ABSTRACT
Influenza B strains represent on average 23% of all circulating strains in Europe and when there is a vaccine mismatch on B strains, additional influenza-related hospitalizations and deaths as well as substantial additional costs are observed. The objective was to estimate the public health and economic impact of seasonal influenza vaccination with quadrivalent influenza vaccines (QIV) compared to trivalent influenza vaccines (TIV) in Europe (EU).
Based on data from 5 EU countries (France, Germany, Italy, Spain and UK) during 10 influenza seasons from 2002 to 2013, epidemiological and associated economic outcomes were estimated for each season for the actual scenario where the TIV was used, and for a hypothetical scenario where QIV could have been used instead.
By using QIV, this study estimated that for the 5 EU countries, an additional 1.03 million (327.9/100,000 inhabitants) influenza cases, 453,000 (143.9/100,000) general practitioners consultations, 672,000 (213.1/100,000) workdays lost, 24,000 (7.7/100,000) hospitalizations and 10,000 (3.1/100,000) deaths could have been avoided compared to the use of TIV over the 10-seasons-period. This study estimates that QIV can be of economic value since from a societal perspective 15 million Euros would have been saved on general practitioners consultations (14 million Euros from third-party payer perspective), 77 million on hospitalizations (74 million Euros from third-party payer perspective) and 150 million Euros on workdays lost, across the 5 EU countries.
In conclusion, the present study estimates that, compared to TIV, QIV may result in a substantial decrease in epidemiological burden and in influenza-related costs.
Introduction
Influenza is a highly infectious viral illness causing significant morbidity and mortality among high risk populations, including the elderly,Citation1,2 pregnant women,Citation3 children,Citation4 persons with specific underlying health conditions Citation5 and healthcare workers.Citation6 Annual epidemics result in about 250,000 to 500,000 deaths worldwideCitation7 and an average of 38,500 estimated deaths in EuropeCitation8 but with considerable season-to-season variation. Influenza occurs globally with an annual attack rate estimated between 5%–10% in adults and between 20%–30% in children.Citation7
Mild and moderate influenza cases have a substantial socioeconomic impact in terms of medical care, healthcare utilization (e.g. increase in consultations, hospitalizations and length of stay) and work absenteeism. The total estimated direct and indirect costs of an influenza epidemic in high income countries may reach €56.7 million per million people.Citation9 The cost of primary care physician visits due to influenza for all EU 25 countries in 2005 was estimated at €267.2 million and the cost of hospital visits at €11.5 billion.Citation10 In Europe, influenza is responsible for approximately 10% of sickness related absence from work,Citation11 while the cost of lost productivity due to influenza in France and Germany alone has been estimated at between €6.4 billion to €9.8 billion per year.Citation12
Vaccination is currently the most effective means of preventing influenza infection. Since the adoption of the EU council recommendation on seasonal flu vaccination in 2009,Citation13 almost all member states have national and/or regional vaccination policies for seasonal influenza that typically target groups with higher susceptibility to infection and greater risk of complications. Several types of influenza vaccine are available in the EU, including live-attenuated or inactivated formulations with or without adjuvant, offering the option of intramuscular, nasal or intradermal delivery routes and now also containing either 3 (TIV) or 4 (QIV) strains. The specific strains to be included in seasonal influenza vaccines are predicted annually by the WHO. Trivalent vaccines which include 2 strains of influenza A (H1N1 and H3N2) and one lineage of the influenza B virus (Yamagata or Victoria) are currently the most widely used flu vaccines in Europe.
Surveillance data show that B strains represent on average 23% (1% to 60%) of all circulating strains in Europe.Citation1 The Yamagata and Victoria lineages of influenza B co-circulate during each influenza season in Europe since 2001,Citation14-17 with one lineage dominating the other in many seasons. Predicting which lineage will predominate can be challenging, and in some seasons, the lineage chosen for the vaccine has differed from the predominant circulating influenza B virus lineage, as it has been reported in Europe and the US.Citation1,18
When there is a vaccine mismatch, the responses against the heterologous B virus are significantly reduced which decreases the expected benefits from the vaccines,Citation19 leading to increased influenza-related hospitalizations and deaths,Citation20 and resulting in substantial additional costs associated with influenza BCitation21 across all age groups.
