ABSTRACT
Influenza is a highly contagious respiratory acute viral disease which imposes a very heavy burden both in terms of epidemiology and costs, in the developed countries as well as in the developing ones. It represents a serious public health concern and vaccination constitutes an important tool to reduce or at least mitigate its burden. Despite the existence of a broad armamentarium against influenza and despite all the efforts and recommendations of international organisms to broaden immunization, influenza vaccination coverage is still far from being optimal. This, taken together with logistic and technical difficulties that can result into vaccine shortage, makes intra-dermal (ID) vaccines, such as Fluzone® ID and Intanza®, particularly attractive. ID vaccines are comparable and, in some cases, superior to intra-muscular/sub-cutaneous vaccines in terms of immunogenicity, safety, reactogenicity, tolerability and cross-protection profiles, as well as in terms of patient preference, acceptance and vaccine selection. Further advances, such as Fluzone® ID with alternative B strains and Quadrivalent Fluzone® ID or the possibility of self-administering the vaccines, make influenza ID vaccines even more valuable.
Introduction
Influenza
Influenza is a highly contagious respiratory acute viral disease characterized by a worldwide distribution, a short incubation period (1–3 days, generally 2 days), usually mild respiratory and systemic symptoms, such as high fever, cough, sore throat, headache, chills, anorexia and fatigue.Citation1,2 It can be asymptomatic in 30–50% of subjects.Citation2,3 On the other hand, it may result into severe complications, such as bacterial pneumonia and exacerbation of underlying chronic conditions (including heart or respiratory failure, chronic obstructive pulmonary diseases or COPDs, among others), hospitalization and deaths, especially in frail and high-risk subjects.Citation2
The burden of seasonal influenza as well as of influenza pandemics is very heavy, both in terms of epidemiology and costs, in the developed countriesCitation4 as well as in the developing ones.Citation5 Indeed, the World Health Organization (WHO) estimates that annual epidemics affect up to 15% of the total population worldwide, causing up to 4–5 million severe cases and up to 500,000 deaths.Citation6
For these reasons, influenza represents a serious public health concern and vaccination constitutes an important tool to reduce and mitigate the tremendous socioeconomic burden generated by influenza.
Influenza is caused by a single-stranded, negative-sense RNA virus, Myxovirus influenzae, which belongs to the Orthomyxoviridae family together with Thogotovirus and Isavirus, and includes 3 serotypes or genera, A, B, and C. The genus A, first isolated in 1933,Citation7 is the most clinically relevant and has the capacity to induce minor or major changes in its structure (antigenic drifts and antigenic shifts, respectively). In case of antigenic drifts, the virus causes interpandemic influenza (known also as annual epidemics or seasonal influenza). In case of antigenic shifts, it causes pandemic influenza. Pandemic influenza is caused by influenza virus genus A, while seasonal influenza by influenza genus A and genus B.Citation2
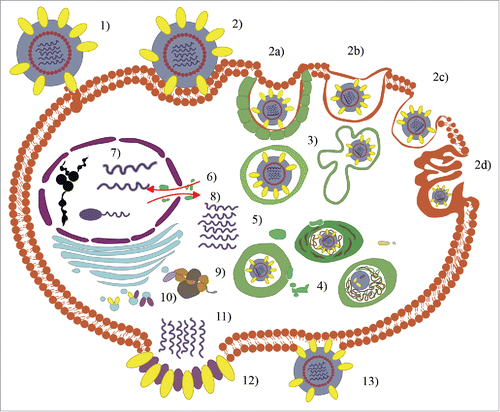
Influenza virus has a quite complex replication cycle, achieved through various stages ().Citation1 First, the virus attaches to the (α-2,3)- or (α-2,6)-linked sialic acid receptors present on the free surface of the cells of the upper respiratory tract or erythrocytes. During this step (termed as virus adsorption), the role of hemagglutinin (HA), a cylinder-shaped, homotrimeric integral type 1 membrane glycoprotein found on the surface of influenza viruses, is crucial. The virus can then enter the cells (internalization) by exploiting different routes (clathrin-mediated endocytosis or CME, caveolae-dependent endocytosis or CDE, clathrin-caveolae-independent endocytosis, or macropinocytosis). CME is the most usual pathway; the virus is internalized into an endosomal compartment, from which it must emerge in order to release its nucleic acid into the cytosol (endosomal trafficking via endosomes/caveosomes/macropinosomes/lysosomes to the perinuclear compartment). During the phase of fusion of the viral envelope with the endosome membrane, after the pH has been reduced (pH-dependent fusion of viral and endosomal/organellar membranes and uncoating), HA plays again a major role. The ribonucleoprotein must then reach the nucleus (nuclear importation) in order to begin the process of translation of its genes and to transcribe and replicate its nucleic acid (transcription and replication). Subsequently, the RNA segments, surrounded by the nucleoproteins, must migrate to the cell membrane (nuclear exportation) in order to enable further molecular processing (namely, protein synthesis, post-translational processing and trafficking, and viral progeny assembly and packaging). Finally, the virus must be freed to invade other cells of the respiratory tract (budding and release). All this is achieved through a complex, finely tuned, highly dynamic, and synchronized action of a vast array of molecular complexes that perform multiple enzymatic and catalytic reactions, whose details are currently known only in part.Citation1
Figure 1. The steps of the replication cycle of the influenza virus are the following: 1) virus adsorption; 2) internalization into cellular regions by means of clathrin-mediated endocytosis (CME), caveolae-dependent endocytosis (CDE), clathrin-caveolae-independent endocytosis, and macropinocytosis; 3) endosomal trafficking; 4) pH-dependent fusion of viral and endosomal / organellar membranes; 5) uncoating; 6) nuclear importation; 7) transcription and replication; 8) nuclear exportation; 9) protein synthesis; 10) post-translational processing and trafficking; 11) viral progeny assembly and packaging; 12) budding; and 13) release (modified from referencesCitation1 and Citation4 ).
