ABSTRACT
Variability in rotavirus gastroenteritis (RVGE) epidemiology can influence the optimal vaccination schedule. We evaluated regional trends in the age of RVGE episodes in low- to middle- versus high-income countries in three continents. We undertook a post-hoc analysis based on efficacy trials of a human rotavirus vaccine (HRV; Rotarix™, GSK Vaccines), in which 1348, 1641, and 5250 healthy infants received a placebo in Europe (NCT00140686), Africa (NCT00241644), and Asia (NCT00197210, NCT00329745). Incidence of any/severe RVGE by age at onset was evaluated by active surveillance over the first two years of life. Severity of RVGE episodes was assessed using the Vesikari-scale. The incidence of any RVGE in Africa was higher than in Europe during the first year of life (≤2.78% vs. ≤2.03% per month), but much lower during the second one (≤0.86% versus ≤2.00% per month). The incidence of severe RVGE in Africa was slightly lower than in Europe during the first year of life. Nevertheless, temporal profiles for the incidence of severe RVGE in Africa and Europe during the first (≤1.00% and ≤1.23% per month) and second (≤0.53% and ≤1.13% per month) years of life were similar to those of any RVGE. Any/severe RVGE incidences peaked at younger ages in Africa vs. Europe. In high-income Asian regions, severe RVGE incidence (≤0.31% per month) remained low during the study. The burden of any RVGE was higher earlier in life in children from low- to middle- compared with high-income countries. Differing rotavirus vaccine schedules are likely warranted to maximize protection in different settings.
Introduction
Rotavirus is a leading cause of severe acute gastroenteritis, resulting in approximately 450,000 annual deaths among children <5 years of age, with almost 90% of these deaths occurring in low-income Asian and African countries.Citation1,2 In children, the first rotavirus infection is generally the most severe, and subsequent infections result in less serious disease outcomes, probably because older children have acquired protective immunity via natural infection.Citation2
Vaccination is considered the most effective strategy to control the global burden associated with rotavirus gastroenteritis (RVGE). The World Health Organization (WHO) recommends inclusion of rotavirus vaccines in all national immunisation programmes and considers rotavirus vaccination as a priority, particularly in countries with high RVGE mortality rates.Citation2 Although the WHO recommends that first doses of rotavirus vaccines be administered as early as possible, the previous recommendations to initiate rotavirus immunisation in children <15 weeks of age was removed, along with the upper age limit restriction, from the new policy in order to optimise coverage.Citation2 However, the removal of both limits of the age restriction do not emphasize early vaccination, leading to a potentially higher burden of disease in settings with high RVGE incidence in early infancy and potentially increasing the risk of intussusception.Citation3
Two live, oral, attenuated rotavirus vaccines are globally available for RVGE prevention: the human rotavirus G1P [8] strain vaccine RIX4414 (HRV; Rotarix™, GSK Vaccines, Belgium) and a pentavalent, human-bovine reassortant rotavirus vaccine (RotaTeq™, Merck & Co, USA). Previous studies have shown that HRV, which is available in over 100 countries for immunisation of infants after 6 weeks of age, is immunogenic, well-tolerated, and effective in preventing severe RVGE.Citation4-11
Since the first rotavirus infection in infants is generally the most severe, it is important to understand regional trends in the timing of that experience of first symptomatic RVGE episode in order to identify the optimal age for rotavirus vaccination. This paper presents a post-hoc analysis, which descriptively compares the incidence of RVGE at different timepoints over the first two years of life in low- to middle-income countries (Africa) with that in high-income countries (Europe and high-income Asian regions). The analysis was based on data from three randomized, placebo-controlled, multicentre efficacy trials on HRV, conducted in Europe (NCT00140686), Africa (NCT00241644), and Asia (NCT00197210 and NCT00329745), which have been previously presented.Citation9,12-17
Results
Age at first vaccination
The majority of children received their first HRV or placebo dose at the age of 2 months in Europe (≥65.87%), 1 months in Africa (≥93.82%), and 3 months in Asia (≥58.76%) ().