Consequently, quadrivalent influenza vaccines (QIV) that include a second influenza B strain have been developed, and are expected to be increasingly available in Europe over the following years. By minimizing the possible mismatch of vaccine and disease-causing strains, QIV alleviates the unpredictability of B strain circulation and contributes to control of infections caused by influenza B.
In 2012, the public health impact that QIV would have had on influenza-related health outcomes over 10 influenza seasons, had QIV been used instead of TIV in the United States had been estimated.Citation22 The authors concluded that additional protection provided by QIV including a second lineage of influenza B could have resulted in the reduction of influenza-associated outcomes. Generalization of these results to other countries is possible but it is unclear how much additional public health benefit would be gained in Europe by including a second influenza B strain in the vaccine.
The objective of this study was to retrospectively estimate the public health impact that QIV could have potentially had on influenza-related health outcomes over 10 previous influenza seasons, if QIV had been used instead of TIV in Europe.
Results
Epidemiological burden averted
The additional epidemiological burden averted by the use of QIV instead of TIV is presented in . For the 5 EU countries over the 10-seasons period, the total number of additional cases avoided by the use of QIV instead of TIV was 1 million (327.9/100,000), distributed from 150,000 in Spain to 230,000 in Italy and and UK. A total of 670,000 (213.1/100,000) workdays lost could be additionally avoided by the use of QIV instead of TIV, distributed from 19,000 in Spain to 250,000 in Germany. A total of 450,000 (143.9/100,000) additional GP consultations could also be additionally avoided by QIV compared to TIV at the EU-5 level. Total EU-5 hospitalizations and deaths due to influenza additionally avoided by the use of QIV were 24,000 (7.7/100,000) and 10,000 (3.1/100,000) respectively.
Table 5. Proportions (%) of influenza A and B (B Victoria and B Yamagata) strains circulating in the 5-EU countries by influenza season between 2002 and 2013. For France, Germany, Italy and Spain, data from came from ref. Citation42 for the 2002–03 season, from ref. Citation43 for the 2012–13 season and from ref. Citation1 for all other seasons. For UK, data came from ref. Citation26 for the 2003–03 to 2008–09 seasons, from ref. Citation1 for the 2010–11 and 2011–12 seasons and from ref. Citation43 for the 2012–13 season.
Table 6. Influenza vaccination coverage (%) by country, age and risk group for EU-5.
Table 7. Vaccination efficacy/effectiveness against influenza A, matched influenza B and mismatched influenza B used in the model. The lower and upper bound correspond to a variation of +/−20% in vaccine effectiveness. Cross protection was included in the model by considering that VE against mismatched B was 67% of the VE against matched B.
Table 1. Absolute number and incidence per 100,000 inhabitants of influenza cases, GP consultations, workdays lost, hospitalizations and deaths due to influenza avoided for the 5 EU countries for the 2002–03 to 2012–13 influenza seasons. Epidemiological burden avoided are presented with the lower and upper bound corresponding to a variation of +/−20% in vaccine effectiveness.
presented the additional epidemiological burden averted by the use of QIV instead of TIV, by age-group for the 5 EU countries. Most of the cases (393,270), GP consultations (142,902), hospitalizations (21,151) and deaths additionally avoided (9,391) by the use of QIV instead of TIV were in the 65+ age group. Incidence results indicates that the greater impact would be for 6 m-< 2 yrs and 65+ extreme age groups with 604.0 and 663.1 additionally avoided cases per 100,000 respectively. Also, most workdays lost additionally avoided by QIV were found in the 18–49 and 50–64 y population with 673.9 and 680.9 additionally avoided workdays lost per 100,000 respectively.
Table 2. Epidemiological burden averted (absolute numbers and incidence per 100,000 inhabitants) by age-group for the 5 EU countries for the 2002–03 to 2012–13 influenza seasons. Epidemiological burden avoided are presented with the lower and upper bound corresponding to a variation of +/−20% in vaccine effectiveness.
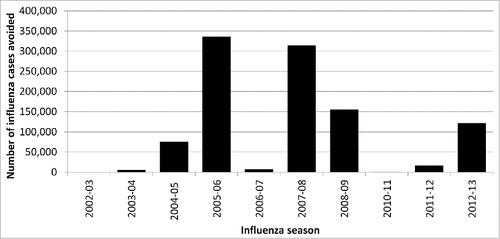
The number of influenza cases additionally avoided by the use of QIV instead of TIV is depicted in according to seasons. The figure demonstrates a great variability according to seasons with less than 1,000 cases additionally avoided by QIV for the 2002–03 and 2010–11 seasons compared to more than 300,000 cases avoided during the 2005–06 and 2007–08 seasons.