Overview of the market
The pharmacological armamentarium against influenza includes different drugs and vaccines, which has been recently reviewed by Gasparini and collaborators.Citation8 Current available drugs include NA inhibitors (NAIs), such as oseltamivir, zanamivir, and peramivir, and adamantane-based M2 protein blockers (amantadine and rimantadine). However, because of the biology of influenza virus and its frequent mutations, these drugs are plagued by the issue of resistance.Citation9,10
A variety of vaccines exists against influenza.Citation8 They can be basically divided into 2 categories: pandemics and seasonal vaccines. Further, they can divided into inactivated influenza vaccines and live, attenuated influenza vaccines (called LAIVs). The first category includes: whole virus vaccine, subunit vaccine made up of purified HA and NA proteins, and split-virion vaccine.
Licensed prepandemic/pandemic vaccines (reviewed in Citation11,12) are shown in . Other investigational vaccines against H7N9 or universal pandemic vaccines are still under clinical experimentation.Citation13
Table 1. Overview of the market with the main available influenza vaccines.
Seasonal vaccines can be divided into inactivated split-virion, inactivated subunit and LAIV vaccines (revised in Citation14-23). Recently, also seasonal adjuvanted vaccines such as those adjuvanted by MF59 or virosomes have become available (reviewed inCitation24). Inactivated vaccines can be administered via the IM route in subjects aged 6 mo and older,Citation25 while LAIVs may be given intra-nasally to healthy, non-pregnant people aged 2–49 y. They may safely be administered at the same time as other vaccines.Citation26 For further details, the reader is referred to .
Generally speaking, the overall efficacy of influenza vaccines is 59% against laboratory-confirmed cases of influenza according to the recent meta-analysis by Osterholm and collaborators.Citation27 According to another meta-analysis it varies from 17% against influenza like illness (ILI) to 73% against confirmed influenza.Citation28 There is therefore the need to develop more effective vaccines.
Influenza vaccination coverage
Despite the fact that annual vaccination represents an important strategy for curbing influenza-related complications, the vaccination coverage is still far from being optimal. For example, in the USA, according to the National Immunization Survey-Flu and the National Internet Flu Survey, only 39.0% of all individuals aged ≥6 mo, that is to say 2 subjects out of 5, were vaccinated against influenza, leaving therefore most population unprotected (CDC, 2015). Also among health-care personnel, the coverage was unacceptably low: according to the most recently available data released by National Health Interview Survey, in the 2007–08 flu season, vaccination coverage among healthcare workers was 48%.Citation29
Further, due to the broadening of vaccination recommendations that extended the suggestion of being vaccinated to all subjects aged 6 mo and older, vaccine shortage can be experienced, together with manufacturing difficulties.
Intradermal (ID) vaccines, such as Fluzone® ID and Intanza®, are therefore an attractive option to properly overcome these critical issues.
Fluzone® ID and intanza®
An ID trivalent split-virion influenza vaccine (Fluzone® ID, Sanofi Pasteur, Swiftwater, PA) has been approved by the Food and Drug Administration (FDA) on 10th May 2011 and been available in the US since the 2011/2012 influenza season for adults aged 18–64 y. Fluzone® ID is available as single-dose, preservative-free pre-filled syringe that contains 9μg hemagglutinin (HA) per strain and exploits the innovative BD's Soluvia™ microinjection device with a glass barrel, a stainless steel barrel with a 1.5-mm, 30-gauge needle and an elastomeric plunger stopper, produced by Becton-Dickinson (BD, Franklin Lakes, NJ).Citation30-32
Intanza 9μg vaccine (also known in some countries as IDflu™ 9μg, Sanofi Pasteur, administered with a micro-injection system), identical to Fluzone® ID for antigen content, way of administration and injection system, received marketing authorization in the EU in February 2009, licensed by the European Medicines Agency (EMA) for adults 18–59 y of age since the 2010/11 season in Europe, and in Canada in September 2010.
Istivac® ID is identifical to Fluzone ID and is commercialized in Argentina for adults 18–59 y of age, used successfully since 2010.
For subjects older than 64 y in the USA and 60 y in Europe and Canada, Fluzone® ID high Dose and Intanza® 15μg (also known in some countries as IDflu™ 15μg, Sanofi Pasteur; administered with a micro-injection system) are available.
High-quality reviews on Intanza® and Fluzone® are already available in the extant literatureCitation30,33-36 and the reader is recommended to refer to them for information concerning trials and studies published until 2012. The thrust of our current manuscript intends to update the current evidences on ID vaccines and to provide new insights and prospects on future developments in the field.
