http://dx.doi.org/10.1016/j.vaccine.2012.07.048
- Yang S, Yu C, Chen P, Deng M, Cao Q, Li Y, Ren J, Xu K, Yao J, Xie T, et al. Protective immune barrier against hepatitis B is needed in individuals born before infant HBV vaccination program in China. Sci Rep 2015; 5:18334; PMID:26655735; http://dx.doi.org/10.1038/srep18334