Table 1. Age of the children at the time of first vaccination in the European, African and Asian studies (total vaccinated cohort).
Incidence of any RVGE episodes by age
In Europe, peaks in incidence of RVGE of similar magnitudes were observed during the first (2.03% at 9–10 months of age) and second (2.00% at 20–21 months of age) years of life among unvaccinated children (). In children who had received two doses of HRV, incidence of any RVGE episodes remained low during the entire study (range: 0.00–0.52%).
Figure 1 Incidence of any rotavirus gastroenteritis cases by age at onset in (A) Europe and (B) Africa Footnote: Data obtained from the total vaccinated cohort. D = dose; HRV = human rotavirus vaccine.
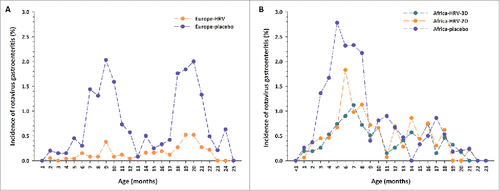
In Africa, incidence of any RVGE in unvaccinated children showed a peak in the first year of life (2.78% at 5 months of age) and was lower (range: 0.0–0.86%) in the second year of life (). The incidence of any RVGE during the first year of life peaked at 1.83% at 6 months of age in children who had received two doses of HRV and at 1.12% at 7 months of age in children who had received three doses of HRV.
In unvaccinated children in the African study, differences in terms of incidence of any RVGE were observed between countries. In South Africa, two peaks were observed: a main peak in the first year of life and a lower peak in the second year of life. In Malawi, where rotavirus is known to circulate year-round, data were more variable, but there was still a trend for higher incidence of any RVGE during the first year of life.
Comparisons between studies suggest that the incidence of any RVGE in unvaccinated children was 40% higher in Africa than in Europe in the first year of life, with a higher incidence of early RVGE (2–5 months of age). In the second year of life, the incidence of any RVGE was still high in Europe, but was much lower in Africa. The incidence of any RVGE tended to be lower in European children who had received two doses of HRV than in African children who had received two or three doses of HRV, especially during the first year of life.
Incidence of severe RVGE episodes by age
In general, the temporal profile for severe RVGE episodes (a score of ≥11 in the 20-point Vesikari scale) was similar to that for any RVGE episode.
In Europe, peaks in incidence of severe RVGE were observed in the first (1.23% at 9 months of age) and second (1.13% at 18 months of age) years of life among unvaccinated infants reflecting the cyclic nature of rotavirus infection in Europe where rotavirus circulates predominantly during the winter months ().
Figure 2 Incidence of severe rotavirus gastroenteritis cases by age at onset in (A) Europe (B) Africa and (C) Asia Footnote: Data obtained from the total vaccinated cohort. D = dose; HRV = human rotavirus vaccine.
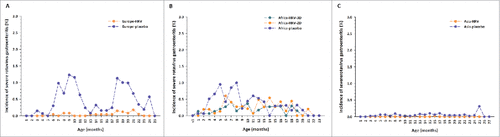
In Africa, the peak in incidence of severe RVGE observed during the first year among unvaccinated infants (1.00% at 8 months of age) was less evident than that observed for any RVGE ().
The incidence of severe RVGE in unvaccinated children seemed comparable in Africa and in Europe in the first year of life, but tended to be higher in Europe than in Africa during the second year of life. Moreover, first severe RVGE episodes seemed to occur at a younger age in African children. The incidence of severe RVGE was low in vaccinated children in both the European and the African study.
In Asia, the incidence of severe RVGE during the first two years of life was low among both unvaccinated (range: 0.00–0.31%) and vaccinated (range: 0.00–0.02%) children ().
Discussion
To maximise their impact, effective vaccines against rotavirus should be administered early in life and before exposure to the first symptomatic natural infection, which is usually the most severe. Since rates of exposure to rotavirus vary by geographic region and socio-economic situation, it is important to understand the incidence of RVGE and the age at which symptomatic infection occurs in both low- to middle- and high-income countries.