Mechanisms of action
ID vaccination is not a novelty, being already known and performed since 1908.Citation33
Although the intradermal route of vaccine delivery was extensively studied for several vaccination including typhoid fever, measles, cholera, rabies, hepatitis B and poliomyelitis,Citation33 only influenza vaccines have been broadly administered by this route: possible issues that hampered the intradermal delivery for other vaccines include the high costs of technical development and the unacceptable local reactogenicity due to adjuvants contained in traditional formulations.Citation37
Skin represents an optimal site for vaccination and elicits both innate and adaptive immune responses. Skin, covering an impressive surface area of 1.6–1.85 m2 and being situated at the interface between human body and environment,Citation38 is an important barrier, both passive and active, against chemical, physical and microbial insults. From an anatomic point of view, skin is composed of an upper laying (the epidermis), a basement membrane and a lower laying (the dermis). The epidermis is 150–200 μm thick and comprises the stratum corneum, the stratum germinativum, the stratum lucidum (present only in some specific parts of the human body), the stratum granulosum, the stratum of Malpighi or stratum spinosum, and the stratum basale.Citation37 The stratum corneum is particularly thick, consisting of dead cells (corneocytes) surrounded by lipid drafts and regions in the lamellar phases,Citation39-40 and has a great importance, in that optimal strategies for drug/vaccine delivery have to overcome the presence of this ‘formidable’ physical barrier.Citation38 As summarized in an excellent way by Gill and collaborators, there are different ways for an optimal drug/vaccine skin delivery: the first strategy is based on stratum corneum disruption (use of chemical enhancers, ultrasounds, and electroporation), the second is based on stratum corneum removal (tape stripping, abrasion, thermal ablation, and microdermabrasion), and the third is based on stratum corneum penetration (use of jet injectors, gene gun, and micro-needles).Citation41
The dermis is 1.5–3 mm thick and, in its turn, can be divided into a papillary compartment (stratum papillare) and a reticular compartment (stratum reticulare), containing thin and thick collagen fibers, respectively.Citation38 Hypodermis or subcutaneous tissue is 3–100 mm thick and is a layer of loose connective tissue and elastin.Citation38
Further, skin harbours immune cellular components (epidermal dendritic cells or epidermal DCs, known also as Langerhans cells or LCs, dermal DCs or DDCs known also as interstitial or migratory DCs, αβ T cells, γδ T cells, natural killer or NK cells, B cells, mast cells, and macrophages), thus constituting a immunocompetent, multi-tasking organCitation38-44 or a complex system (skin immune system or SIS).Citation45 Skin includes, indeed, skin-associated lymphoid tissues (SALTs),Citation46 which are responsible of a continuous cross-talk between skin (and its cellular components) and lymph nodes.Citation38 SALTs are constituted by lymphoid follicles, vessels, antigen-presenting cells (APCs), and lymphocytes. Some scholars, referring to the unique, natural immune enhancer effect of the skin, have proposed the existence of a skin-mediated ‘adjuvant’ mechanism.Citation38-44 However, the exact nature of this mechanism is complex and still poorly known. It can be hypothesized that the effect of ID vaccination may be the result of 3 concurring, complementary pathways (summarized in and ). Antigens are captured at skin level by resident, highly efficient APCs, like epidermal keratinocytes and specialized DCs, expressing high levels of class II major histocompatibility complex.Citation38 Skin DCs belong to the non-lymphoid tissue (NLT) DC group, while the LT DC group comprises 3 subsets (namely, the conventional DCs or cDCs type 1, the cDCs type 2, and, finally, the plasmacytoid DCs or pDCs).Citation47 Skin NLT DCs can be subdivided into 4 subsets: LCs within the suprabasal layers of the epidermis, expressing langerin; DDCs lacking langerin, which in their turn include DDCs expressing CD1a and DDCs expressing CD14; and finally, recruited macrophages or other innate immune cells, activated by the expression of Toll-like receptors (TLRs) and nucleotide-binding domain leucine-rich repeat receptors.Citation38 CD141, known also as BDCA-3, THBD or thrombomodulin, is an important marker in differentiating the different DC subsets. LCs can mature interacting with e-cadherin expressed by keratinocytes and can crosstalk with Th1, Th2, Th17, Th22 (cells producing IL-22, but not IL-17), Treg, and can prime naïve CD8+ T cells into effector cytotoxic T lymphocytes (CTLs), while CD1a+ DDCs crosstalk with Th2 and CTLs, CD1a+ CD141+ DDCs with Th17, and CD14+ CD141+ DDCs with Th1, Tfh, Treg, and TC2, as well as induce the differentiation of naïve B cells into IgM-secreting plasma cells.Citation48
Figure 2. A pictorial scheme of the different mechanism at the basis of the action of intra-dermal vaccines. Abbreviations: DCs: dendritic cells; DDCs: dermal dendritic cells.
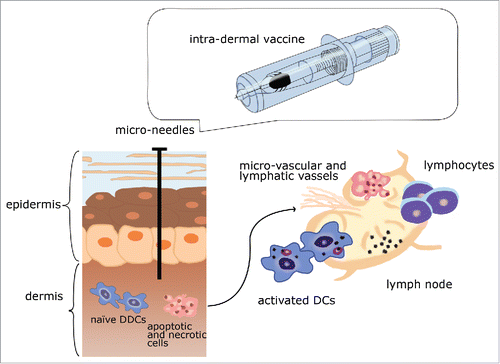
Table 2. Overview of the 3 mechanisms at the basis of the action of intra-dermal vaccines.
This pathway plays undoubtedly a major role. The maturation, the differentiation, the acquisition of adequate immune abilities, and the migration to the paracortical area of the regional draining lymph nodes as afferent lymph veiled cells (ALVCs) through high endothelial venules (HEVs), are modulated by different signaling pathways, including increased expression of MHC antigens, co-stimulatory molecules and pro-inflammatory cytokines such as IL-1β, IL-6, IL-12 and TNF-α. These event lead to the subsequent activation of lymphocyte T CD8+, releasing interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), granzyme B, among other molecules, and high cytotoxic activity and memory B-cells.
