ABSTRACT
This systematic review evaluated the epidemiology of community-acquired pneumonia in children <6 y of age within 90 developing and newly industrialized countries. Literature searches (1990–2011), based on MEDLINE, EMBASE, Cochrane, CAB Global Health, WHO, UNICEF, country-specific websites, conferences, health-technology-assessment agencies, and the reference lists of included studies, yielded 8,734 records; 62 of 340 studies were included in this review. The highest incidence rate among included studies was 0.51 episodes/child-year, for children <5 y of age in Bangladesh. The highest prevalence was in Chinese children <6 months of age (37.88%). The main bacterial pathogens were Streptococcus pneumoniae, Haemophilus influenzae and Mycoplasma pneumoniae and the main viral pathogens were respiratory syncytial virus, adenovirus and rhinovirus. Community-acquired pneumonia remains associated with high rates of morbidity and mortality. Improved and efficient surveillance and documentation of the epidemiology and burden of community-acquired pneumonia across various geographical regions is warranted.
Introduction
Annually, approximately 120–156 million cases of acute lower respiratory infections (ALRI) occur globally, with approximately 1.4 million resulting in death.Citation1-4 Of these, pneumonia kills an estimated 1 million children under the age of 5 every year and accounts for 15% of deaths in children <5 y of age,Citation5 with 90–95% of these deaths occurring in the developing world.Citation2-11 The majority (˜2 thirds) of pneumonia episodes in children <5 y of age occurs in just 15 countries, with South Asia and Sub-Saharan Africa collectively enduring the largest burden of more than half the worldwide total cases of pneumonia in children.Citation1,12 Risk factors for community-acquired pneumonia (CAP) include age (<1 year), malnutrition, prematurity, immunosuppression, overcrowding, passive tobacco exposure, indoor fuel exposure, inadequate housing, overcrowding and the winter season.Citation10,13 The burden of disease has been worsened by the human immunodeficiency virus (HIV) epidemic.Citation14 Other co-existing illnesses, like malaria and diarrhea, are also important contributing factors to the increased CAP burden of disease in African and South Asian settings.Citation3,15,Citation16
The primary cause of CAP is usually bacterial, but isolating bacteria does not necessarily establish causation since the validity of the results might vary depending on the collected body fluid sample, and viral causes also exist.Citation17,18 Streptococcus pneumoniae is the main bacteriological causative pathogen of childhood pneumonia followed by Haemophilus influenzae, Mycoplasma pneumoniae and Chlamydophila pneumoniae. The most common pneumococcal serotypes include 1, 5, 6A, 6B, 14, 19F and 23F, which account for between 58% to 66% of invasive pneumococcal disease (IPD) in Africa and Asia, respectively.Citation19 Serotype 14 is the most common cause of IPD across the regions of Africa, Asia, Europe, Oceania, Latin America and the Caribbean, and North America.Citation19
In general, there is a paucity of data on the epidemiology of CAPCitation1,2,11,20-22 in the developing and newly industrialised countries, as defined by the United Nations (UN);Citation23 one systematic review was published on the epidemiology of CAP in children across Latin America and the Caribbean.Citation24
To further investigate the burden of CAP disease, we conducted a systematic literature review on the epidemiology of bacterial and viral pneumonia in children <6 y of age in 90 countries from the following regions: Africa, India and South Asia, Middle East, China, and Russia and CIS (Commonwealth of Independent States). More specifically, the review incorporates literature on the incidence and seasonality of CAP; the distribution of causative pathogens; antimicrobial resistance; country-specific CAP treatment guidelines; and country-specific vaccination recommendations. In addition, this review identifies gaps in the epidemiological data and research evidence and aims to consolidate all available data from these regions. To the best of our knowledge, no previous systematic review including these specific regions was previously published. The current review is relevant for decision makers when considering vaccine introduction in their countries, and also for the public health authorities interested to monitor childhood CAP over time.
Results
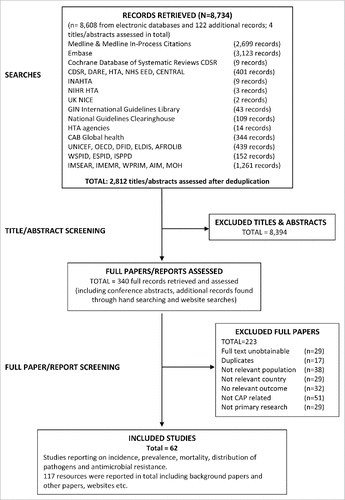
A total of 8,734 records were retrieved from the comprehensive search criteria, of which 62 studies reporting on the epidemiology of CAP were included for this review (). The included studies covered incidence, prevalence, mortality, distribution of pathogens and antimicrobial resistance. Publication dates ranged from 1990 to October 2011 and reported data for locations in Africa, India and South Asia, China, Middle East, and Russia and CIS.Citation25-86 Study designs included randomized controlled trials (RCTs; n = 7), cross-sectional (n = 51), case-series (n = 3) and case-control (n = 1) studies. The majority of studies (81%; 50/62) were of children <6 y of age with a diagnosis of CAP and covered geographical regions of Africa (n = 20); India and South Asia (n = 14); China (n = 13); and Middle East (n = 3). However, data for this age group were lacking for Russia and CIS. In order to identify further potentially relevant data, 12 studies of children <18 y of age (but including children <6 years) were also included; these studies covered Africa (n = 3), India and South Asia (n = 1), China (n = 3), Middle East (n = 3), and Russia and CIS (n = 2). The incidence, prevalence and mortality were reported by age, CAP severity, pathogen, presence of risk factors, and by season.
The majority of studies, including those of children >6 y of age, used the CAP definition as recommended by the WHO guidelines.Citation87
Incidence
Data on the incidence of CAP were reported in 14 studiesCitation25-38 (11 cross-sectional studies and 3 RCTsCitation30,31,Citation36), covering various regions in Africa (n = 7),Citation25-31 India and South Asia (n = 5),Citation32-36 and China (n = 2)Citation37,38 (). Data from Middle East, Russia and CIS were non-existent. The 3 RCTs had a low risk of bias (based on Downs and Black's checklist),Citation88 with sample sizes ranging from 17,400 to 42,848. In contrast, most cross sectional studies had a high risk of bias with sample sizes ranging from 1,078 to 8,198. Therefore, the generalizability of results from cross-sectional studies is rather limited compared to that of RCTs.
Table 1. Summary of included studies reporting on overall incidence and, by age, CAP severity, concomitant disease and seasonal variations.
Africa
Reported overall incidence rates ranged from 1.91 to 698 per 100,000 child-years in the 7 included studies.Citation25-31 Due to the high prevalence of reported cases in children <5 years, age is considered the most important risk factor for CAP. The highest incidence rate when assessed by age was reported in Kenyan infants <1 y of age (1,370/100,000 child-years).Citation30 The highest incidence rate of CAP assessed by season was reported at 1,175 per 100,000 child-years during the month of July in Kenya.Citation28
India and South Asia
Overall incidence rates ranged from 31 to 50,526 per 100,000 child-years.Citation32-36 The highest incidence rate for this review was reported at 51 pneumonia episodes per 100 child-years in children <5 y of age from Bangladesh.Citation32 Children <11 months of age endured the highest burden of CAP at 65/100 child-years.Citation35 The highest incidence rate of CAP by season was reported during the months of March to June (summer season) at 14.1/100 child-years in Indian pre-schoolers.Citation34
China
Only 2 studies from China reported on incidence rates of CAP.Citation37,38 The highest overall incidence rate was reported at 206.2/100,000 child-years in children <5 y of age from Hong Kong.Citation38
Prevalence
Twenty-one studies in total reported data on the prevalence of CAP ( and Supplementary Table 1). Eighteen studies were of children <6 y of age covering geographical regions of Africa (n = 6),Citation26,29,Citation39-42 China (n = 4),Citation37,43-45 India and South Asia (n = 5),Citation35,46-49 and Middle East (n = 3).Citation50-52 In addition, 3 studies from Africa (n = 1),Citation53 India and South Asia (n = 1)Citation54 and Russia and CIS (n = 1)Citation55 were of children <18 y of age (including children <6 years). The majority of these studies (86%; 18/21) were cross-sectional, except for 2 case-seriesCitation39,40 and one case-control.Citation44 The sample sizes were generally small, with the highest reported at 4,155. All of the studies had a high risk of bias (based on Downs and Black's checklist).Citation88 The majority of studies were hospital-based, making it difficult to generalize findings to the general population.
Table 2. Summary of included studies reporting on prevalence by age, CAP severity, concomitant disease and seasonal variations.