This analysis showed that the burden of any RVGE was higher in young children from low- to middle-income African compared to high-income European countries during the first year of life. Moreover, the peak age of disease was observed in younger children in Africa versus Europe, in line with previously reported data.Citation3,18 While the incidence of any RVGE in unvaccinated children between 2 and 6 months of age was approximately two times higher in Africa than in Europe, the opposite tendency was observed during the second year of life, highlighting the earlier symptomatic infection in Africa. The lower incidence of any RVGE in the second year of life in Africa (≤0.86%) could be explained by the higher rate of natural immunity to wild-type infection, which is acquired at a younger age in unvaccinated infants from low- to middle-income vs. high-income countries.Citation19 These results suggest that early universal primary immunization may be useful in Europe and in Africa, while the benefits of any booster dose should be evaluated in future studies.
In Europe and Africa, the age-distribution of severe RVGE was similar to that of any RVGE: incidence was higher in Africa than in Europe during the first 2–6 months of age and decreased during the second year of life in Africa, while peaks of incidence of similar magnitude were observed during the first and second years in Europe. However, in contrast with observations made for any RVGE, peaks of incidence of severe RVGE seemed higher in both the first and second years of life in Europe compared to Africa. A potential explanation for this observation could be that the really early cases (before two months of age) are not captured in these studies; this hypothesis is supported by the high IgA positivity rates in Malawi at enrolment, demonstrating early exposure to wild-type infection.Citation12 The lower incidence of severe RVGE in Africa could also be due to differences in intensity of surveillance between sites, which may have impacted the detection rate. To underscore whether this is due to differences in case ascertainment specific to RVGE or more generalizable, analyses in terms of all-cause gastroenteritis are needed.
The incidence of severe RVGE observed in Asia ranged between 0.00% and 0.31% in both vaccinated and unvaccinated children; the low incidence observed in these three high-income regions may not correctly reflect the situation in the rest of Asia.Citation15 Indeed, a sub-analysis of the data from Hong Kong showed that 1.5% (23/1499) of placebo- and 0.1% (1/1494) of vaccine-recipients had severe RVGE during the first two years of life.Citation13 Moreover, in a previous hospital-based surveillance conducted between 2005 and 2008 in Singapore, the annual incidence of RVGE hospitalisations (per 1000 Person-year) was 5.40 (95% CI: 4.65, 6.23) in children <1 year of age and 5.77 (95% CI: 5.21, 6.37) in children <2 years of age, with yearly peaks between January and March.Citation20 The incidence of severe RVGE was also significantly higher in a previous study conducted in China, where 4.8% (95% CI: 3.8, 5.9) of unvaccinated children had reported an episode of severe RVGE during the first two years of life.Citation21 The low incidence in the Asia study could reflect differences in ascertainment, particularly in the Singapore sample.