This schematic representation, even though more complex, basically corresponds to the classical Ralph Steinman's theory, which postulates the existence of at least 2 distinct states of DCs: a) immature DCs committed to antigen capture and b) mature DCs working as APCs.Citation49,50
There is also another complementary pathway, independent on the first: antigens and especially small vaccine components (<400 nm) are passively drained by the unique micro-vascular and lymphatic structures of the skin and transported directly to the lymph nodes, where they are captured by lymph node resident DCs, medullary macrophages and subcapsular sinus macrophages.Citation51-53 This second path, even though less known and studied, is not nevertheless less fundamental.Citation53
Moreover, there are some physical aspects due to the mechanic action of the adopted injection system. The microneedle device generates localized transient stresses invoking cell necrosis and apoptosis around each projection,Citation54 favoring the release of damage associated molecular patterns (DAMPs). This is the well-known Matzinger's ‘danger hypothesis.’Citation55,56 Further, the device generates high immunoglobulin G (IgG) responses. Also immunoglobulin E (IgE) in immunity against influenza virus could play a role, as recently demonstrated by Smith-Norowitz and colleagues.Citation57
On the contrary muscle quite inefficient to capture antigensCitation26 and the IM route is therefore inferior to ID route in terms of biological mechanism and action.The molecular mechanisms of Fluzone® ID have been recently uncovered also using innovative cutting-edge biotechnologies, such as transcriptomic assays, which have shown a strong signature of adaptive immunity activation following vaccination.Citation58 In particular, Fluzone® ID seems to elicit temporary transcriptional changes in the circulating myeloid compartment. Vaccination upregulates modules linked to NF-κB-driven inflammation, IFN-γ response, TNF and CD40 signaling. These pathways, involved in T-cell activation and development of adaptive immunity, were detected in symptomatic individuals, but not asymptomatic ones.
As regard as the cross-protection potential of influenza ID vaccines, that is the ability of an influenza vaccine to elicit an effective antibody response against circulating viruses presenting antigenic patterns different from those of the vaccine strains, the exact involved mechanism is still not understood and under debate, even though a number of studies provided evidences of cross-protection elicited by ID vaccines (see Cross-protection section). In particular, Ansaldi et al. attributed the enhanced and broaden antibody response elicited by ID vaccine to the re-stimulation of B cells specific for selected antigenic sites of hemagglutinin surface protein, previously primed either by vaccination or infection. However, new and specific evaluations are needed in order to deeply investigate this aspect.
Cutaneous drug and vaccine delivery
Current ID drug and vaccine delivery relies mainly upon 6 technologies: namely, jet injection, ballistic injection, tattooing devices, transcutaneous patches, micro-injection and microneedles. First generation approaches relied upon Mantoux and bifurcated needles.Citation26
Jet injectors consist in a needle-free syringe and in a high-pressured propulsion system made of reusable, disposable pre-filled cartridges. While jet injectors are used to deliver liquids, ballistic injectors are able to transfer solid particles, such as gold nanoparticles. Tattooing devices consist in a short injection needle (or multiple needles) penetrating the skin through vibrations at a high frequency, while transcutaneous patches contain a dry formulation of the vaccine antigen, the adjuvant or of the vaccine and adjuvant mix.
Microneedle device represents an important achievement within the experimental technologies for a proper skin drug delivery. Solid-silicon microneedles and solid metal microneedle arrays, hollow-silicon microneedles, polymers- or carbohydrates-based microneedles, macroneedles, patches are other possible devices, which are being currently investigated.Citation58-60
ID devices are used in other fields, such as diabetology (such as SQ-PEN technology, InsuJetTM, Injex23TM, Injex30TM, Vision® MediJector IITM, or ClickSoftTM for the delivery of insulin),Citation61,62 immunology/allergology,Citation63 anaesthesiology (J-Tip Needle-Free Injector, Medajet-XL),Citation64-65 endocrinology (for the delivery of growth hormones, including for example Bioject® Cool Click™, SeroJet, Antares' Medi-Jector Vision technology),Citation66 dermatology and cosmetology (for example, MTS ROLLER™, ScalpRollerTM, DERMAROLLER®, MICRO HYALA®, or LITE CLEAR®, for the treatment of anti-aging, scars, hair-loss, and acne).Citation67
The Soluvia BD device represents the first FDA-approved delivery system in the field of vaccinology. Subsequently, the USA FDA has approved on August 14, 2014 the use of the PharmaJet Stratis 0.5 ml Needle-free Jet Injector for delivery of one particular flu vaccine (AFLURIA® by bioCSL Inc.) in people 18 through 64 y of age.