Africa
No data were found for the overall prevalence of CAP in Africa. However, data were found for prevalence related to age, malnutrition, CAP severity, and pathogen. A study from Uganda on the prevalence of CAP by severity suggested that children with severe pneumonia had a higher prevalence rate (83%; 117/140) compared to children with very severe pneumonia (16%; 23/140).Citation39 The most prevalent pathogen in studies with neonates was Klebsiella pneumoniae at 22%.Citation40 In children aged between 3 months and 5 y of age, S. pneumoniae was reported as the main causative agent for CAP.Citation41 The most prevalent viral pathogens were reported by several studies and included adenovirus (ADV), rhinovirus (RV) and respiratory syncytial virus (RSV).Citation29,41,Citation42 The most prevalent viral pathogens during the rainy season were RV (56%), ADV (60%) and RSV (63%) and viral co-infections at 71%.Citation29 S. pneumoniae was the most prevalent pathogen in Gabon, reported at 35% (35/99) in children with a median age of 21 months (interquartile range 11–49 months).Citation53
India and South Asia
A study by Naheed (2009) from Bangladesh reported on the prevalence of pathogens in CAP by age.Citation46 The results showed that 4% (161/4,155) of the specimens obtained from children aged 2–59 months were either S. pneumoniae (6%; 10/161) or H. influenzae type b (Hib) (3%; 5/161). The prevalence rate in children aged 2–11 months was reported at 4% (5/116) and 3% (4/116) for S. pneumoniae and Hib, respectively. Additionally, a prevalence rate of 11% (5/45) for S. pneumoniae and 2% (1/45) for Hib was reported among children with CAP aged 12–59 months.Citation46
China
A study by Zhao et al.Citation43 reported on the prevalence of S. pneumoniae by age. The prevalence rate of S. pneumoniae was reported at 8.9% (90/1,011). By age, the prevalence rate of S. pneumoniae in CAP was between 5.7% (22/383) in children <6 months of age and 14.3% (5/35) in children aged 48–60 months.Citation43 Bacterial pathogens were more prevalent than viral pathogens, although mixed viral and bacterial pathogens were often reported.Citation45
Middle East
Overall prevalence data were non-existent. The highest prevalence of CAP by age was reported at 66.3% (65/98) in children aged between 29 d and 12 months from Iran.Citation50 Somer et al.Citation52 reported that out of a sample of 140 children with CAP aged between 2 months to 15 years, 27% (38/140) were infected with M. pneumoniae and 5% (7/140) with C. pneumoniae.
Mortality
Data on mortality were reported by 12 studies from Africa (n = 7)Citation26-29,Citation56-58 and India and South Asia (n = 5)Citation36,46,Citation59-61 (Supplementary Table 2). Where available, data were reported according to age, CAP severity, concomitant disease and season and these data have been highlighted; however in many cases this level of detail was not available. All of the studies used a cross-sectional design with the exception of one RCT.Citation36 The studies consisted of very small sample sizes and had a high risk of bias (ranging between 3 and 13 items on the Downs and Black's checklist). No data were found for China, Middle East, Russia and CIS.
Africa
Tornheim et al.Citation28 reported the overall mortality rate of CAP at 65/100,000 person-years for children <5 y of age, and 24/100,000 person-years for children >5 y of age in Kenya. The overall mortality rates ranged from 8% to 15.3%, respectively.Citation29,56 The highest overall mortality rates were from studies of children with either HIV, severe malnutrition, unvaccinated and very severe pneumonia.Citation26,29,Citation56 The differences in CAP mortality according to season were reported by only 1 study, which included Kenyan children living in various slums across Nairobi.Citation58 The reported mortality rates varied across the year. The highest mortality rate was reported during the month of June at 60.1/100,000 person years and the lowest was reported in the month of November at 16.4/100,000 person years. Thereafter, a steady rise in mortality was reported from December to March.Citation58
India and South Asia
Naheed et al.Citation46 reported on the mortality rates in Indian children aged <5 y of age. The mortality rates were reported by age, CAP severity and malnutrition. The results showed that children <12 months of age had a mortality rate of 4% (123/2,897), while the rate for those aged 12–59 months was 2% (27/1,258).Citation46 One study reported on mortality rates across 3 villages in India.Citation36 The overall mortality rates ranged from 0.89% to 3.32% for children with severe pneumonia and from 0.77% to 2.35% for children with pneumonia.Citation36
Distribution of viral and bacterial pathogens
Twenty-two studies in total reported on the distribution of pathogens ( and Supplementary Table 3). The studies covered geographical regions of Africa (n = 3),Citation29,41,Citation62 India and South Asia (n = 2),Citation48,59 and China (n = 8).Citation37,43,Citation45,63-67 Nine studies from Middle East (n = 3),Citation68-70 Russia and CIS (n = 1),Citation71 Africa (n = 2),Citation72,73 and China (n = 3)Citation74-76 included children of various age groups.
Table 3. Summary of included studies reporting on the distribution of pathogens and serotypes.
Africa
Adegbola et al.Citation41 reported data on 159 malnourished children with pneumonia. The most common pathogens were S. pneumoniae (6.9%; 11/159), followed by H. influenzae (3.7%; 6/159) and Salmonella spp (2.5%; 4/159); however the total number of isolates were not clearly reported. The results were consistent with the data on 119 well-nourished children with pneumonia, where S. pneumoniae was identified in 26.1% (31/119) of children; H. influenzae in 6.7% (8/119); Staphylococcus aureus in 2.5% (3/119) and Salmonella spp in only one child. The distribution of viral pathogens was reported by only one study of children ≤60 months of age from Mozambique.Citation29 The study reported that the highest number of patients infected with the viral pathogens were children aged 3–12 months and 12–60 months. Around 50% (67/135) of the RV isolates were obtained from children aged 3–12 months, and 40% (54/135) from children aged 12–60 months; 30% (26/135) were from children with HIV. Additionally, 57 ADV isolates were obtained, of which 77% (44/57) were detected in children between the ages of 12–60 months and 21% (12/57) in children aged between 3–12 months; 24% (9/57) of the ADV isolates were found in children with HIV.
India and South Asia
A study by Rahman et al.Citation59 of children <5 y from Bangladesh identified H. influenzae as the dominant pathogen accounting for 60% (15/25) of the total isolates from invasive diseases. The non typeable Haemophilus influenzae (NTHi) isolates accounted for 24% (6/25). A sentinel study from Sri Lanka in children aged 2 months to 5 y identified S. pneumoniae as the dominant pathogen (0.6%; 9/1,468 children) followed by H. influenzae (0.5%; 7/1,468 children). However, other species (not reported by study) were reported in 11% (161/1468 children) of the children with pneumonia.Citation48
China
A study by Yao et al.Citation64 reported on the most common serotypes of S. pneumoniae. The data showed that serotypes 19F accounted for 56% (188/338), followed by 19A at 14% (47/338), 23F at 10% (34/338), 6B at 5%(16/338), and 14 at 4% (12/338) of the causative serotype for CAP. In children aged between 1–60 months, K. pneumoniae was the most dominant pathogen accounting for 22.3% (170/761), followed by Escherichia coli at 17.1% (130/761), S. pneumoniae at 11.7% (89/761), S. aureus at 8.3% (63/761), and H. influenzae and Haemophilus parainfluenzae at 7.9% (60/761) collectively.Citation66 Additionally, human RV species C (HRV-C) was detected in children <6 months of age at 7.32 % (18/246) and at 9.9% (8/81) of the 6–12 months old. Human RV species B (HRV-B) was only detected in children <6 y of age and those aged 12–24 months, at 2.03% (5/246), and 5.9% (2/34), respectively.Citation37 A similar study of children with CAP from China reported S. pneumoniae serotypes of 19F, 23F, 6B and 4 to be the most dominant in children aged <5 y.Citation43 The results indicated that 19F accounted for 62% (56/90), 23F for 16% (14/90), 6B for 10% (9/90) and 4 for 7% (6/90). In neonates (<28 days of age), the most frequently detected pathogen out of 425 isolates was E. coli (27%; 115/425 isolates), followed by K. pneumoniae (18%; 77/425 isolates) and H. influenzae (7%; 31/425 isolates).Citation63 No data on the seasonal distribution of pathogens across all geographical regions were reported.
Antimicrobial resistance
In total, 9 studies reported data on antimicrobial resistance among bacterial pathogens causing CAP (Supplementary Table 4). The studies covered regions from China (n = 4),Citation63,64,Citation67,77 Bangladesh (n = 2)Citation32,35 and Sri Lanka (n = 1).Citation48 Additionally, 2 studies from China were included (children <18 y of age)Citation75,76 (Supplementary Table 5). No data were reported for Africa. Given this lack of data, supplementary information was also reported from an additional 8 studies which included data for children <18 y of age with IPD (including children <6 years).Citation89-96 These studies covered China (n = 3),Citation89-91 Middle East (n = 4),Citation92-95 and Russia and CIS (n = 1).Citation96
India and South Asia
Studies by Brooks et al.Citation29 and Arifeen et al.Citation32 established that S. pneumoniae isolates from children with CAP were susceptible to penicillin (97% and 85%, respectively), which is the most widely used antibiotic in developing countries. Contrarily, a sentinel study by Batuwanthudawe et al.Citation48 reported data for Sri Lanka which suggested that isolates of S. pneumoniae (predominant serotypes 19F and 23F) were highly resistant to penicillin (91.30%), and also showed considerable resistance to other antimicrobial agents including co-trimoxazole (73.91%), chloramphenicol (26.09%), erythromycin (60.87%) and cefotaxime (47.83%).