Figure 1. Influenza cases avoided per season for the 5 EU countries if QIV vaccine was used instead of a TIV vaccine during the 2002–03 to 2012–13 influenza seasons.
Extrapolation of the results to the 27 EU countries led to a total number of additional avoided cases by QIV of 1.6 million (range of variation for −/+20% of vaccine effectiveness: 1.3 million; 1,9 million], mostly in the 65+ age group with 600,000 [480,000;710,000]. Corresponding results for the number of additional deaths avoided by the use of QIV instead of TIV were 15,000 [11,000; 19,000] for the 27 EU countries, with 14,000 [11,000; 19,000] for the 65+ age group.
Economic burden averted
The total economic burden additionally averted by the use of QIV instead of TIV from the TPP and SP was €87.2 million and €241.4 million, respectively. Details for GP costs, hospitalizations costs and workdays lost costs by countries is presented in . From the TPP, most of total costs additionally avoided by QIV came from the 65+ age group while from the SP, costs additionally avoided by QIV were uniformly distributed across all age groups (), due to the substantial impact of the additional avoided workdays lost (Fig. S1). Indeed, 84% of costs avoided are hospital costs in the TPP perspective, while 63% of costs avoided are workdays saved costs in the SP.
Table 3. Total costs for GP, hospitalizations and workdays lost avoided for the 5 EU countries for the 2002–03 to 2012–13 influenza seasons.
Table 4. Total costs avoided for the 5 EU countries according to age and risk groups, and perspectives in million Euros for the 2002–03 to 2012–13 influenza seasons.
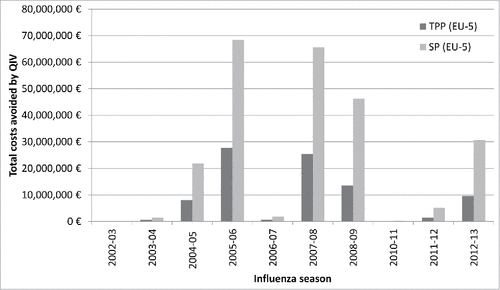
shows that the total costs additionally avoided by QIV instead of TIV for the 5 EU countries varied greatly according to the influenza season. But in 2005–06 and 2007–08, total costs additionally avoided could have been considerable, if QIV had been used instead of TIV, with more than €25 million and more than €65 million additionally avoided for the TPP and SP respectively.
Figure 2. Total costs avoided if QIV vaccine was used instead of a TIV vaccine according to TPP and SP perspectives for the 5 EU countries for the 2002–03 to 2012–13 influenza seasons.
Extrapolation to the 27 EU countries led to a total number of additional avoided costs by QIV of €133.6 million [€100.2; €171.8] for the TPP, mostly in the 65+ age group with €103.7 million [€77.1; €134.7]. For the SP, the total cost additionally avoided by QIV was €381.3 million [€292.9; €478.0] mainly distributed in the 18–64 age group with €232.9 million [€180.7; €288.7].
Sensitivity analysis
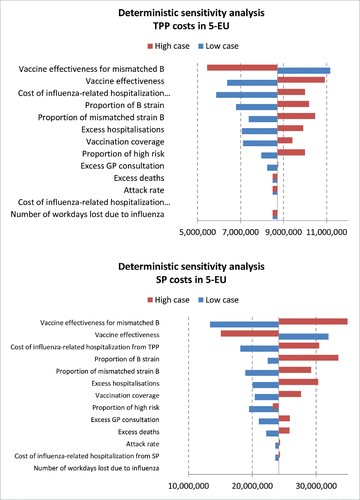
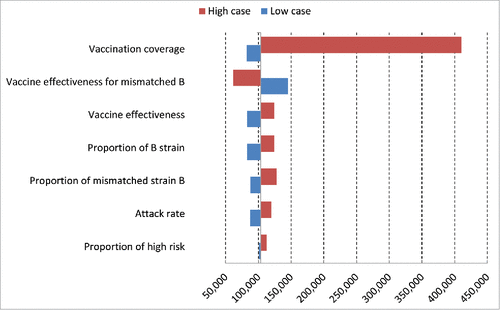
Results of the sensitivity analysis are presented in . For avoided cases estimation per season (), the most influential parameter was the vaccine coverage (from 82,437 cases in the low case scenario to 410,278 in the high case scenario), followed by the vaccine effectiveness for mismatched B strain, increasing the number of influenza-related cases up to 145,371 in the low case scenario and decreasing this outcome to 61,575 in the high case scenario, corresponding to a variation of over a third around the base case amount of influenza cases (103,473).