The comparison of results obtained in unvaccinated and vaccinated children in the low- to middle-income country setting indicates that early vaccination against rotavirus could decrease the RVGE burden in the first year of life, when infants are the most vulnerable to the rapid onset and outcomes of symptomatic disease. This is in line with results of previous studies.Citation5-7,9-11 The WHO recommends the completion of the immunisation schedule early in infancy; this should especially be implemented in low- to middle-income countries, where disease burden is high and occurs early.Citation2 A previous analysis showed that >30% of children receive their childhood vaccines later than recommended in most low- and middle-income countries.Citation22 Unfortunately, this puts the infants in the highest burden settings at an added disadvantage. Improved timing of childhood immunisations, along with rotavirus vaccine, and strengthening of health care systems in developing countries is urgently needed, as suggested by data from numerous epidemiology studies in low- to middle-income countries.Citation12,23,24
Regarding the timing of vaccination, initiating immunisation during the neonatal period could provide early protection and would likely reduce the risk of intussusception.Citation3,25 Previous studies showed that vaccination with a rhesus rotavirus-based tetravalent vaccine during the neonatal period triggered good immune responses and demonstrated efficacy during the first year of life.Citation26,27 Moreover, vaccination with the neonatal RV3 strain during the first 7 days of life triggered immune responses similar to the conventional immunisation schedule.Citation28 However, before neonatal immunisation can be recommended, the actual timing for first dose and the protection of the vaccine against disease later in life should be evaluated. However, it has been suggested that these orally administered, live attenuated vaccines might be associated with a waning of immunity. We know that re-infection in developing countries is common and is, in general, associated with milder disease.Citation19,29,30 However, a recent cohort study in India has shown that infants can be symptomatically infected multiple times, even with a similar strain.Citation19 If the waning of immunity is true, then a neonatal schedule is unlikely to be the solution to long-term protection into the second year of life. Furthermore, we may need to consider alternate immunisation strategies for rotavirus vaccines that give early protection against severe symptomatic infection in the first few months of life, and longer term protection when children are still vulnerable to the disease.
This analysis has several strengths, such as the facts that episodes of any and severe RVG were identified by active surveillance, captured using a clinical definition that focused on hospitalization and rehydration, and graded with the use of the validated 20-point Vesikari scale. Moreover, we compared results from three placebo controlled trials with the same endpoints and including children aged between 6 and 20 weeks. This study has several limitations, such as the fact that it was a retrospective analysis, differences in case ascertainment might have occurred between sites, really early cases of RVGE were not captured because they were excluded per protocol within the primary evaluation, and duration of the surveillance during the first year of life was different in each study due to variability in age at first dose. Moreover, analysis of data by child-year of exposition would be needed to address the question of the importance of seasonality. Indeed, as previously reported for regions with a temperate climate, a clear seasonality of the rotavirus epidemiology, with a peak of incidence during the winter, was observed in Europe and South Africa,Citation2 although rotavirus does circulate year round in South Africa, albeit at a lower level.Citation31,32 In contrast, no peak of incidence was observed in Malawi, where the climate is sub-tropical, and in Asia, where the incidence of RVGE remained low during the entire study. In Europe and South-Africa, all infants were recruited within a limited age range, and most of them shortly before the start of rotavirus season. Age at the time of RVGE episodes should therefore not be completely dissociated from the exposure time to rotavirus. Finally, this study was also limited by the the low incidence of severe RVGE in Asia, which may be partly due to a lower case ascertainment in Singapore, where 61.1% of participants were enrolled. Other contributing factors could be the facts that (i) in these three high-income regions the age of onset of rotavirus diarrhea may be shifted to an older age, (ii) rotavirus incidence peaks every few years in these regions with periods of relatively low rotavirus circulation between the peaks, and (iii) admissions to private hospitals might have been missed by the computerised system used in this trial.
In conclusion, this study showed that the incidence of any RVGE episodes in unvaccinated children was higher in Africa than in Europe during the first year of life, while that of severe RVGE seemed comparable in both regions. During the second year of life, the incidence of any or severe RVGE in placebo-recipients was lower in Africa. In high-income regions of Asia, the incidence of severe RVGE remained very low during the first two years of life. Our results confirmed that vaccination against rotavirus should be given early in infancy to all children, in particular those living in low- and middle-income countries, to reduce the burden of rotavirus disease.