China
Wang et al.Citation63 reported on the degree of antimicrobial resistance of various pathogens like S. aureus, Staphylococcus epidermidis, E. coli, H. influenzae and K. pneumoniae to the different antibiotics used in China. The data showed that H. influenzae and K. pneumoniae were highly sensitive to meropenem (100% each) whereas S. aureus was highly sensitive to quinupristin/dalfopristin (98.6%) and resistant to penicillin (3.3% sensitivity). In addition, S. epidermidis was highly sensitive to quinupristin/dalfopristin (96.7%) but not to penicillin (2.3%). E. coli was fully sensitive to meropenem and imipenem (100% each) but less sensitive to ampicillin (48.9%) and amoxicillin (4.3%).Citation63 A similar study by Zhao et al.Citation43 presented data on the resistance of antibiotics to Hib. The highest resistance of 22.2% to ampicillin was reported compared to ampicillin/sulbactam and cefaclor which were 100% effective against the pathogen.
Another study by Yao et al.Citation64 tested the resistance of antibiotics to S. pneumoniae and the data showed the bacteria were susceptible to the majority of the antibiotics. On the other hand, erythromycin showed the highest level of resistance at 99.7% compared to penicillin (1.8%), ofloxacin (0.3%) and imipenem (1.5%). Additionally, Liu et al.Citation67 reported S. pneumoniae to be fully resistant to penicillin and erythromycin (at 100% each) followed by clindamycin at 96.8%, tetracycline at 93.5% and trimethoprim-sulfamethoxazole (TMP-SMX) at 83.9%.
Liu et al.Citation67 reported that, in China, 96.6% of S. pneumoniae isolates were resistant to erythromycin, tetracycline and clindamycin. In addition, there was also some evidence of resistance to TMP-SMX (82.8%), cefaclor (65.5%), and penicillin (55.2%). However, a previous study by Yao et al.Citation89 reported that S. pneumoniae isolates showed no evidence of full resistance to any of the studied antibiotics, although penicillin had the highest intermediate resistance (54.5%). Li et al.Citation90 reported the highest full resistance of S. pneumoniae to tetracycline at 79% followed by erythromycin at 72% and TMP-SMX at 70%. In addition, another study from Hong Kong reported that 85.2% of S. pneumoniae isolates were resistant to erythromycin followed by cefotaxime (33.0%).Citation91
Two additional studies from China (of subjects with a wide age range) of children with CAP, reported on antimicrobial resistance of various pathogens against the most commonly used antibiotics.Citation75,76 A study by Zeng et al.Citation76 reported that of the 48 strains of S. pneumoniae isolated, 50% were fully resistant to penicillin followed by 45.8% to erythromycin and 45.8% to cephazolin. Also, 52.5% and 56.5% of S. haemolyticus isolates were resistant to penicillin and cephazolin. Of the 16 strains of S. aureus isolated, 100% showed full resistance to penicillin and 87.5% to erythromycin and cephazolin each. A similar study by Wang et al.Citation75 reported 100% full resistance of the S. pneumoniae isolates to erythromycin followed by 90.6% to penicillin, and 94.3% to clindamycin. Hib was reported to have full resistance to meropenem and ciprofloxacin.
Middle East
A study by Percin et al.Citation92 from Turkey reported full resistance of S. pneumoniae isolates to TMP-SMX at 36% followed by penicillin at just 6%. Similarly, a study by Ercan et al.Citation93 from Turkey, reported on the resistance of S. pneumoniae to TMP-SMX at 63.3% followed by erythromycin at 40% and tetracycline at 33.3%. A study by Shibl et al.Citation94 from Saudi Arabia reported S. pneumoniae resistance of 26% to erythromycin and 12% to penicillin. Finally, a study from Kuwait reported that serotypes 19F and 23F from S. pneumoniae showed intermediate resistance to penicillin in comparison to serotypes 6B, 6A, 19A and 14, for which no significant resistance was reported.Citation95
Russia and CIS
Due to the lack of data in children <6 y of age with CAP in Russia, a study by Katz et al.Citation96 was included. The study included children with IPD aged 16–70 months. The results showed the highest resistance of 61.4% to TMP-SMX, followed by tetracycline at 32.5%, clindamycin at 19.3%, erythromycin at 16.7% and the lowest resistance to chloramphenicol at 6%.Citation96 On average, data suggested that the high resistance levels are seen where TMP-SMX, erythromycin and tetracycline antibiotics are used, suggesting that the use of penicillin (as a major antibiotic) for treatment of IPDs is very low in comparison to TMP-SMX, erythromycin and tetracycline. However, this could be due to the natural resistance of the pathogens to penicillin after its extensive use in recent years.
Discussion
This is the first comprehensive review of the epidemiology and burden of CAP in children <6 y of age within developing and newly industrialized countries using a rigorous search strategy. Several CAP-related outcomes were covered, including incidence, prevalence, mortality, seasonal variation, distribution of pathogens and antimicrobial resistance.
The incidence rates among children <6 y of age varied greatly between the included studies according to age, severity of CAP and season. In general, incidence rates were higher for infants, for more severe CAP episodes and during wet/rainy seasons. The highest incidence rate from this review was reported in Bangladesh,Citation33 with 0.51 episodes per child-year, for children <5 y of age hospitalized with pneumonia. A previous systematic review reported an incidence rate of pneumonia in children <5 y at 0.29 episodes per child-year in developing countries in comparison with 0.05 episodes per child-year in developed countries.Citation12 Other systematic reviews assessed the incidence rate of pneumonia in Chinese children <5 y of age at ˜0.13 episodes per child-year between 1980 and 2008Citation97 while the incidence rate of CAP or hospitalized pneumonia ranged from 0.06 to 0.27 episodes per person-year between 1985 and 2008.Citation98 A more recent estimate by Rudan et al.Citation2 has shown a decreasing trend in the burden of pneumonia from 2000 to 2010. Although there is a paucity of data on CAP burden in the Russian Federation, previous expert evaluations estimated an incidence of pneumococcal CAP of 490–1,300 cases per 100,000 child-years in children <6 years, according to a recent publication.Citation99
Prevalence data from developing countries were very scarce. There was variation in the prevalence rate by age, pathogen, co-infections (HIV, malaria) and season. Infants had a higher CAP prevalence rate compared to those >12 months of age.Citation50 HIV positive children had a higher prevalence rate of pneumonia.Citation39 Children with malaria are at risk of bacterial infection, which results in an increased risk of mortality.Citation16 Moreover, in highly affected malaria endemic regions, the diagnosis of pneumonia may be uncertain because malaria and severe pneumonia in hospitalized young children show remarkable clinical similarities.Citation100,101 Wrong diagnosis often leads to under-treatment of pneumoniaCitation102 or to inappropriate prescription of antibiotics to children with malaria,Citation103 increasing antimicrobial resistance levels in the community.
There were very limited data on the overall mortality of CAP from the included studies. Pneumonia was accountable for at least 19% of worldwide deaths of children <5 y of age, 70% of these deaths occurring in Sub-Saharan Africa, India and South Asia.Citation12 Nigeria had the greatest burden of mortality of children <5 y with pneumonia at 177,000 children, considered the highest in Africa and the second largest worldwide, after India.Citation104 Also, the highest mortality rates were recorded in Kenya during the wet and rainy season.Citation58 A systematic literature review from China covering the period between 1980 and 2008 reported the mortality rate of all-cause pneumonia at 526 per 100,000 child-years in children aged between 1 and 59 months.Citation97
Higher mortality rates were generally reported for: un-vaccinated, malnourished and HIV-positive children;Citation56 children with severe (45%) and very severe pneumonia (51%);Citation46 infants compared to those >12 months of age.Citation27
In the studies included, bacteria were more frequent than viral pathogens, although a trend of co-infections was noted. A recent review has shown that the true incidence of pulmonary bacterial co-infection with a viral respiratory infection in hospitalized infants and children is difficult to assess, but can vary widely from under 1 to 44%.Citation105 However, viral pathogens were not explored as causal agents at laboratory level. Furthermore, even the bacterial pathogens were probably underreported due to a lack of established protocols for specific pathogens. S. pneumoniae was the most common bacterial cause of CAP, followed by H. influenzae and M. pneumoniae. Hib was not highly reported. The most prevalent pneumococcal serotypes were 19F, 19A, 23F, 6B, and 14; however, they were reported only from China. Serotypes 1, 6A/6B, 14 and 23F were reported as the most dominant in children in Malawi.Citation72 A similar research carried out in children with CAP from Latin America and the Caribbean,Citation24 showed that S. pneumoniae was the dominant pathogen followed by H. influenzae, while the dominant pneumococcal serotypes were 14, 1 and 5.Citation24 The burden of viral pathogens in children from the included studies is consistent with the results from other regions of the world.Citation2,24
Data from the Russian Federation and CIS covering the screened period were very limited. However, a recent publication reported on serotyping and antibiotic susceptibility testing performed on 863 non-invasive pneumococcal isolates from children <6 y of age who sought medical care at 5 pediatric hospitals in Moscow between 2009 and 2013.Citation99 The most common pneumococcal serotypes were 19F, 6B, 23F, 14, 6A, 3 and 19A.