Figure 3. Deterministic sensitivity analysis represented by tornado charts reports the impact of the parameters on the number of influenza cases per season in EU-5.
From the TPP perspective economic results () were most sensitive to the vaccine effectiveness for the mismatched B strain, with the average savings per season related to the use of the QIV increasing up to €11.2 million for low vaccine effectiveness for the mismatched B strain and decreasing to €5.4 million for high vaccine effectiveness on mismatched B strain. The main driver for SP () were the number of workdays lost per influenza-related consultation with the average savings per season implied by the recourse to QIV instead of TIV increasing up to €34,9 million for high values of this parameter and decreasing to €13,4 million with low values. Results were then most sensitive to the vaccine effectiveness both overall and for the mismatched B strain.
Discussion
The objective of this study was to retrospectively estimate the public health impact that QIV could have had on influenza-related health outcomes and associated influenza costs over 10 previous influenza seasons, if QIV had been used instead of TIV in Europe.
By substituting TIV with QIV, this study estimated that for the 5 EU countries, 1.03 million (327.9/100,000) influenza cases, 453,000 (143.9/100,000) GP consultations, 672,000 (213.1/100,000) workdays lost, 24,000 (7.7/100,000) hospitalizations and 10,000 (3.1/100,000) deaths could have been additionally avoided over the 10-seasons-period. The largest number of influenza-related events could have been avoided among elderly people. High-risk groups and 65+ generate most of the savings as the vaccine coverage and the consequences if affected are more important than in the low-risk groups.
Consequently, this study suggests that, although seasonally varying, substantial public health benefits could have been achieved if QIV had been used instead of TIV from 2002 to 2013, excluding the 2009–2010 pandemic season. The benefits estimated in this study are in agreement with studies conducted in other countries.Citation22-25 These results supports the 2012 WHO recommendation to include a second influenza B strain from opposite lineage in influenza vaccines and not to limit the production and distribution of influenza vaccines to TIV.Citation26,27
The results show the implementation of QIV instead of TIV could have been associated with the avoidance of a significant economic burden. Indeed, from SP 15 million Euros could have been saved on GP consultations (14 million from TPP), 77 million on hospitalizations (74 million from TPP) and 150 million on workdays lost, across the 5 EU countries. Again, these numbers varied greatly according to seasons and age groups. These economic benefits compared to the TIV are in line with previous studies in other countries.Citation22,24,28,29 Although, vaccination costs were not considered in the present study, existing literature suggests the public health and economic burden averted by QIV could be important enough to make this intervention cost-effectiveCitation24,30 or even cost-savingCitation31 compared to TIV.
Substantial season-by-season variability was observed in epidemiological and cost-related benefits. On a seasonal basis, our model estimated logically that there could have been negligible public health and economic benefit gained had QIV been used instead of TIV when B circulation is low or when there was no antigenic mismatch between circulating influenza virus B and that in the vaccine for those years.
Influenza B mismatches were the strongest for seasons 2004–05, 2005–06, 2007–08, 2008–09 and 2012–13. Years 2005–06 and 2008–09 had also higher proportions of circulating influenza B virus. This resulted in significant numbers of influenza-related events avoided with significant cost savings made in these years if QIV had been used instead of TIV. Mismatch arises from various mechanisms. Firstly, the level of match between circulating and vaccine strains is difficult to predict at the time of the strains selection (i.e. February of the year for the influenza season of the year). Secondly, influenza strains circulation differs from one place to another, which means that a match could be observed in some regions, but not in other regions. Lastly, Victoria and Yamagata lineages often co-circulate during the same season.Citation14 Using QIV instead of TIV could overcome these situations.