The need of a more effective activation of the immune system than that achieved by traditional IM vaccine delivery, along with the concerns of influenza vaccine shortages and the possibility of using ID route to spare vaccine dose, connected the research for new ID devices with the clinical development of ID influenza vaccines, in particular Fluzone®. A number of clinical and pre-clinical trials, evaluating the ID flu vaccine efficiency, have been performed by using the Soluvia BD device or with precursors of this micro-injection system.Citation68,69
Microneedle allows precise tissue localization of delivery and overcome the limitations of other chemical, biochemical and physical methods of transdermal drug delivery.Citation70-72
Immunogenicity
The immunogenicity profile of Fluzone® ID has been extensively investigated.Citation73-76
Immunogenicity has been explored also in immunocompromised patients: in transplanted patients,Citation77,78 in HIV-infected patients,Citation79 and in cancer patients.Citation80
Immunogenicity profiles of ID vaccines are not inferior to IM vaccines, as proven by some recently published meta-analyses: those of Marra and colleagues carried out among immunocompetent subjectsCitation81 and of Pileggi and collaborators performed among immunocompromised individuals.Citation82 Marra and collaborators found an IM/ID GMTR ratio of 0.92 (95% CI = 0.77-1.09), of 0.97 (95% CI = 0.80–1.18) and of 0.93 (95% CI = 0.80–1.08), a seroconversion ratio of 0.94 (95% 0.86–1.02), of 0.89 (95% CI 0.80–0.99) and of 0.91 (95% CI = 0.80–1.04), and a seroprotection ratio of 0.97 (95% CI = 0.94–1.00), of 0.98 (95% CI = 0.96–0.99) and of 0.97 (95% CI = 0.91–1.03) for A/H1N1, A/H3N2 and B strains, respectively.Citation81 Pileggi and co-workers found an overall risk ratio (RR) of seroprotection was 1.00 (95 % CI = 0.91–1.10) for A/H1N1 strain, 1.00 (95 % CI = 0.90–1.12) for A/H3N2 and 0.99 (95 % CI = 0.84–1.16) for B strain.Citation82
In conclusion ID vaccines proved to be statistically non inferior to other influenza vaccines, such as Fluzone® IM, Fluad®, Vaxigrip®, Inflexal V® and Flumist®.Citation73,83-87
Cross-protection
Ansaldi and collaborators performed a randomized trial to investigate the ability of Intanza® 15μg versus Vaxigrip® of conferring cross-protection against heterologous circulating H3N2 strains in 50 adults aged 60 y and older during the 2006–2007 influenza season. This study demonstrated the broader immune response elicited by an ID influenza vaccine vs. a standard IM influenza vaccine against heterologous viruses.Citation87,88
Also Camilloni and coworkers found that ID vaccine confers cross-protection against drifted H3N2 strains.Citation73
Safety and reactogenicity
Both pre-licensure and post-licensure studies have confirmed the safety profile of Fluzone® ID. According to the most recently available data, based on the Vaccine Adverse Effects Reporting System (VAERS), 9 out of 466 received reports (1.9%) were serious, including one reported fatality in an 88-year-old vaccinee. The most common adverse effects (AEs) included injection site reactions, which accounted for approximately half of all the reported AEs.Citation89
Tsang and collaborators performed a randomized, controlled, multicenter, phase II study in older adults (≥65 years of age) who were randomly assigned to ID vaccine with 15μg (HA)/strain (n = 636), ID vaccine with 21μg HA/strain (n = 634), standard IM vaccine with 15μg HA/strain (n = 319) and high-dose IM vaccine with 60μg HA/strain (n = 320), respectively. Subjects immunized with ID vaccine were more likely to report more injection-site reactions.Citation85
An interesting study carried out by Gorse and co-workers assessed the safety and reactogenicity in case of revaccination with ID preparation, performing a phase II, active-controlled, multi-center, open-label trial in 1,250 adults aged 18–64 y randomly assigned to ID or IM vaccine. Vaccines with ID vaccine reported erythema, induration, swelling, pruritus and ecchymosis more often than those receiving IM vaccine, especially if they had been immunized with ID vaccine in the previous year.Citation90,91
Patient acceptance and preference
Besides the unique properties of ID vaccination, due to the immunological and micro-vascular properties of the skin and its extreme richness in specific resident and recruited antigen-presenting cells (APCs), capable of eliciting stronger immune responses, ID vaccination offers further advantages in terms of patient acceptance and preference. ID vaccination is, indeed, painless and the microneedle device exorcises the fear of needle-sticks.Citation41
Frenck and coworkers, indeed, found that adults rated the experience of receiving Fluzone ID vaccine as less (50%) or the same (24%) painful as their previous experience of receiving the vaccine via IM route.Citation75
Eizenberg and collaborators carried out a study among 1,666 vaccinees with Intanza 9μg and 46 prescribers in Australia and Argentina during the 2010 influenza. 98% of vaccinees were satisfied or very satisfied. 95% of vaccinees reported that they would prefer to receive the same vaccination next year. Furthermore, 85% of prescribers were satisfied or very satisfied with the vaccine.Citation92
Durando and collaborators performed an observational multicenter study in Italy among 1,600 subjects aged 60 y and older, using a validated, self-administered questionnaire, namely the 21-items Vaccinees' Perception of Injection (VAPI®), which assessed 4 dimensions (bother, arm movements, sleep, and acceptability). 75.5% and 94.9% of the interviewees were favorable and very favorable to Intanza® 15 μg, respectively. Also the compliance by healthcare professionals (n = 130) with the novel ID vaccine was favorable. No serious adverse event occurred during the 6-month follow-up period, while solicited local reactions were significantly higher in the ID-vaccine group than in the IM-vaccine group.Citation93
Arnou and collaborators performed an online survey in France and Germany among 483 physicians and 2,778 members of the general public aged 50 y and older. 61–78% of practitioners would strongly recommend Intanza® over the corresponding IM vaccine. More than two-thirds of the unvaccinated general public would prefer Intanza®. More than 82% of the physicians agreed that Intanza may help increase vaccination coverage rates.Citation94
Further, from some studies it is emerging that subjects would prefer ID versus IM vaccine in terms of pre-injection anxiety and post-injection pain, and in particular self-administration vs. nurse-led administration, if given the possibility of freely choosing.