There was even a greater variation in the type of antibiotic used to treat CAP, depending on geographical region. This is potentially due to the variation in the resistance patterns of some antibiotics across the various countries, suggesting that some antibiotics would be less effective for the treatment of pneumonia. Some adaptations may be required especially where high-risk patients are concerned (e.g. patients with HIV, malnutrition, sickle-cell anaemia, or in combination), who may need various drug combinations to effectively treat pneumonia.
HIV-infected children are treated with various antibiotics, in combination with antiretroviral drugs.Citation106 Due to the increased penicillin resistance in recent years, modifications in the use of penicillin as the preferred drug has ceased with the recommendation for the use of co-trimoxazole and amoxicillin as recommended by the WHO/UNICEF.Citation13 In our review we observed a trend for a higher resistance of S. pneumoniae and H. influenzae compared to (in descending order) erythromycin, TMP-SMX, tetracycline and penicillin across China, Africa, India and South Asia. In China, S. pneumoniae and H. influenzae showed very high resistance to other antibiotics, including meropenem and imipenemCitation63 and very low resistance to amoxicillin and ampicillin compared to India and South Asian countries.
Antimicrobial resistance is a major problem in countries where antibiotic use is unregulated and are available without prescription in combination with the higher density populations.Citation107 Therefore, concerns still remain about combating the spread of antimicrobial resistance especially in children with pneumonia. In Russia, for example, the rate of multidrug-resistant pneumococci was reported at 22%, while the resistance rate to penicillin and erythromycin was 28% and 26%, respectively.Citation99
Recent developments in reducing antimicrobial resistance have been supported by the WHO; the 2011 WHO Health Day campaign demonstrated the need for further research into antimicrobial resistance worldwide. Additionally, in 2013, WHO and UNICEF launched the integrated Global action plan for pneumonia and diarrhea (GAPPD).Citation108 The aim of GAPPD is to end preventable childhood deaths due to pneumonia and diarrhea by 2025.Citation108
Immunization of children with conjugate vaccines has proven to be a successful strategy to prevent infections caused by various encapsulated bacteria. For the prevention of childhood pneumonia, several effective vaccines are currently available. These include the relatively new Hib conjugate vaccine and pneumococcal conjugate vaccines (PCVs). PCVs against S. pneumoniae have been proven to reduce morbidity and mortality of CAP.Citation109,110 Since its introduction in 2000, the heptavalent PCV (serotypes 4, 6B, 9V, 14, 18C, 19F, 23F), PCV7-CRM (Pfizer, New York, NY, USA), currently licensed for use worldwide, was shown to be effective in providing protection against pneumonia.Citation111 Recently, 2 extended valency PCVs (the 10-valent NTHi protein D conjugate vaccine [PHiD-CV; GSK Vaccines, Rixensart, Belgium] and the 13-valent CRM197 conjugate vaccine [PCV13-CRM; Pfizer, New York, NY, USA]) that also contain the important serotypes 1, 5 and 7F, gradually replace PCV7-CRM.Citation112
A comprehensive comparison of CAP epidemiology pre- and post-vaccination would be very useful since it was shown that national PCV-7 vaccination has reduced the incidence of vaccine-type IPD.Citation113,114 However, as of December 2012, only 44%(86/194) of the WHO member states had introduced PCV into national immunization programs.Citation115 PCV was introduced in national immunization programs in 41% (19/46) of member states in the African Region, 33% (9/27) of member states in the Western Pacific Region, and none of 11 member states in the South East Asia Region.Citation115 In 2014, PCV was introduced in 117 countries, up from 103 countries in 2013. Global coverage was estimated at 31% in 2014, up from 25% in 2013. Coverage levels were estimated at 83% in the Americas, 50% in the African Region and at only 2% in the Western Pacific Region.Citation116
The results of our study support the WHO recommendation of vaccination against S. pneumoniae and Hib, since pneumonia is still a high risk in children (<5 years). Along with vaccination, proper management of emerging cases is needed in the community, health centers and hospitals. Further effective strategies recommended by WHO include exclusive breastfeeding for the first 6 months of life, improvement of nutrition and prevention of low birth weight, pollution control and prevention of HIV infections.Citation117
The strength of this review lies in its general adherence to the established methods for conducting systematic reviews, including extensive literature searching methods, an inclusive publication date range, and the screening and inclusion of non-English language papers.
However, our systematic review has several limitations. Firstly, due to the heterogeneity in reporting the outcomes, statistical pooling or a meta-analysis could not be performed. Some of the studies presented unreliable data on incidence, mortality and prevalence rates, making it very difficult to draw comparisons. The variations in methodological quality of the studies, with majority of studies having a high risk of bias, implied that data may be over/under-estimated, therefore, unreliable. Additionally, although the WHO definition of childhood pneumonia is the most frequently used in field studies, a broader definition of CAP was adopted for this review to ensure that all studies describing CAP as an infection acquired outside the hospital environment. Depending on the case definition, the burden of CAP in children may be over– or underestimated.Citation118 Finally, as the focus of this review was to provide a literature overview as sensitive as possible, sample size was not considered as an exclusion criterion.
Conclusions
In conclusion, CAP within the 90 developing and newly industrialised countries included in the current review remains a disease associated with very high rates of incidence and mortality. This overview is useful to policy and decision making within these countries when considering the implementation and monitoring of CAP preventive measures. The data presented provides an overview of the best available evidence on the burden of CAP data up to 2012, prior to pneumococcal vaccines being widely used in the countries considered, and show critical gaps in the pathophysiology, etiology and epidemiology of pneumonia in the included countries. Therefore, several preventative and management measures aimed at reducing the burden of CAP in developing and newly industrialised countries are essential. Additionally, new research should also aim to use strict diagnostic criteria in assembling a sample and adequately report this in publications. Studies of bacterial etiology should include much larger samples to add further reliability to the evidence-base.
Materials and methods
Search strategy
The methods for this systematic literature review adhered, wherever possible, to recommendations and guidance published by the Center for Reviews & Dissemination (CRD), York, UK and the Cochrane Collaboration Handbook.Citation119,120 Searches were undertaken in several stages to identify relevant information, such as epidemiological data and statistics, systematic reviews, burden of disease studies, guidelines, national guidance and vaccination status. Searches were not limited by language or publication status. Electronic databases included MEDLINE, EMBASE, Cochrane Library, CAB Global Health, country-specific health ministries, UNICEF, WHO and health-technology-assessment (HTA) agencies (Supplementary Material, Appendix 1). The review included all study designs, including RCTs, cross-sectional, cohort, case-control studies as well as case-series. The outcomes of interest included incidence, prevalence, mortality, distribution of pathogens and antimicrobial resistance in children with CAP.
Study Selection and Eligibility Criteria
Eligible studies had to report data for children <6 y of age (where data were scarce, studies of children with a wide age range were also included) within 90 developing and newly industrialised countries (the newly industrialised countries are an intermediate category between fully developed and developing countries) across 5 regions of interest: Africa, India and South Asia, China, Middle East, and Russia and CIS (please see Supplementary Material, Appendix 2 for a list of the countries included). As described previously, we used the United Nations classification, but also geographic criteria to include the regions were knowledge gaps about CAP were identified. Latin America and the Caribbean were not included in our search, since these regions were covered by another systematic review, published in 2012.Citation24 Children had to have been diagnosed with CAP (using any reported definition) or be at-risk of pneumonia due to the following bacteria or viruses: S. pneumoniae, H. influenzae (encapsulated [a, b, c, d, e and f] and unencapsulated/ NTHi), M. pneumoniae, S. aureus and Legionella pneumophila, influenza A and B viruses, parainfluenza virus (PIV), RSV, ADV or RV. Risk factors of interest were HIV, malnutrition, sickle cell disease and vaccination status.
For this review, we adopted a broad definition of CAP which ensured that all studies describing CAP as an infection acquired outside the hospital environment with some relation to the definition as recommended by the WHO guidelines.Citation87 The WHO definition states that “mild pneumonia is reported as tachypnea (fast breathing) in a child (defined as ≥50 breaths/min in children under 12 months of age and ≥40 breaths/min in children over 12 months of age) in the absence of lower chest wall in-drawing or other signs and symptoms of WHO-defined severe pneumonia.” A summary of findings on the guidelines used across the included studies is presented in Supplementary data, Appendix 3.
Screening and data collection
Two reviewers working independently screened the titles and abstracts of the retrieved literature for relevance. Full papers were then ordered and screened in detail for inclusion in the review. Any disagreements between the 2 reviewers at any stage were resolved through a consensus or by the involvement of a third reviewer. Data were extracted using a pre-piloted excel extraction sheet in Microsoft Excel (version 2010).