The strength of our study lies in the quality of the inputs used to populate the model. We collected up-to-date, country-specific, EU-5 data from national sources for most of the economic and epidemiological data, including seasonal influenza data. In addition, our study took into account the variable impact of influenza as we stratified by age and also took cross protection against the B virus into account. Our analysis was conducted over a large period from 2001 to 2013 with the exclusion of the 2009–2010 pandemic season to avoid bias. The comprehensive sensitivity analyses we conducted helped to assess the impact of the uncertainty around VE estimates.Citation32
Nevertheless, some limitations should be discussed. First, attack rates were not available by age group and were estimated using data from placebo arms of clinical trials and not observational data. Then, surveillance data are not collected using similar methods across the countries which can lead in a lack of consistency between countries. Moreover, data are scarce regarding cross-protection and herd immunity, the latter being ignored in this study. To our knowledge, no clinical studies directly studied the vaccine efficacy against matched and mismatched influenza B strains. So the data used in our study were derived from 2 Lature reviews.Citation33,34 The model assumes the same severity for all influenza strains, a fixed VE and close matching to serotypes which may continuously change over a season or from year-to-year. While the hypothetical scenario we used in this study can be theoretically plausible, there are no empirical data yet to show benefit of QIV over TIV.
Plenty of vaccine effectiveness estimates have been reported in the literature covering a wide range of values. In the study, we used age specific vaccine efficacy estimates from meta-analysis.Citation19,35-37 The comprehensive sensitivity analyses we conducted helped to assess the impact of the uncertainty around VE estimates on the study conclusions. Besides, the evidence suggesting that the proportion of B cases is lower among elderly, which is the group with highest risk of hospitalization and mortality may introduce an overestimation of the benefits of QIV against hospitalizations and deaths.Citation38 Finally, extrapolation to the 27 EU countries was only based on population figures. Although the 5 EU countries represent 2 thirds of the EU population, it is unsure they are representative of the EU-27.
In conclusion, this study estimates that, compared to TIV, QIV could result in substantial decrease in epidemiological burden and influenza-related costs. Moreover, it would avoid the challenges of dealing with influenza B lineages co-circulation and predicting which lineage of influenza B will circulate in the upcoming seasons. This will result in avoiding influenza related diseases and boosting population confidence in influenza vaccines' performance, and increasing vaccination adoption closer to 75% coverage rate for influenza as recommended by WHO.
Material and methods
Study period
The analysis covered 10 influenza seasons, from 2002–2003 to 2012–2013. Data for the pandemic influenza season 2009–2010 were excluded because the viral circulation in this season was almost all H1N1 pandemic strain, rendering the year atypical and introducing a bias.
Geographical scope
The primary analysis was conducted for France, Germany, Italy, Spain and UK (EU-5). Indeed, these 5 countries were selected because they represented about 2 thirds of the total population of the European Union which comprise 27 countries. In addition, they contain a greater proportion of the number of vaccinated persons and more available data compared to other countries. In a secondary analysis, the results were extrapolated to the 27 countries of EU (EU-27: Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, UK).
Study populations
The population numbers by age for all EU-5 countries were obtained from Eurostat, 2012.Citation39 To account for heterogeneity between age groups in regard of attack rates, risks of complications, vaccine effectiveness and vaccination coverage, the analysis was stratified by age and risk group as follows: children aged 6 months to under 2 years, children aged 2 y to 17 years, adults aged 18 to 49 y stratified according to low-risk and high-risk conditions, adults aged 50 to 64 y stratified low-risk and high-risk conditions, elderly aged 65 and above.
High-risk conditions were the following: persons with pulmonary and cardiovascular illnesses, metabolic diseases including diabetes mellitus, renal dysfunction, and various types of immunosuppression including acquired immunodeficiency syndrome (AIDS) or induced immunosuppression in transplant recipients.Citation26,40,41
Assessment of the additional outcomes avoided with the use of QIV instead of TIV
The model used in this study was similar to that used by Reed et al, 2012, for the US.Citation22 Epidemiological and economic outcomes were estimated for each season for the actual scenario where the TIV was used, and for a hypothetical scenario where QIV would have been used instead. Results are therefore presented as the additional epidemiological and economic outcomes avoided with the use of QIV instead of TIV.
Epidemiological outcomes include the numbers of influenza cases, general practitioners (GP) visits, hospitalizations and deaths, and workdays lost due to influenza. They are presented as absolute number and per 100,000 inhabitants. In the results section, to facilitate the reading of the results, absolute numbers of cases > 1 million, between 100,000 and 999,999, and between 10,000 and 99,999 have been rounded to the nearest hundred thousands, 10 thousands and thousands respectively.