Self-administration has been previously investigated and used with live attenuated influenza vaccines, and the performed studies reported high percentage of compliance with this practice and a few or no problems with administration.Citation95,96
Self-administration has the potential to reduce the time of influenza vaccination, in comparison with nurse-administration, and to increase influenza vaccine uptake, especially among healthcare workers. This approach would be particularly useful for the management of possible pandemics or urgent mass vaccination settings, such as in response to a severe influenza epidemic, both at country and global level.
In order to assure a safe and effective self-administration, a proper education about the correct techniques and an appropriate responses to any immediate adverse reactions, such as hypersensitivity reactions, should be guaranteed. For these reason, a supervision by trained staff must be available to manage possible anaphylactic reactions following administration of the vaccine
Coleman and colleagues recruited 228 adults aged 18–59 y who were randomized to either self-administered (n = 115) or nurse-administered (n = 113) ID vaccine. Self-administering participants rated pain less than nurse-led vaccines, even though they reported larger areas of redness post-vaccination. 70% of all participants said they would prefer ID vaccinations in the future.Citation97
Foy and colleagues compared Fluzone® ID versus Fluzone® IM and Flumist (Medimmune) intranasal vaccine. Of the 367 participants vaccinated, 249 (67.8%) chose the ID vaccine and 99.6% of these subjects reported being satisfied with the route and method of administration.Citation32
Dhont and colleagues investigated the acceptability of Intanza® 15 g among 837 vaccinees and 105 general practitioners (GPs) during the 2010–2011 influenza season. The majority of vaccinees was very satisfied (70.0%) or satisfied (27.9%) with the ID vaccine. Most vaccinees (91.1%) who had previously received IM influenza vaccination preferred the ID vaccine, and 98.5% of vaccinees reported they would consider receiving the ID vaccine the following year. The majority of GPs was very satisfied (78.6%) or satisfied (18.4%) with the ID vaccine, and most GPs (87.6%) expressed a preference for the ID vaccine over IM influenza vaccine.Citation98
Coleman and co-workers performed an interesting trial among 810 healthcare workers and found that ID self-administration could be a valuable asset for increasing the coverage of influenza vaccination among people working in health-care settings. 401 subjects were randomized to self-administered ID influenza vaccine (Intanza®, n = 401), while 409 were assigned to receiving nurse-administered IM vaccine (Vaxigrip®). Acceptability was high: 96% were very or somewhat certain that they administered the vaccine correctly, 83% would choose ID influenza vaccine again and of those, 75% would choose self-administration again.Citation99
Future prospects
Future prospects concern the increase in influenza strain coverage, adding for example a second B-lineage strain or using an alternative B-lineage strain. In the 1970s, the influenza B virus split into 2 main antigenically different lineages, named B/Yamagata and B/Victoria after named after their first representatives, B/Victoria/2/87 and B/Yamagata/16/88, respectively. Further, since 2001, the 2 B strain lineages have co-circulated with varying prevalence, making it difficult to predict the next season's dominant lineage.Citation100,101 The result has been frequent mismatches between the B strain lineage in influenza vaccines and the B strain lineage circulating in the community. Between 1999–2000 and 2012–2013, the B strain lineage in trivalent influenza vaccines has not matched the dominant circulating strain in half of the influenza seasons. For this reason, quadrivalent vaccines containing both B strain lineages have been developed.Citation102 Quadrivalent Fluzone® IM has already shown non inferiority to trivalent Fluzone® IM for all matched strains and superior immunogenicity for the additional B-lineage strain in a study carried out in the USA during the 2013/2014 influenza season for persons ≥6 mo of age.Citation103
Gorse and collaborators investigated the immunogenicity and safety of an ID quadrivalent split-virion influenza vaccine administered to 1,672 adults 18–64 y of age in the US during the 2012–2013 influenza season vs. 837 participants vaccinated with Fluzone® ID and 846 vaccinated with an investigational version of Fluzone® ID. The randomized, double-blind, active-controlled multicentre trial showed that the quadrivalent version of Fluzone® ID proved to be statistically non-inferior to the 2 vaccines both in terms of antibody responses and adverse effects in case of A and matched B strains, and even superior for the unmatched B strains.Citation102 This further advance could overcome mismatched B strains in previous influenza vaccines.
Conclusion
The broadening of recommendations for receiving influenza vaccine, logistic and technical difficulties together with an unacceptably low influenza coverage make ID vaccines particularly attractive. ID vaccines are comparable and in some cases superior to IM vaccines in terms of immunogenicity, safety, reactogenicity, tolerability and cross-protection profiles, as well as in terms of patient preference, acceptance and vaccine selection.Citation103-108 Further advances, such as Fluzone® ID with alternative B strains and Quadrivalent Fluzone® ID or the possibility of self-administering the vaccines, make Fluzone® ID and Intanza® even more valuable.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Gasparini R, Amicizia D, Lai PL, Bragazzi NL, Panatto D. Compounds with anti-influenza activity: present and future of strategies for the optimal treatment and management of influenza. Part I: Influenza life-cycle and currently available drugs. J Prev Med Hyg 2014 Sep; 55(3):69-85.