Assessment of risk of bias
The risk of bias of observational studies was assessed using Downs and Black's 27 item checklist for the methodological quality assessment of non-RCTs.Citation88 The checklist items for observational studies focused on the use of an appropriate recruitment strategy, response rates, sample representativeness of the general population, objective and reliable outcome measures, use of a power calculation, appropriate use of statistical methods and evidence of bias within the studies (see Supplementary Material, Appendix 4). Due to the heterogeneity in the reporting of outcomes, it was not possible to carry out a planned meta-analysis. The number of items met for the studies ranged from 3 to 23 of the 27 checked items on the list. The majority of the studies had a cross-sectional design with a number of items met ranging from 6 to 11. The average number of items met for the included RCTs was 20. The data were grouped by geographical region.
Data synthesis and analysis
The heterogeneity of the included studies precluded statistical pooling of data. Instead, results have been narratively summarized, taking into account the reliability of the data and generalizability of the findings. Gaps in the research base were also highlighted.
Abbreviations
| ADV | = | adenovirus |
| ALRI | = | acute lower respiratory infections |
| CAP | = | community-acquired pneumonia |
| CIS | = | Commonwealth of Independent States |
| CRD | = | Center for Reviews & Dissemination |
| GAPPD | = | Global action plan for pneumonia and diarrhea |
| Hib | = | Haemophilus influenzae type b |
| HIV | = | human immunodeficiency virus |
| HTA | = | health-technology-assessment |
| IPD | = | invasive pneumococcal disease |
| NTHi | = | non typeable Haemophilus influenza |
| PIV | = | parainfluenza virus |
| PCV | = | pneumococcal conjugate vaccines |
| RCT | = | randomized controlled trial |
| RV | = | rhinovirus |
| RSV | = | respiratory syncytial virus |
| TMP-SMX | = | trimethoprim-sulfamethoxazole |
Disclosure of potential conflicts of interest
KSR Ltd received project funding from GlaxoSmithKline Biologicals SA, RDA, JPY, JPC, and JES are employees of GSK group of companies and own GSK shares.
Author contributions
JK contributed to developing the protocol, writing and critical revision of the report and provided project supervision. RDA, JPY, JPC, and JES participated to the concept of the literature review and were involved in the analysis and interpretation of the data. All authors have contributed to the manuscript development and have reviewed all drafts and provided approval prior submission. All authors are accountable for all aspects of the work.
KHVI_A_supplementary_material.zip
Download Zip (379.7 KB)Acknowledgments
The authors would like to thank the staff at Kleijnen Systematic Reviews Ltd for carrying out the systematic and literature review. The authors also thank Vasile Coman and Abdelilah Ibrahimi (both XPE Pharma & Science on behalf of GSK) for editing and coordinating the publication development.
Funding
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study and analysis. GlaxoSmithKline Biologicals SA also took in charge all costs associated with developing and publishing the present manuscript. All authors had full access to the data.
References
- Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet 2013; 381:1405-16; PMID:23582727; http://dx.doi.org/10.1016/S0140-6736(13)60222-6
- Rudan I, O'Brien KL, Nair H, Liu L, Theodoratou E, Qazi S, Lukšić I, Fischer Walker CL, Black RE, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health 2013; 3:010401; PMID:23826505; http://dx.doi.org/10.7189/jogh.03.010101
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012; 379:2151-61; PMID:22579125; http://dx.doi.org/10.1016/S0140-6736(12)60560-1
- Sonego M, Pellegrin MC, Becker G, Lazzerini M. Risk factors for mortality from acute lower respiratory infections (ALRI) in children under five years of age in low and middle-income countries: a systematic review and meta-analysis of observational studies. PLoS One 2015; 10:e0116380; PMID:25635911; http://dx.doi.org/10.1371/journal.pone.0116380
- WHO. Fact sheet N°331; Updated November 2015. Available at: http://www.who.int/mediacentre/factsheets/fs331/en/.
- WHO. The world health report 1998: life in the 21st century a vision for all. Available at: http://www.who.int/whr/1998/en/whr98_en.pdf. Geneva: WHO, 1998.
- Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet 2005; 365:1147-52; PMID:15794969; http://dx.doi.org/10.1016/S0140-6736(05)71877-8
- Rudan I, Tomaskovic L, Boschi-Pinto C, Campbell H. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ 2004; 82:895-903; PMID:15654403.
- Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis 2002; 2:25-32; PMID:11892493; http://dx.doi.org/10.1016/S1473-3099(01)00170-0
- Zar HJ, Madhi SA. Childhood pneumonia: progress and challenges. S Afr Med J 2006; 96:890-900; PMID:17077915
- Zar HJ, Ferkol TW. The global burden of respiratory disease-impact on child health. Pediatr Pulmonol 2014; 49:430-4; PMID:24610581; http://dx.doi.org/10.1002/ppul.23030
- Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 2008; 86:408-16; PMID:18545744; http://dx.doi.org/10.2471/BLT.07.048769
- Wardlaw T, White Johansson E, Hodge M. Pneumonia: the forgotten killer of children. Available at: http://www.childinfo.org/files/Pneumonia_The_Forgotten_Killer_of_Children.pdf. New York: The United Nations Children's Fund (UNICEF) & World Health Organization (WHO), 2006.
- Zar HJ, Jeena P, Argent A, Gie R, Madhi SA, McNally L, Working Groups of the Paediatric Assembly of the South African Thoracic Society. Diagnosis and management of community-acquired pneumonia in childhood: South African Thoracic Society guildelines. S Afr Med J 2005; 95:977-90; PMID:16482985.
- Walker CL, Perin J, Katz J, Tielsch JM, Black RE. Diarrhea as a risk factor for acute lower respiratory tract infections among young children in low income settings. J Glob Health 2013; 3:010402; PMID:23826506; http://dx.doi.org/10.7189/jogh.03.010402
- Church J, Maitland K. Invasive bacterial co-infection in African children with Plasmodium falciparum malaria: a systematic review. BMC Med 2014; 12:31; PMID:24548672; http://dx.doi.org/10.1186/1741-7015-12-31
- Ashraf H, Chisti MJ, Alam NH. Treatment of childhood pneumonia in developing countries In: Smigorski K, ed. Health Management. Rijeka, Croatia: InTech, 2010
- British Thoracic Society Standards of Care Committee. BTS guidelines for the management of community acquired pneumonia in childhood. Thorax 2002; 57:1-24; PMID:11809978; http://dx.doi.org/10.1136/thorax.57.1.1
- Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, Muenz LR, O'Brien KL. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med 2010; 7:pii:e1000348; http://dx.doi.org/10.1371/journal.pmed.1000348
- Lassi ZS, Das JK, Haider SW, Salam RA, Qazi SA, Bhutta ZA. Systematic review on antibiotic therapy for pneumonia in children between 2 and 59 months of age. Arch Dis Child 2014; 99(7):687-93; PMID:24431417.
- Zar HJ, Madhi SA, Aston SJ, Gordon SB. Pneumonia in low and middle income countries: progress and challenges. Thorax 2013; 68:1052-6; PMID:23956020; http://dx.doi.org/10.1136/thoraxjnl-2013-204247
- Madhi SA, De Wals P, Grijalva CG, Grimwood K, Grossman R, Ishiwada N, Lee PI, Nascimento-Carvalho C, Nohynek H, O'Brien KL, et al. The burden of childhood pneumonia in the developed world: a review of the literature. Pediatr Infect Dis J 2013; 32:e119-27; PMID:23099423; http://dx.doi.org/10.1097/INF.0b013e318271f369
- United Nations Industrial Development Organization. 2013. Country grouping in UNIDO statistics. Available at: https://www.unido.org/fileadmin/user_media/Services/PSD/Country_Grouping_in_UNIDO_Statistics_2013.pdf
- Gentile A, Bardach A, Ciapponi A, Garcia-Marti S, Aruj P, Glujovsky D, Calcagno JI, Mazzoni A, Colindres RE. Epidemiology of community-acquired pneumonia in children of Latin America and the Caribbean: a systematic review and meta-analysis. Int J Infect Dis 2012; 16:e5-15; PMID:22056731; http://dx.doi.org/10.1016/j.ijid.2011.09.013
- Roca A, Sigauque B, Quinto L, Morais L, Berenguera A, Corachan M, Ribó JL, Naniche D, Bassat Q, Sacoor Ch, et al. Estimating the vaccine-preventable burden of hospitalized pneumonia among young Mozambican children. Vaccine 2010; 28:4851-7; PMID:20392430; http://dx.doi.org/10.1016/j.vaccine.2010.03.060
- Campbell JD, Kotloff KL, Sow SO, Tapia M, Keita MM, Keita T, Diallo S, Hormazabal JC, Murray P, Levine MM. Invasive pneumococcal infections among hospitalized children in Bamako, Mali. Pediatr Infect Dis J 2004; 23:642-9; PMID:15247603; http://dx.doi.org/10.1097/01.inf.0000130951.85974.79
- Campbell JD, Sow SO, Levine MM, Kotloff KL. The causes of hospital admission and death among children in Bamako, Mali. J Trop Pediatr 2004; 50:158-63; PMID:15233192; http://dx.doi.org/10.1093/tropej/50.3.158
- Tornheim JA, Manya AS, Oyando N, Kabaka S, Breiman RF, Feikin DR. The epidemiology of hospitalized pneumonia in rural Kenya: the potential of surveillance data in setting public health priorities. Int J Infect Dis 2007; 11:536-43; PMID:17537660; http://dx.doi.org/10.1016/j.ijid.2007.03.006
- O'Callaghan-Gordo C, Bassat Q, Morais L, Diez-Padrisa N, MacHevo S, Nhampossa T, Nhalungo D, Sanz S, Quintó L, Alonso PL, et al. Etiology and epidemiology of viral pneumonia among hospitalized children in rural mozambique: a malaria endemic area with high prevalence of human immunodeficiency virus. Pediatr Infect Dis J 2011; 30:39-44; PMID:20805786; http://dx.doi.org/10.1097/INF.0b013e3181f232fe
- Usen S, Adegbola R, Mulholland K, Jaffar S, Hilton S, Oparaugo A, Omosigho C, Lahai G, Corrah T, Palmer A, et al. Epidemiology of invasive pneumococcal disease in the Western Region, The Gambia. Pediatr Infect Dis J 1998; 17:23-8; PMID:9469390; http://dx.doi.org/10.1097/00006454-199801000-00006
- Enwere G, Cheung YB, Zaman SMA, Akano A, Oluwalana C, Brown O, Vaughan A, Adegbola R, Greenwood B, Cutts F. Epidemiology and clinical features of pneumonia according to radiographic findings in Gambian children. Trop Med Int Health 2007; 12:1377-85; PMID:18045264; http://dx.doi.org/10.1111/j.1365-3156.2007.01922.x
- Brooks WA, Breiman RF, Goswami D, Hossain A, Alam K, Saha SK, Nahar K, Nasrin D, Ahmed N, El Arifeen S, et al. Invasive pneumococcal disease burden and implications for vaccine policy in urban Bangladesh. Am J Trop Med Hyg 2007; 77:795-801; PMID:17984328.