Economic outcomes were the associated costs to epidemiological and were reported from the third-party payer (TPP) and the societal perspectives (SP). Costs from the TPP perspective were estimated by aggregating the costs associated with GP consultations and hospitalizations. Costs from the SP include costs associated with GP consultations, hospitalizations and workdays lost.
Data on influenza viruses circulation (), attack rate (AR) of the different strains and incidence rates of different influenza-associated outcomes (GP visits, hospitalization and death) by country and age group, on vaccination coverage by country, age and risk group (), on B strain included in the vaccine and therefore degree of mismatch (for TIV scenario), and on vaccine effectiveness against the different strains () were incorporated in the analysis. Model description, epidemiological and economic inputs are presented in Supplementary material.
No mismatch in the QIV scenario was considered and the vaccine was assumed to be equally effective against both B strains. As in Reed et al, the observed seasonal vaccine effectiveness against the included B strain was applied to both B strains in the QIV vaccine. However, whereas the effectiveness of the vaccine against the mismatched B strain was assumed to be zero by Reed et al.,Citation22 a cross protection for the mismatched B strain similar across age groups was assumed in this study ().Citation25,34 In addition, contrary to the analysis by Reed et al.,Citation22 stratification by age group and high-risk group for complications was done in this study in order to take into account difference in epidemiology, vaccine effectiveness and vaccine coverage in these different age groups.
Additional epidemiological and economic outcomes avoided were extrapolated proportionally from EU-5 to EU-27 according to the EU-5 and EU-27 population figures in each age group.
Sensitivity analyses
Sensitivity analyses regarding effectiveness of QIV were conducted. The epidemiological burden averted is presented with an interval corresponding to a variation of +/− 20% of the QIV effectiveness (). In addition, deterministic sensitivity analyses were performed to assess the impact of variability in model data inputs on the model estimates by varying key parameters within ranges reflecting possible parameter values. Deterministic sensitivity analysis is represented by tornado charts and concerned the absolute number of influenza cases and average cost savings per season according to TPP and SP for the 5 EU countries during the 2002–03 to 2012–13 influenza seasons.
All analyses were performed using Microsoft Excel®.
Disclosure of potential conflicts of interest
MU, HB and NL are employed by Sanofi Pasteur MSD who commercializes influenza vaccines in Europe; EC is employed by Creative who received consultancy fees for this project.
Supplementary files
Download MS Word (202 KB)Acknowledgments
The authors would like to thank Julie Roïz for her help during model development, Murielle Cornier (Sanofi Pasteur MSD) for useful advices during manuscript preparation, and Nicolas Voirin and Pierre Pradat (www.alpha005.com and www.epibm.com) for manuscript writing.
References
- Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother 2012; 8:81-8; PMID:22252006; http://dx.doi.org/10.4161/hv.8.1.17623
- Mazick A, Gergonne B, Nielsen J, Wuillaume F, Virtanen MJ, Fouillet A, Uphoff H, Sideroglou T, Paldy A, Oza A, et al. Excess mortality among the elderly in 12 European countries, February and March 2012. Euro Surveill 2012; 17; PMID:22516003
- Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis 2008; 8:44-52; PMID:18156088; http://dx.doi.org/10.1016/S1473-3099(07)70311-0
- Paget WJ, Balderston C, Casas I, Donker G, Edelman L, Fleming D, Larrauri A, Meijer A, Puzelli S, Rizzo C, et al. Assessing the burden of paediatric influenza in Europe: the European Paediatric Influenza Analysis (EPIA) project. Eur J Pediatr 2010; 169:997-1008; PMID:20229049; http://dx.doi.org/10.1007/s00431-010-1164-0
- Nokleby H, Nicoll A. Risk groups and other target groups - preliminary ECDC guidance for developing influenza vaccination recommendations for the season 2010–11. Euro Surveill 2010; 15(12):19525; PMID:20350496
- Kuster SP, Shah PS, Coleman BL, Lam PP, Tong A, Wormsbecker A, McGeer A. Incidence of influenza in healthy adults and healthcare workers: a systematic review and meta-analysis. PLoS One 2011; 6:e26239; PMID:22028840; http://dx.doi.org/10.1371/journal.pone.0026239
- WHO Influenza Factsheet 211: Prevention and control of influenza pandemics and annual epidemics. World Health Organisation, 2012. Available from: http://www.who.int/mediacentre/factsheets/fs211/en/. (Last access: 11/24/2015).