- Fiore AE, Bridges CB, Katz JM, Cox NJ. Inactivated influenza vaccines. In (Eds. Plotkin SA, Orenstein WA, Offit PA): Vaccines. Sixth edition. Section 2: Licensed vaccines. 2013; 257-93. Elsevier, ISBN: 978-1-4557-0090-5
- Carrat F, Vergu E, Ferguson NM, Lemaitre M, Cauchemez S, Leach S, Valleron AJ. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol 2008 Apr 1; 167(7):775-85; http://dx.doi.org/10.1093/aje/kwm375
- Gasparini R, Amicizia D, Lai PL, Panatto D. Clinical and socioeconomic impact of seasonal and pandemic influenza in adults and the elderly. Hum Vaccin Immunother 2012 Jan; 8(1):21-8; http://dx.doi.org/10.4161/hv.8.1.17622
- de Francisco Shapovalova N, Donadel M, Jit M, Hutubessy R. A systematic review of the social and economic burden of influenza in low- and middle-income countries. Vaccine 2015 Nov 27; 33(48):6537-44; http://dx.doi.org/10.1016/j.vaccine.2015.10.066
- World Health Organization (WHO). Influenza (Seasonal), factsheet n° 211. Available at http://www.who.int/mediacentre/factsheets/fs211/en/
- Smith W, Andrewes CH, Laidlaw PP. A virus obtained from influenza patients. Lancet 1933; ii:66-88; http://dx.doi.org/10.1016/S0140-6736(00)78541-2
- Gasparini R, Amicizia D, Lai PL, Bragazzi NL, Panatto D. Compounds with anti-influenza activity: present and future of strategies for the optimal treatment and management of influenza. Part II: Future compounds against influenza virus. J Prev Med Hyg 2014 Dec; 55(4):109-29
- Shen Z, Lou K, Wang W. New small-molecule drug design strategies for fighting resistant influenza A. Acta Pharm Sin B 2015 Sep; 5(5):419-30; http://dx.doi.org/10.1016/j.apsb.2015.07.006
- Treanor JJ. Prospects for Broadly Protective Influenza Vaccines. Am J Prev Med 2015 Dec; 49(6 Suppl 4):S355-63; http://dx.doi.org/10.1016/j.amepre.2015.09.012
- Stöhr K, Kieny MP, Wood D. Influenza pandemic vaccines: how to ensure a low-cost, low-dose option. Nat Rev Microbiol 2006 Aug; 4(8):565-6; http://dx.doi.org/10.1038/nrmicro1482
- Gasparini R, Amicizia D, Lai PL, Panatto D. Aflunov(®): a prepandemic influenza vaccine. Expert Rev Vaccines 2012 Feb; 11(2):145-57; http://dx.doi.org/10.1586/erv.11.170
- He F, Leyrer S, Kwang J. Strategies towards universal pandemic influenza vaccines. Expert Rev Vaccines 2015 Dec 5:1-11. [Epub ahead of print]
- Cox MM, Hollister JR. FluBlok, a next generation influenza vaccine manufactured in insect cells. Biologicals 2009 Jun; 37(3):182-9; http://dx.doi.org/10.1016/j.biologicals.2009.02.014
- Cox MM, Izikson R, Post P, Dunkle L. Safety, efficacy, and immunogenicity of Flublok in the prevention of seasonal influenza in adults. Ther Adv Vaccines 2015 Jul; 3(4):97-108; http://dx.doi.org/10.1177/2051013615595595
- Cox MM, Patriarca PA, Treanor J. FluBlok, a recombinant hemagglutinin influenza vaccine. Influenza Other Respir Viruses 2008 Nov; 2(6):211-9; http://dx.doi.org/10.1111/j.1750-2659.2008.00053.x
- Yang LP. Recombinant trivalent influenza vaccine (flublok(®)): a review of its use in the prevention of seasonal influenza in adults. Drugs 2013 Aug; 73(12):1357-66; http://dx.doi.org/10.1007/s40265-013-0103-6
- Manini I, Domnich A, Amicizia D, Rossi S, Pozzi T, Gasparini R, Panatto D, Montomoli E. Flucelvax (Optaflu) for seasonal influenza. Expert Rev Vaccines 2015 Jun; 14(6):789-804; http://dx.doi.org/10.1586/14760584.2015.1039520
- McKeage K. Inactivated quadrivalent split-virus seasonal influenza vaccine (Fluarix® quadrivalent): a review of its use in the prevention of disease caused by influenza A and B. Drugs 2013 Sep; 73(14):1587-94; http://dx.doi.org/10.1007/s40265-013-0114-3
- McKeage K. Inactivated quadrivalent split-virus seasonal influenza vaccine (Fluarix® quadrivalent): a review of its use in the prevention of disease caused by influenza A and B. Drugs 2013 Sep; 73(14):1587-94; http://dx.doi.org/10.1007/s40265-013-0114-3
- Curran MP, Leroux-Roels I. Inactivated split-virion seasonal influenza vaccine (Fluarix): a review of its use in the prevention of seasonal influenza in adults and the elderly. Drugs 2010 Aug 20; 70(12):1519-43; http://dx.doi.org/10.2165/11205020-000000000-00000
- El Sahly HM, Keitel WA. Clinical data on Fluarix: an inactivated split seasonal influenza vaccine. Expert Rev Vaccines 2008 Aug; 7(6):713-9; http://dx.doi.org/10.1586/14760584.7.6.713
- Rose GW, Cooper CL. Fluarix, inactivated split-virus influenza vaccine. Expert Opin Biol Ther 2006 Mar; 6(3):301-10; http://dx.doi.org/10.1517/14712598.6.3.301
- Gasparini R, Lai P. Utility of virosomal adjuvated influenza vaccines: a review of the literature. J Prev Med Hyg 2010 Mar; 51(1):1-6
- Childress BC, Montney JD, Albro EA. Making evidence-based selections of influenza vaccines. Hum Vaccin Immunother 2014; 10(9):2729-32; PMID:25483499; http://dx.doi.org/10.4161/hv.29340
- Gasparini R, Amicizia D, Lai PL, Panatto D. Live attenuated influenza vaccine–a review. J Prev Med Hyg 2011 Sep; 52(3):95-101
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012 Jan; 12(1):36-44; http://dx.doi.org/10.1016/S1473-3099(11)70295-X
- Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2010 Feb 17; (2):CD004876.