- Brooks WA, Goswami D, Rahman M, Nahar K, Fry AM, Balish A, Iftekharuddin N, Azim T, Xu X, Klimov A, et al. Influenza is a major contributor to childhood pneumonia in a tropical developing country. Pediatr Infect Dis J 2010; 29:216-21; PMID:20190613; http://dx.doi.org/10.1097/INF.0b013e3181bc23fd
- Awasthi S, Pande VK. Seasonal pattern of morbidities in preschool slum children in Lucknow, north India. Indian Pediatr 1997; 34:987-93; PMID:9567528.
- Arifeen SE, Saha SK, Rahman S, Rahman KM, Rahman SM, Bari S, Naheed A, Mannan I, Seraji MH, Ahmed NU, et al. Invasive pneumococcal disease among children in rural Bangladesh: results from a population-based surveillance. Clin Infect Dis 2009; 48 Suppl 2:S103-13; PMID:19191605; http://dx.doi.org/10.1086/596543
- Gupta M, Kumar R, Deb AK, Bhattacharya SK, Bose A, John J, Balraj V, Ganguly NK, Kant L, Kapoor AN, et al. Multi-center surveillance for pneumonia & meningitis among children. Indian J Med Res 2010; 131:649-58; PMID:20516536.
- Xiang Z, Gonzalez R, Xie Z, Xiao Y, Liu J, Chen L, Liu C, Zhang J, Ren L, Vernet G, et al. Human rhinovirus C infections mirror those of human rhinovirus A in children with community-acquired pneumonia. J Clin Virol 2010; 49:94-9; PMID:20728404; http://dx.doi.org/10.1016/j.jcv.2010.07.013
- Ho P-L, Chiu SS, Chow FKH, Mak GC, Lau YL. Pediatric hospitalization for pneumococcal diseases preventable by 7-valent pneumococcal conjugate vaccine in Hong Kong. Vaccine 2007; 25:6837-41; PMID:17714837; http://dx.doi.org/10.1016/j.vaccine.2007.07.039
- Hildenwall H, Nantanda R, Tumwine JK, Petzold M, Pariyo G, Tomson G, Peterson S. Care-seeking in the development of severe community acquired pneumonia in Ugandan children. Ann Trop Paediatr 2009; 29:281-9; PMID:19941751; http://dx.doi.org/10.1179/027249309X12547917869005
- Delport SD, Brisley T. Aetiology and outcome of severe community-acquired pneumonia in children admitted to a paediatric intensive care unit. S Afr Med J 2002; 92:907-11; PMID:12506595.
- Adegbola RA, Falade AG, Sam BE, Aidoo M, Baldeh I, Hazlett D, Whittle H, Greenwood BM, Mulholland EK. The etiology of pneumonia in malnourished and well-nourished Gambian children. Pediatr Infect Dis J 1994; 13:975-82; PMID:7845751; http://dx.doi.org/10.1097/00006454-199411000-00008
- Johnson A-W, Osinusi K, Aderele WI, Gbadero DA, Olaleye OD, Adeyemi-Doro FAB. Etiologic agents and outcome determinants of community-acquired pneumonia in urban children: a hospital-based study. J Natl Med Assoc 2008; 100:370-85; PMID:18481475; http://dx.doi.org/10.1016/S0027-9684(15)31269-4
- Zhao R, Zheng Y, Deng Q, Wang H, Chen Q, Deng J. [Serotype/serogroup distribution and antimicrobial resistance of streptococcus pneumoniae in children with community-acquired pneumonia in Shenzhen area]. Chinese Journal of Infection and Chemotherapy 2010; 10:205-8.
- Liu G, Talkington DF, Fields BS, Levine OS, Yang Y, Tondella MLC. Chlamydia pneumoniae and mycoplasma pneumoniae in young children from China with community-acquired pneumonia. Diagn Microbiol Infect Dis 2005; 52:7-14; PMID:15878436; http://dx.doi.org/10.1016/j.diagmicrobio.2005.01.005
- Zhang Q, Guo Z, MacDonald NE. Vaccine preventable community-acquired pneumonia in hospitalized children in Northwest China. Pediatr Infect Dis J 2011; 30:7-10; PMID:20625346; http://dx.doi.org/10.1097/INF.0b013e3181ec6245
- Naheed A, Saha SK, Breiman RF, Khatun F, Brooks WA, El Arifeen S, Sack D, Luby SP, Pneumococcal Study Group. Multihospital surveillance of pneumonia burden among children aged. Clin Infect Dis 2009; 48:S82-9; PMID:19191623; http://dx.doi.org/10.1086/596485
- Agarwal J, Awasthi S, Rajput A, Tiwari M, Jain A. Atypical bacterial pathogens in community-acquired pneumonia in children: a hospital-based study. Trop Doct 2009; 39:109-11; PMID:19299299; http://dx.doi.org/10.1258/td.2008.080248
- Batuwanthudawe R, Karunarathne K, Dassanayake M, De Silva S, Lalitha MK, Thomas K, Steinhoff M, Abeysinghe N. Surveillance of invasive pneumococcal disease in Colombo, Sri Lanka. Clin Infect Dis 2009; 48:S136-40; PMID:19191609; http://dx.doi.org/10.1086/596492
- Thomas K. Prospective multicentre hospital surveillance of streptococcus pneumoniae disease in India. Lancet 1999; 353:1216-21; PMID:10217081; http://dx.doi.org/10.1016/S0140-6736(98)07228-6
- Habibinejad HA, Riahin AA, Heidari A, Mahjourian F. Infectious diseases in hospitalized children of central Iran. Pakistan Journal of Medical Sciences 2010; 26:901-4.
- Howidi M, Muhsin H, Rajah J. The burden of pneumococcal disease in children less than 5 years of age in Abu Dhabi, United Arab Emirates. Ann Saudi Med 2011; 31:356-9; PMID:21808110; http://dx.doi.org/10.4103/0256-4947.83214
- Somer A, Salman N, Yalcin I, Agacfidan A. Role of mycoplasma pneumoniae and chlamydia pneumoniae in children with community-acquired pneumonia in Istanbul, Turkey. J Trop Pediatr 2006; 52:173-8; PMID:16627487; http://dx.doi.org/10.1093/tropej/fml017
- Lassmann B, Poetschke M, Ninteretse B, Issifou S, Winkler S, Kremsner PG, Graninger W, Apfalter P. Community-acquired pneumonia in children in Lambarene, Gabon. Am J Trop Med Hyg 2008; 79:109-14; PMID:18606773.
- Chaudhry R, Nazima N, Dhawan B, Kabra SK. Prevalence of mycoplasma pneumoniae and chlamydia pneumoniae in children with community acquired pneumonia. Indian J Pediatr 1998; 65:717-21; PMID:10773927; http://dx.doi.org/10.1007/BF02731050
- Baranovich T, Zaraket H, Shabana II, Nevzorova V, Turcutyuicov V, Suzuki H. Molecular characterization and susceptibility of methicillin-resistant and methicillin-susceptible staphylococcus aureus isolates from hospitals and the community in Vladivostok, Russia. Clin Microbiol Infect 2010; 16:575-82; PMID:19681959; http://dx.doi.org/10.1111/j.1469-0691.2009.02891.x
- Nantanda R, Hildenwall H, Peterson S, Kaddu-Mulindwa D, Kalyesubula I, Tumwine JK. Bacterial aetiology and outcome in children with severe pneumonia in Uganda. Ann Trop Paediatr 2008; 28:253-60; PMID:19021940; http://dx.doi.org/10.1179/146532808X375404
- Hussey G, Hitchcock J, Schaaf H, Coetzee G, Hanslo D, van Schalkwyk E, Pitout J, Clausen J, van der Horst W. Epidemiology of invasive haemophilus influenzae infections in Cape Town, South Africa. Ann Trop Paediatr 1994; 14:97-103; PMID:7521637.