Materials and methods
Study designs
We undertook a post-hoc analysis based on data from three randomized, placebo-controlled, multicentre efficacy trials on HRV, which were conducted in Europe (NCT00140686), Africa (NCT00241644), and Asia (NCT00197210 and NCT00329745). The results of the primary study objectives, and the inclusion and exclusion criteria of the study participants have been previously presented.Citation9,12-17
In the European study, which was conducted between September 2004 and August 2006, healthy infants aged 6–14 weeks from the Czech Republic, Finland, France, Germany, Italy, and Spain were randomized (2:1) to receive two doses of HRV (N = 2646) or placebo (N = 1348) co-administered with routine childhood vaccines according to the national plan of each country. Infants were enrolled between 08 September 2004 and 01 February 2005; 3271 infants (82%) received the first dose of HRV or placebo before the beginning of the annual rotavirus epidemic season, and 723 infants (18%) received the first dose during the first rotavirus epidemic season (from December to May).Citation9
In the African study, which was conducted between October 2005 and January 2009, healthy infants aged 5–10 weeks from South Africa and Malawi were randomized (1:1:1) to receive three doses of HRV (N = 1651), a placebo dose followed by two doses of HRV (N = 1647), or three doses of placebo (N = 1641) at 6, 10, and 14 weeks of age. In South Africa, infants were enrolled from October 2005 through January 2006 (cohort 1) and from November 2006 through early February 2007 (cohort 2), and were vaccinated before the anticipated rotavirus seasons (winter–spring peak).Citation31 In Malawi, where rotavirus is known to circulate year-round, infants were enrolled between October 2006 and July 2007.Citation14
In the Asian study, which was conducted between December 2003 and July 2008, healthy infants aged 6–12 weeks from Hong Kong and Taiwan or aged 11–17 weeks from Singapore were enrolled between December 2003 and August 2005, and were randomized (1:1) to receive two doses of HRV (N = 5250) or placebo (N = 5250) co-administered with routine childhood vaccines according to each local country regulation. Infants were continuously enrolled and vaccinated before, during, and after rotavirus seasons.
The three studies were conducted in accordance with Good Clinical Practice guidelines, the Declaration of Helsinki, and local rules and regulations of the countries. Parents/guardians of the participating infants provided written consent before any study-related procedure. The study protocols and related documents were approved by the ethics committee of the individual study centers.
Analysis of the incidence of rotavirus gastroenteritis and clinical severity.
This post-hoc analysis used data collected between the time of first dose administration and the end of follow-up in the second year of life.
RVGE episodes were assessed through active follow-up surveillance. In Europe, weekly or bi-weekly contacts were made with parents/guardians to inquire about the occurrence of gastroenteritis, and any gastroenteritis-related medical care, advice and hospitalisation. In Africa, active surveillance for gastroenteritis episodes was done through weekly visits from the day of first dose until one year of age for children in cohort 1 in South Africa, and until approximately two years of age for children in cohort 2 in South Africa and all children in Malawi. In Asia, where only episodes of severe RVGE were recorded, hospital/medical facility surveillance was put in place in the study area to ensure that all gastroenteritis cases requiring hospitalisation or re-hydration therapy in a medical facility were captured. In addition, weekly or bi-weekly contacts were made with parents/guardians/caretakers to determine whether there had been any admissions to hospitals.
An episode of gastroenteritis was defined as the occurrence of diarrhea (≥3 looser than normal stools within a 24 hour period) with or without vomiting. If there was an interval of ≥5 symptom-free days between two gastroenteritis episodes, they were considered as different episodes. Stool samples were collected during each episode of gastroenteritis and were analyzed for rotavirus using enzyme-linked immunosorbent assay (ELISA; RotaClone™ assay, Meridian Biosciences, USA).
For each suspected gastroenteritis episode in the European and African studies, and each severe gastroenteritis episode in the Asian study, a diary card was completed daily. The following information was recorded: axillary/rectal temperature, number of vomiting episodes and looser than normal stools, rehydration or other medication given during the gastroenteritis episode, medical attention sought, and behavioral symptoms.
The severity of RVGE episodes was assessed using the 20-point Vesikari scale, where a score of ≥11 was considered as severe.Citation33 The incidence of RVGE was calculated as the number of children with RVGE multiplied by 100 and divided by the number of children at risk. The incidence of any and severe RVGE was evaluated in the different geographical regions by age at onset over the first two years of life. The results are presented per age group: children aged 1 – <2 months at onset were included in the 1 month age group, children aged 2 – <3 months at onset in the 2 months age group, etc.