- Revised estimates of deaths associated with seasonal influenza in the US. European Centre for Disease Prevention and Control, 2010. Available from: http://ecdc.europa.eu/en/activities/sciadvice/_layouts/forms/Review_DispForm.aspx?List=a3216f4c-f040-4f51-9f77-a96046dbfd72&ID=394. (Last access: 11/24/2015).
- European Centre for Disease Prevention and Control. Factsheet for health professionals. Available from: http://ecdc.europa.eu/en/healthtopics/seasonal_influenza/basic_facts/Pages/factsheet_professionals_seasonal_influenza.aspx. (Last access: 11/24/2015).
- Ryan J, Zoellner Y, Gradl B, Palache B, Medema J. Establishing the health and economic impact of influenza vaccination within the European Union 25 countries. Vaccine 2006; 24:6812-22; PMID:17034909; http://dx.doi.org/10.1016/j.vaccine.2006.07.042
- Keech M, Scott AJ, Ryan PJ. The impact of influenza and influenza-like illness on productivity and healthcare resource utilization in a working population. Occup Med (Lond) 1998; 48:85-90; PMID:9614766; http://dx.doi.org/10.1093/occmed/48.2.85
- Keech M, Beardsworth P. The impact of influenza on working days lost: a review of the literature. Pharmacoeconomics 2008; 26:911-24; PMID:18850761; http://dx.doi.org/10.2165/00019053-200826110-00004
- Commission of the European Communities. Proposal for a Council recommendation on seasonal influenza vaccination. Brussels; Commission of the European Communities; 2009. Available from: http://ec.europa.eu/health/ph_threats/com/Influenza/docs/seasonflu_rec2009_en.pdf. (Last access: 11/24/2015).
- Caini S, Huang QS, Ciblak MA, Kusznierz G, Owen R, Wangchuk S, Henriques CM, Njouom R, Fasce RA, Yu H, et al. Epidemiological and virological characteristics of influenza B: results of the Global Influenza B Study. Influenza Other Respir Viruses 2015; 9(Suppl 1):3-12; PMID:26256290; http://dx.doi.org/10.1111/irv.12319
- Harvala H, Smith D, Salvatierra K, Gunson R, von Wissmann B, Reynolds A, Frew C, MacLean A, Hunt A, Yirrell D, et al. Burden of influenza B virus infections in Scotland in 2012/13 and epidemiological investigations between 2000 and 2012. Euro Surveill 2014; 19; PMID:AMBIGUOUS
- Heikkinen T, Ikonen N, Ziegler T. Impact of influenza B lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999–2012. Clin Infect Dis 2014; 59:1519-24; PMID:25139969; http://dx.doi.org/10.1093/cid/ciu664
- Mosnier A, Caini S, Daviaud I, Bensoussan JL, Stoll-Keller F, Bui TT, Lina B, Van der Werf S, Cohen JM; GROG network. Ten influenza seasons in France: distribution and timing of influenza A and B circulation, 2003–2013. BMC Infect Dis 2015; 15:357; PMID:26289794; http://dx.doi.org/10.1186/s12879-015-1056-z
- Centers for Disease Control and Prevention (CDC). Influenza activity–United States and worldwide, 2007–08 season. MMWR Morb Mortal Wkly Rep 2008; 57:692-7; PMID:18583957
- Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2010:CD001269; PMID:20614424
- Lamure M, Cohen JM, Pribil C, Garassus P, Auray J, Fleming D, Pujol P. Impact of Influenza B in France. Value Health 2014;17(7):A554; PMID:27201811; http://dx.doi.org/10.1016/j.jval.2014.08.1818
- Silva ML, Perrier L, Späth HM, Grog I, Mosnier A, Havet N, Cohen JM; IBGP team. Economic burden of seasonal influenza B in France during winter 2010-2011. BMC Public Health 2014;14:56; PMID:24443900; http://dx.doi.org/10.1186/1471-2458-14-56
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993-8; PMID:22226861; http://dx.doi.org/10.1016/j.vaccine.2011.12.098
- Crepey P, de Boer PT, Postma MJ, Pitman R. Retrospective public health impact of a quadrivalent influenza vaccine in the United States. Influenza Other Respir Viruses 2015; 9 (Suppl 1):39-46; PMID:26256294; http://dx.doi.org/10.1111/irv.12318
- Van Bellinghen LA, Meier G, Van Vlaenderen I. The potential cost-effectiveness of quadrivalent versus trivalent influenza vaccine in elderly people and clinical risk groups in the UK: a lifetime multi-cohort model. PLoS One 2014; 9:e98437; PMID:24905235; http://dx.doi.org/10.1371/journal.pone.0098437
- Clements KM, Meier G, McGarry LJ, Pruttivarasin N, Misurski DA. Cost-effectiveness analysis of universal influenza vaccination with quadrivalent inactivated vaccine in the United States. Hum Vaccin Immunother 2014; 10:1171-80; PMID:24609063; http://dx.doi.org/10.4161/hv.28221
- Reingold L. Proposed Revisions to the 2005 WHO Position Paper on Influenza Vaccines. 2012.
- Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 Influenza Season. MMWR Morb Mortal Wkly Rep 2015; 64:818-25; PMID:26247435; http://dx.doi.org/10.15585/mmwr.mm6430a3
- Eichner M, Schwehm M, Hain J, Uphoff H, Salzberger B, Knuf M, Schmidt-Ott R. 4Flu - an individual based simulation tool to study the effects of quadrivalent vaccination on seasonal influenza in Germany. BMC Infect Dis 2014; 14:365; PMID:24993051; http://dx.doi.org/10.1186/1471-2334-14-365
- Lee BY, Bartsch SM, Willig AM. The economic value of a quadrivalent versus trivalent influenza vaccine. Vaccine 2012; 30:7443-6; PMID:23084849; http://dx.doi.org/10.1016/j.vaccine.2012.10.025
- Meier G, Gregg M, Poulsen Nautrup B. Cost-effectiveness analysis of quadrivalent influenza vaccination in at-risk adults and the elderly: an updated analysis in the UK. J Med Econ 2015; 18:746-61; PMID:25903831; http://dx.doi.org/10.3111/13696998.2015.1044456
- Duru G, Carrat F, Pribil C, Bricaire F, Pujol P, Robert J, Lafuma A. Cost Effectiveness of Quadrivalent Influenza Vaccine Over Trivalent Vaccine in France. Value Health 2014;17(7):A678; PMID:27202501; http://dx.doi.org/10.1016/j.jval.2014.08.2525
- Quinn E, Jit M, Newall AT. Key issues and challenges in estimating the impact and cost-effectiveness of quadrivalent influenza vaccination. Expert Rev Pharmacoecon Outcomes Res 2014; 14:425-35; PMID:24734967; http://dx.doi.org/10.1586/14737167.2014.908713
- DiazGranados CA, Denis M, Plotkin S. Seasonal influenza vaccine efficacy and its determinants in children and non-elderly adults: a systematic review with meta-analyses of controlled trials. Vaccine 2012; 31:49-57; PMID:23142300; http://dx.doi.org/10.1016/j.vaccine.2012.10.084
- Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, et al. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med 2013; 11:153; PMID:23800265; http://dx.doi.org/10.1186/1741-7015-11-153
- Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2010; 2010:CD004876.
- Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2008:CD004879; PMID:18425905
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2011; 12:36-44; PMID:22032844; http://dx.doi.org/10.1016/S1473-3099(11)70295-X
- Influenza in Canada 2010–2011 season. Public health agency of canada, 2013
- Eurostat. Your Key to European Statistics. 2013. Available from: http://ec.europa.eu/eurostat. (Last access: 03/12/2014).
- ECDC SIIP Team. Priority risk groups for Influenza vaccination. Available from: http://ecdc.europa.eu/en/publications/Publications/0808_GUI_Priority_Risk_Groups_for_Influenza_Vaccination.pdf. (Last access: 11/24/2015).
- Centers for Disease Control and Prevention (CDC). Influenza activity--United States and worldwide, 2007-08 season. MMWR Morb Mortal Wkly Rep 2008; 57(25):692-7; PMID:18583957.
- European Centre for Disease Prevention and Control. European Influenza Surveillance Network (EISN). Available at: http://ecdc.europa.eu/en/healthtopics/influenza/EISN/Pages/index.aspx. (Last access: 11/24/2015).
- European Centre for Disease Prevention and Control. Influenza virus characterisation, May 2013. Available at: http://ecdc.europa.eu/en/publications/Publications/influenza-virus-characterisation-may-2013.pdf. (Last access: 03/12/2014).