- Ye Y, Zulu E, Mutisya M, Orindi B, Emina J, Kyobutungi C. Seasonal pattern of pneumonia mortality among under-five children in Nairobi's informal settlements. Am J Trop Med Hyg 2009; 81:770-5; PMID:19861609; http://dx.doi.org/10.4269/ajtmh.2009.09-0070
- Rahman M, Hossain S, Baqui AH, Shoma S, Rashid H, Nahar N, Zaman MK, Khatun F. Haemophilus influenzae type-b and non-b-type invasive diseases in urban children. J Infect 2008; 56:191-6; PMID:18280571; http://dx.doi.org/10.1016/j.jinf.2007.12.008
- Luby SP, Halder AK, Saha SK, Naheed A, Sazzad HM, Akhter S, Gurley ES, Brooks WA, El-Arifeen S, Najnin N, et al. A low-cost approach to measure the burden of vaccine preventable diseases in urban areas. Vaccine 2010; 28:4903-12; PMID:20653079; http://dx.doi.org/10.1016/j.vaccine.2010.05.040
- Hussain H, Waters H, Khan AJ, Omer SB, Halsey NA. Economic analysis of childhood pneumonia in Northern Pakistan. Health Policy Plan 2008; 23:438-42; PMID:18755733; http://dx.doi.org/10.1093/heapol/czn033
- Falade AG, Lagunju IA, Bakare RA, Odekanmi AA, Adegbola RA. Invasive pneumococcal disease in children aged. Clin Infect Dis 2009; 48:S190-6; PMID:19191615; http://dx.doi.org/10.1086/596500
- Wang H, Tang J, Xiong Y, Li X, Gonzalez F, Mu D. Neonatal community-acquired pneumonia: pathogens and treatment. J Paediatr Child Health 2010; 46:668-72; PMID:20796185; http://dx.doi.org/10.1111/j.1440-1754.2010.01814.x
- Yao K-H, Wang L-B, Zhao G-M, Zheng Y-J, Deng L, Huang J-F, Wang JX, Zhao RZ, Deng QL, Hu YH, et al. Pneumococcal serotype distribution and antimicrobial resistance in Chinese children hospitalized for pneumonia. Vaccine 2011; 29:2296-301; PMID:21276441; http://dx.doi.org/10.1016/j.vaccine.2011.01.027
- Chen H, Huang Y, Cui Z. A study of viral pathogens in 280 cases of children with community acquired pneumonia(CAP). J Med Res 2009; 38:73-5.
- Hou L, Zheng Y, Deng J, Zhao R. Bacterial etiology and antimicrobial resistance patterns of community- acquired pneumonia in hospitalized children in Shenzhen. Zhongguo Weishengtaxixue Zazhi 2008; 20:586-8.
- Liu Y, Wang H, Chen M, Sun Z, Zhao R, Zhang L, Wang H, Zhang H, Wang L, Chu Y, et al. Serotype distribution and antimicrobial resistance patterns of streptococcus pneumoniae isolated from children in China younger than 5 years. Diagn Microbiol Infect Dis 2008; 61:256-63; PMID:18358662; http://dx.doi.org/10.1016/j.diagmicrobio.2008.02.004
- Al-Ali MK, Batchoun RG, Al-Nour TM. Etiology of community-acquired pneumonia in hospitalized patients in Jordan. Saudi Med J 2006; 27:813-6; PMID:16758041.
- Secmeer G, Ciftci AO, Kanra G, Ceyhan M, Kara A, Cengiz AB, Kiper N, Haliloğlu M, Ozçelik U, Cağdaş DN. Community-acquired pneumonia and parapneumonic effusions in developing countries. Turk J Pediatr 2008; 50:51-7; PMID:18365592.
- Balkhy HH, Cunningham G, Chew FK, Francis C, Al Nakhli DJ, Almuneef MA, Memish ZA. Hospital- and community-acquired infections: a point prevalence and risk factors survey in a tertiary care center in Saudi Arabia. Int J Infect Dis 2006; 10:326-33; PMID:16678467; http://dx.doi.org/10.1016/j.ijid.2005.06.013
- Kaijalainen T, Kharit SM, Kvetnaya AS, Sirkia K, Herva E, Parkov OV, Nohynek H. Invasive infections caused by neisseria meningitidis, haemophilus influenzae and streptococcus pneumoniae among children in St Petersburg, Russia. Clin Microbiol Infect 2008; 14:507-10; PMID:18318743; http://dx.doi.org/10.1111/j.1469-0691.2008.01967.x
- Cornick JE, Everett DB, Broughton C, Denis BB, Banda DL, Carrol ED, Parry CM. Invasive streptococcus pneumoniae in children, Malawi, 2004-2006. Emerg Infect Dis 2011; 17:1107-9; PMID:21749782; http://dx.doi.org/10.3201/eid/1706.101404
- Deraz TE, El SSA, Shaheen MA, Motawea AA, Gomaa HE, Fawzy SH, et al. Atypical pathogens in community acquired pneumonia of Egyptian children. Asian Pac J Trop Med 2009; 2:1-8.
- Wang Y-J, Vuori-Holopainen E, Yang Y, Wang Y, Hu Y, Leboulleux D, Hedman K, Leinonen M, Peltola H. Relative frequency of haemophilus influenzae type b pneumonia in Chinese children as evidenced by serology. Pediatr Infect Dis J 2002; 21:271-7; PMID: 12075755; http://dx.doi.org/10.1097/00006454-200204000-00002
- Wang Z, Ji W, Guo HB, Tao YZ, Ding YF. ; Comparative studies on the composition and antibiotic-resistance of pathogenic bacteria between children with community-acquired and hospital-acquired pneumonia]. Zhonghua Yu Fang Yi Xue Za Zhi 2011; 45:211-6; PMID:21624231.
- Zeng L, Liao B. Pathogens and drug resistance analysis in children with community- acquired and hospital-acquired pneumonia. Chinese Journal of Nosocomiology 2010; 20:3231-3
- Zhao R, Zheng Y, Ma D, Deng J, Wang H, Sun L. Study on antibiotic antibacterial activity in vitro of hemophilus influenza isolated in children with CAP in Shenzhen area. Zhongguo Weishengtaxixue Zazhi 2007; 19:533-4
- Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, Oluwalana C, Vaughan A, Obaro SK, Leach A, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 2005; 365:1139-46; PMID:15794968; http://dx.doi.org/10.1016/S0140-6736(05)71876-6
- Madhi SA, Kuwanda L, Cutland C, Klugman KP. The impact of a 9-valent pneumococcal conjugate vaccine on the public health burden of pneumonia in HIV-infected and -uninfected children. Clin Infect Dis 2005; 40:1511-8; PMID:15844075; http://dx.doi.org/10.1086/429828
- Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med 2003; 349:1341-8; PMID:14523142; http://dx.doi.org/10.1056/NEJMoa035060
- Adegbola RA, Secka O, Lahai G, Lloyd-Evans N, Njie A, Usen S, Oluwalana C, Obaro S, Weber M, Corrah T, et al. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: a prospective study. Lancet 2005; 366:144-50; PMID:16005337; http://dx.doi.org/10.1016/S0140-6736(05)66788-8
- Ayieko P, Akumu AO, Griffiths UK, English M. The economic burden of inpatient paediatric care in Kenya: household and provider costs for treatment of pneumonia, malaria and meningitis. Cost Eff Resour Alloc 2009; 7:3; PMID:19161598; http://dx.doi.org/10.1186/1478-7547-7-3
- Aurangzeb B, Hameed A. Comparative efficacy of amoxicillin, cefuroxime and clarithromycin in the treatment of community -acquired pneumonia in children. J Coll Physicians Surg Pak 2003; 13:704-7; PMID:15569557.
- Hussain H, Waters H, Omer SB, Khan A, Baig IY, Mistry R, Halsey N. The cost of treatment for child pneumonias and meningitis in the Northern Areas of Pakistan. Int J Health Plann Manage 2006; 21:229-38; PMID:17044548; http://dx.doi.org/10.1002/hpm.847
- Yuan L, Zhang J-P, Chen C. Retrospective analysis of clinic and epidemiology of respiratory syncytial virus pneumonia in 309 neonates. Journal of Applied Clinical Pediatrics 2009; 24:1064-7
- Yan FQ, Li DM, Sun HP, LüJuan Li, Liu Y. Cost-effectiveness analysis of three therapeutic schemes in treatment of children's pneumonia. Chinese Journal of Nosocomiology 2010; 20:3026-7.