Statistical analysis
All analyses were performed on the total vaccinated cohorts, including children who received ≥1 dose of HRV or placebo. All comparisons of RVGE incidence between the different regions and age groups were descriptive.
Trademarks
Rotarix is a trademark of the GSK group of companies.
Rotateq is a trademark of Merck and Co., USA.
RotaClone is a trademark of Meridian Bioscience, Inc.
Abbreviations
| CI | = | Confidence Interval |
| ELISA | = | enzyme-linked immunosorbent assay |
| HRV | = | human rotavirus vaccine |
| IgA | = | immunoglobulin A |
| RVGE | = | rotavirus gastroenteritis |
| WHO | = | World Health Organization |
Disclosure of potential conflicts of interest
Authors KBP, LMH, EASN, FSL, NAC, SAM and YLL declare to have received institutional funding/grants from the GSK group of companies for the conduct of Rota trials. NAC is member of the GSK Rotavirus vaccine Advisory Panel. LMH and SAM declare to have received personal funding/grants and consulting fees or honorarium from the GSK group of companies. SAM, EASN and TV declare to have received payment for lectures in the past from GSK group of companies. FSL declares to have received grants and support for travel for trial related matters, presentations and conferences from GSK group of companies. Authors BB, HHH, SD and NK are employees of the GSK group of companies. BB and HHH also hold stock options from the sponsoring company. ADS declares no conflict of interest.
Author contributions
EASN, FSL, KBP, LMH, NAC, SAM, TV and YLL were principal investigators that participated in the study. BB, NAC, ADS, FSL, HHH, LMH, NK, KBP, SD, SAM, TV and YLL contributed to the conception, design and planning of the study. NK was the statistician. ADS, KBP, EASN and YLL participated in the recruitment of centers and investigators. All authors participated in the design or implementation or analysis, and interpretation of the study; and the development of this manuscript. All authors had full access to the data, gave final approval before submission and are accountable for all aspects of the work.
Acknowledgments
The authors thank Claire Verbelen and Quentin Godechal (XPE Pharma and Science, on behalf of GSK Vaccines) for writing support and incorporation of comments received from the authors and Manjula K (employee of the GSK group of companies) and Angeles Ceregido (XPE Pharma and Science, on behalf of GSK Vaccines) for publication management.
Funding
This study was sponsored and funded by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis; and also took charge of all costs associated with the development and the publishing of the manuscript.
References
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. Network WH-cGRS. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136-41; PMID:22030330; http://dx.doi.org/10.1016/S1473-3099(11)70253-5
- World Health Organization. Rotavirus vaccines. WHO position paper - January 2013. Wkly Epidemiol Rec 2013; 88:49-64
- World Health Organization. Rotavirus vaccines. Wkly Epidemiol Rec 2007; 82:285-95; PMID:17691162
- Dennehy PH, Brady RC, Halperin SA, Ward RL, Alvey JC, Fischer FH, Jr, Innis BL, Rathfon H, Schuind A, De Vos B, et al. Comparative evaluation of safety and immunogenicity of two dosages of an oral live attenuated human rotavirus vaccine. Pediatr Infect Dis J 2005; 24:481-8; PMID:15933555; http://dx.doi.org/10.1097/01.inf.0000164763.55558.71
- Phua KB, Emmanuel SC, Goh P, Quak SH, Lee BW, Han HH, Ward RL, Bernstein DI, De Vos B, Bock HL. A rotavirus vaccine for infants: the Asian experience. Ann Acad Med Singapore 2006; 35:38-44; PMID:16470273
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11-22; PMID:16394298; http://dx.doi.org/10.1056/NEJMoa052434
- Salinas B, Perez Schael I, Linhares AC, Ruiz Palacios GM, Guerrero ML, Yarzabal JP, Cervantes Y, Costa Clemens S, Damaso S, Hardt K, et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: A randomized, placebo-controlled trial in Latin American infants. Pediatr Infect Dis J 2005; 24:807-16; PMID:16148848; http://dx.doi.org/10.1097/01.inf.0000178294.13954.a1