- WHO, UNICEF. Integrated management of childhood illness (IMCI) chart booklet. Available at: http://apps.who.int/iris/bitstream/10665/43993/1/9789241597289_eng.pdf. Geneva: WHO, 2008.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998; 52:377-84; PMID:9764259; http://dx.doi.org/10.1136/jech.52.6.377
- Yao KH, Lu Q, Deng L, Yu SJ, Zhang H, Deng QL, Tong YJ, Gao W, Yuan L, Shen XZ, et al. [Serotype distribution and resistance to beta-lactams of streptococcus pneumoniae isolated from children in Beijing, Shanghai and Guangzhou, 2000 – 2002]. Zhonghua Er Ke Za Zhi 2006; 44:928-32; PMID:17254463.
- Li J, Yuan L, Yu S, Yang Y. Nasal carriage of streptococcus pneumoniae among children in Beijing. Chin Med J 2001; 114:1196-200; PMID:11729519.
- Ho PL, Lam KF, Chow FKH, Lau YL, Wong SSY, Cheng SLE, Chiu SS. Serotype distribution and antimicrobial resistance patterns of nasopharyngeal and invasive streptococcus pneumoniae isolates in Hong Kong children. Vaccine 2004; 22:3334-9; PMID:15308357; http://dx.doi.org/10.1016/j.vaccine.2004.02.038
- Percin D, Ay Altintop Y, Sumerkan B. Ten-year surveillance of invasive streptococcus pneumoniae isolates in central Turkey prior to the introduction of a conjugate vaccine. J Infect Dev Ctries 2010; 4:560-5; PMID:21045368; http://dx.doi.org/10.3855/jidc.834
- Ercan TE, Severge B, Topkaya A, Ercan RG, Altinkaya N. Effect of the pneumococcal conjugate vaccine on pneumococcal carriage in Turkish children. Pediatr Int 2011; 53:224-30; PMID:21501306; http://dx.doi.org/10.1111/j.1442-200X.2010.03212.x
- Shibl AM. Distribution of serotypes and antibiotic resistance of invasive pneumococcal disease isolates among children aged 5 years and under in Saudi Arabia (2000–2004). Clin Microbiol Infect 2008; 14:876-9; PMID:18844690; http://dx.doi.org/10.1111/j.1469-0691.2008.02058.x
- Mokaddas EM, Rotimi VO, Albert MJ. Implications of streptococcus pneumoniae penicillin resistance and serotype distribution in Kuwait for disease treatment and prevention. Clin Vaccine Immunol 2008; 15:203-7; PMID:18077618; http://dx.doi.org/10.1128/CVI.00277-07
- Katz A, Leibovitz E, Timchenko VN, Greenberg D, Porat N, Peled N, Dagan R, Ossipov IB. Antibiotic susceptibility, serotype distribution and vaccine coverage of nasopharyngeal and oropharyngeal streptococcus pneumoniae in a day-care centre in St. Petersburg, Russia. Scand J Infect Dis 2007; 39:293-8; PMID:17454891; http://dx.doi.org/10.1080/00365540600987741
- Chen Y, Deng W, Wang SM, Mo QM, Jia H, Wang Q, Li SG, Li X, Yao BD, Liu CJ, et al. Burden of Pneumonia and Meningitis Caused by Streptococcus pneumoniae in China among Children under 5 Years of Age: A Systematic Literature Review. PLoS One 2011; 6:e27333; PMID:22110628; http://dx.doi.org/10.1371/journal.pone.0027333
- Guan X, Silk BJ, Li W, Fleischauer AT, Xing X, Jiang X, Yu H, Olsen SJ, Cohen AL. Pneumonia incidence and mortality in Mainland China: systematic review of Chinese and English literature, 1985–2008. PLoS One 2010; 5:e11721; PMID:20668535; http://dx.doi.org/10.1371/journal.pone.0011721
- Mayanskiy N, Alyabieva N, Ponomarenko O, Lazareva A, Katosova L, Ivanenko A, Kulichenko T, Namazova-Baranova L, Baranov A. Serotypes and antibiotic resistance of non-invasive Streptococcus pneumoniae circulating in pediatric hospitals in Moscow, Russia. Int J Infect Dis 2014; 20:58-62; PMID:24462930; http://dx.doi.org/10.1016/j.ijid.2013.11.005
- Bassat Q, Machevo S, O'Callaghan-Gordo C, Sigauque B, Morais L, Diez-Padrisa N, Ribó JL, Mandomando I, Nhampossa T, Ayala E, et al. Distinguishing malaria from severe pneumonia among hospitalized children who fulfilled integrated management of childhood illness criteria for both diseases: a hospital-based study in Mozambique. Am J Trop Med Hyg 2011; 85:626-34; PMID:21976562; http://dx.doi.org/10.4269/ajtmh.2011.11-0223
- Mahende C, Ngasala B, Lusingu J, Butichi A, Lushino P, Lemnge M, Premji Z. Aetiology of acute febrile episodes in children attending Korogwe District Hospital in north-eastern Tanzania. PLoS One 2014; 9:e104197; PMID:25090651; http://dx.doi.org/10.1371/journal.pone.0104197
- Acacio S, Verani JR, Lanaspa M, Fairlie TA, Nhampossa T, Ruperez M, Aide P, Plikaytis BD, Sacoor C, Macete E, et al. Under treatment of pneumonia among children under 5 years of age in a malaria-endemic area: population-based surveillance study conducted in Manhica district- rural, Mozambique. Int J Infect Dis 2015; 36:39-45; PMID:25980619; http://dx.doi.org/10.1016/j.ijid.2015.05.010
- Means AR, Weaver MR, Burnett SM, Mbonye MK, Naikoba S, McClelland RS. Correlates of inappropriate prescribing of antibiotics to patients with malaria in Uganda. PLoS One 2014; 9:e90179; PMID:24587264; http://dx.doi.org/10.1371/journal.pone.0090179
- Akanbi II A, Taiwoo S, Babatunde S, Onile B, Abdulraheem I. Antibiotic susceptibility pattern of streptoccocus pneumoniae in Ilorin, Nigeria. African journal of clinical and experimental microbiology 2004; 5:173-6.
- Thorburn K, Riordan A. Pulmonary bacterial coinfection in infants and children with viral respiratory infection. Expert Rev Anti Infect Ther 2012; 10:909-16; PMID:23030330; http://dx.doi.org/10.1586/eri.12.80
- World Health Organization (WHO). Immunization monitoring: vaccination schedule [Excel] [Internet]. WHO/UNICEF Joint Reporting Form, 2011 [cited 22.2.12].
- Okeke IN, Laxminarayan R, Bhutta ZA, Duse AG, Jenkins P, O'Brien TF, Pablos-Mendez A, Klugman KP. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis 2005; 5:481-93; PMID:16048717; http://dx.doi.org/10.1016/S1473-3099(05)70189-4
- WHO. Ending Preventable Child Deaths from Pneumonia and Diarrhoea by 2025. The integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD). Available at: http://www.who.int/maternal_child_adolescent/documents/gappd_report_2013_en.pdf. 2013.
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32-41; PMID:19947881; http://dx.doi.org/10.1086/648593
- Theodoratou E, Johnson S, Jhass A, Madhi SA, Clark A, Boschi-Pinto C, Bhopal S, Rudan I, Campbell H. The effect of Haemophilus influenzae type b and pneumococcal conjugate vaccines on childhood pneumonia incidence, severe morbidity and mortality. Int J Epidemiol 2010; 39 Suppl 1:i172-85; PMID:20348119; http://dx.doi.org/10.1093/ije/dyq033
- Fitzwater SP, Chandran A, Santosham M, Johnson HL. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2012; 31:501-8; PMID:22327872; http://dx.doi.org/10.1097/INF.0b013e31824de9f6
- Pneumococcal vaccines WHO position paper–2012. Wkly Epidemiol Rec 2012; 87:129-44; PMID:24340399
- Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999-2011(1.). Emerg Infect Dis 2013; 19:1074-83; PMID:23763847; http://dx.doi.org/10.3201/eid1907.121830
- Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378:1962-73; PMID:21492929; http://dx.doi.org/10.1016/S0140-6736(10)62225-8
- WHO. Progress in Introduction of Pneumococcal Conjugate Vaccine — Worldwide, 2000–2012. MMWR Weekly 2013; 62:308-11
- WHO. Global Immunization Data. July 2015. Available at: http://www.who.int/immunization/monitoring_surveillance/Global_Immunization_Data.pdf?ua = 1 and http://www.who.int/mediacentre/factsheets/fs378/en/.
- WHO. Treatment and prevention of pneumonia. March 2010. Available at: http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_26-en.pdf
- Campbell H, Biloglav Z, Rudan I. Reducing bias from test misclassification in burden of disease studies: use of test to actual positive ratio–new test parameter. Croat Med J 2008; 49:402-14; PMID:18581619; http://dx.doi.org/10.3325/cmj.2008.3.402
- Dissemination CfRa. Systematic Reviews: CRD's guidance for undertaking reviews in health care. Available at: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf. York: University of York, 2009.
- Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Available at: http://community.cochrane.org/handbook. Version 5.1.0: The Cochrane Collaboration, 2011.