- Vesikari T, Karvonen A, Korhonen T, Espo M, Lebacq E, Forster J, Zepp F, Delem A, De Vos B. Safety and immunogenicity of RIX4414 live attenuated human rotavirus vaccine in adults, toddlers and previously uninfected infants. Vaccine 2004; 22:2836-42; PMID:15246619; http://dx.doi.org/10.1016/j.vaccine.2004.01.044
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370:1757-63; PMID:18037080; http://dx.doi.org/10.1016/S0140-6736(07)61744-9
- Vesikari T, Karvonen A, Puustinen L, Zeng SQ, Szakal ED, Delem A, De Vos B. Efficacy of RIX4414 live attenuated human rotavirus vaccine in Finnish infants. Pediatr Infect Dis J 2004; 23:937-43; PMID:15602194; http://dx.doi.org/10.1097/01.inf.0000141722.10130.50
- Linhares AC, Velazquez FR, Perez-Schael I, Saez-Llorens X, Abate H, Espinoza F, Lopez P, Macias-Parra M, Ortega-Barria E, Rivera-Medina DM, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet 2008; 371:1181-9; PMID:18395579; http://dx.doi.org/10.1016/S0140-6736(08)60524-3
- Cunliffe NA, Witte D, Ngwira BM, Todd S, Bostock NJ, Turner AM, Chimpeni P, Victor JC, Steele AD, Bouckenooghe A, et al. Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: a randomized, double-blind, placebo controlled trial. Vaccine 2012; 30 Suppl 1:A36-43; http://dx.doi.org/10.1016/j.vaccine.2011.09.120
- Lau YL, Nelson EA, Poon KH, Chan PK, Chiu S, Sung R, Leung CW, Ng D, Ma YM, Chan D, et al. Efficacy, safety and immunogenicity of a human rotavirus vaccine (RIX4414) in Hong Kong children up to three years of age: a randomized, controlled trial. Vaccine 2013; 31:2253-9; PMID:23499605; http://dx.doi.org/10.1016/j.vaccine.2013.03.001
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289-98; PMID:20107214; http://dx.doi.org/10.1056/NEJMoa0904797
- Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, Lee BW, Teoh YL, Tang H, Boudville I, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine 2009; 27:5936-41; PMID:19679216; http://dx.doi.org/10.1016/j.vaccine.2009.07.098
- Steele AD, Neuzil KM, Cunliffe NA, Madhi SA, Bos P, Ngwira B, Witte D, Todd S, Louw C, Kirsten M, et al. Human rotavirus vaccine Rotarix provides protection against diverse circulating rotavirus strains in African infants: a randomized controlled trial. BMC Infect Dis 2012; 12:213; PMID:22974466; http://dx.doi.org/10.1186/1471-2334-12-213
- Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, Lee BW, van Doorn LJ, Teoh YL, Tang H, et al. Rotavirus vaccine RIX4414 efficacy sustained during the third year of life: a randomized clinical trial in an Asian population. Vaccine 2012; 30:4552-7; PMID:22497874; http://dx.doi.org/10.1016/j.vaccine.2012.03.030
- Bresee JS, Hummelman E, Nelson EA, Glass RI. Rotavirus in Asia: the value of surveillance for informing decisions about the introduction of new vaccines. J Infect Dis 2005; 192 Suppl 1:S1-5; PMID:16088790; http://dx.doi.org/10.1086/431515
- Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J, Sarkar R, Rehman AM, Jaffar S, Gomara MI, Gray JJ, Brown DW, et al. Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med 2011; 365:337-46; PMID:21793745; http://dx.doi.org/10.1056/NEJMoa1006261
- Phua KB, Tee N, Tan N, Ramakrishnan G, Teoh YL, Bock H, Liu Y. A hospital-based surveillance of rotavirus gastroenteritis in children. Pediatr Infect Dis J 2013; 32:e426-31; PMID:23958814; http://dx.doi.org/10.1097/INF.0b013e31829f2cb0
- Li RC, Huang T, Li Y, Luo D, Tao J, Fu B, Si G, Nong Y, Mo Z, Liao X, et al. Human rotavirus vaccine (RIX4414) efficacy in the first two years of life: a randomized, placebo-controlled trial in China. Hum Vaccin Immunother 2014; 10:11-8; PMID:24013441; http://dx.doi.org/10.4161/hv.26319
- Clark A, Sanderson C. Timing of children's vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet 2009; 373:1543-9; PMID:19303633; http://dx.doi.org/10.1016/S0140-6736(09)60317-2
- Cunliffe NA, Ngwira BM, Dove W, Thindwa BD, Turner AM, Broadhead RL, Molyneux ME, Hart CA. Epidemiology of rotavirus infection in children in Blantyre, Malawi, 1997-2007. J Infect Dis 2010; 202 Suppl:S1 68-74.
- Giaquinto C, van Damme P, Group RS. Age distribution of paediatric rotavirus gastroenteritis cases in Europe: the REVEAL study. Scand J Infect Dis 2010; 42:142-7; PMID:19916900; http://dx.doi.org/10.3109/00365540903380495
- Ruuska T, Vesikari T, Delem A, Andre FE, Beards GM, Flewett TH. Evaluation of RIT 4237 bovine rotavirus vaccine in newborn infants: correlation of vaccine efficacy to season of birth in relation to rotavirus epidemic period. Scand J Infect Dis 1990; 22:269-78; PMID:2164706; http://dx.doi.org/10.3109/00365549009027047
- Armah GE, Kapikian AZ, Vesikari T, Cunliffe N, Jacobson RM, Burlington DB, Ruiz LP, Jr. Efficacy, immunogenicity, and safety of two doses of a tetravalent rotavirus vaccine RRV-TV in Ghana with the first dose administered during the neonatal period. J Infect Dis 2013; 208:423-31; PMID:23599316; http://dx.doi.org/10.1093/infdis/jit174
- Vesikari T, Karvonen A, Forrest BD, Hoshino Y, Chanock RM, Kapikian AZ. Neonatal administration of rhesus rotavirus tetravalent vaccine. Pediatr Infect Dis J 2006; 25:118-22; PMID:16462287; http://dx.doi.org/10.1097/01.inf.0000199288.98370.71
- Danchin M, Kirkwood CD, Lee KJ, Bishop RF, Watts E, Justice FA, Clifford V, Cowley D, Buttery JP, Bines JE. Phase I trial of RV3-BB rotavirus vaccine: a human neonatal rotavirus vaccine. Vaccine 2013; 31:2610-6; PMID:23597719; http://dx.doi.org/10.1016/j.vaccine.2013.04.008
- Fischer TK, Valentiner-Branth P, Steinsland H, Perch M, Santos G, Aaby P, Molbak K, Sommerfelt H. Protective immunity after natural rotavirus infection: a community cohort study of newborn children in Guinea-Bissau, West Africa. J Infect Dis 2002; 186:593-7; PMID:12195345; http://dx.doi.org/10.1086/342294
- Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med 1996; 335:1022-8; PMID:8793926; http://dx.doi.org/10.1056/NEJM199610033351404
- Steele AD, Alexander JJ, Hay IT. Rotavirus-associated gastroenteritis in black infants in South Africa. J Clin Microbiol 1986; 23:992-4; PMID:3711291
- Steele AD, Peenze I, de Beer MC, Pager CT, Yeats J, Potgieter N, Ramsaroop U, Page NA, Mitchell JO, Geyer A, et al. Anticipating rotavirus vaccines: epidemiology and surveillance of rotavirus in South Africa. Vaccine 2003; 21:354-60; PMID:12531632; http://dx.doi.org/10.1016/S0264-410X(02)00615-1
- Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis 1990; 22:259-67; PMID:2371542; http://dx.doi.org/10.3109/00365549009027046