- Centers for Disease Control and Prevention (CDC). FluVaxView. Available at http://www.cdc.gov/flu/fluvaxview/
- Atmar RL, Patel SM, Keitel WA. Intanza(®): a new intradermal vaccine for seasonal influenza. Expert Rev Vaccines 2010; 9:1399-409; PMID:21105776; http://dx.doi.org/10.1586/erv.10.134
- Clayville LR. Influenza update: a review of currently available vaccines. P T 2011 Oct; 36(10):659-84
- Foy JE, Hendriksz T, Malouf P, Tobin A. Acceptability of fluzone intradermal vaccine to patients and vaccine administrators. J Am Osteopath Assoc 2013 Feb; 113(2):134-43.
- Icardi G, Orsi A, Ceravolo A, Ansaldi F. Current evidence on intradermal influenza vaccines administered by Soluvia™ licensed micro injection system. Hum Vaccin Immunother 2012 Jan; 8(1):67-75; http://dx.doi.org/10.4161/hv.8.1.18419
- Ansaldi F, Durando P, Icardi G. Intradermal influenza vaccine and new devices: a promising chance for vaccine improvement. Expert Opin Biol Ther 2011 Mar; 11(3):415-27; http://dx.doi.org/10.1517/14712598.2011.557658
- Sullivan SJ, Jacobson R, Poland GA. Advances in the vaccination of the elderly against influenza: role of a high-dose vaccine. Expert Rev Vaccines 2010 Oct; 9(10):1127-33; http://dx.doi.org/10.1586/erv.10.117
- Sticchi L, Alberti M, Alicino C, Crovari P. The intradermal vaccination: past experiences and current perspectives. J Prev Med Hyg 2010 Mar; 51(1):7-14
- Hickling JK, Jones KR, Friede M, Zehrung D, Chen D, Kristensen D. Intradermal delivery of vaccines: potential benefits and current challenges. Bull World Health Organ 2011 Mar 1; 89(3):221-6; http://dx.doi.org/10.2471/BLT.10.079426
- Di Meglio P, Perera GK, Nestle FO. The multitasking organ: recent insights into skin immune function. Immunity 2011 Dec 23; 35(6):857-69; http://dx.doi.org/10.1016/j.immuni.2011.12.003
- Menon GK, Cleary GW, Lane ME. The structure and function of the stratum corneum. Int J Pharm 2012 Oct 1; 435(1):3-9; http://dx.doi.org/10.1016/j.ijpharm.2012.06.005
- Bouwstra JA, Ponec M. The skin barrier in healthy and diseased state. Biochim Biophys Acta 2006 Dec; 1758(12):2080-95; http://dx.doi.org/10.1016/j.bbamem.2006.06.021
- Gill HS, Kang SM, Quan FS, Compans RW. Cutaneous immunization: an evolving paradigm in influenza vaccines. Expert Opin Drug Deliv 2014 Apr; 11(4):615-27; http://dx.doi.org/10.1517/17425247.2014.885947
- Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine 2008 Jun 19; 26(26):3197-208; http://dx.doi.org/10.1016/j.vaccine.2008.03.095
- Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol 2014 May; 14(5):289-301; http://dx.doi.org/10.1038/nri3646
- Richmond JM, Harris JE. Immunology and skin in health and disease. Cold Spring Harb Perspect Med 2014 Dec 1; 4(12):a015339; http://dx.doi.org/10.1101/cshperspect.a015339
- Bos JD, Kapsenberg ML. The skin immune system Its cellular constituents and their interactions. Immunol Today 1986 Jul-Aug; 7(7-8):235-40; http://dx.doi.org/10.1016/0167-5699(86)90111-8
- Streilein JW. Skin-associated lymphoid tissues (SALT): origins and functions. J Invest Dermatol 1983 Jun; 80 Suppl:12s-16s
- Boltjes A, van Wijk F. Human dendritic cell functional specialization in steady-state and inflammation. Front Immunol 2014 Apr 1; 5:131.
- Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, Reiter Y, Banchereau J, Ueno H. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity 2008 Sep 19; 29(3):497-510; http://dx.doi.org/10.1016/j.immuni.2008.07.013
- Clausen BE, Stoitzner P. Functional Specialization of Skin Dendritic Cell Subsets in Regulating T Cell Responses. Front Immunol 2015 Oct 22; 6:534.
- Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol 2012; 30:1-22; PMID:22136168; http://dx.doi.org/10.1146/annurev-immunol-100311-102839
- Leroux-Roels I, Weber F. Intanza (®) 9 µg intradermal seasonal influenza vaccine for adults 18 to 59 years of age. Hum Vaccin Immunother 2013 Jan; 9(1):115-21; http://dx.doi.org/10.4161/hv.22342
- Al-Zahrani S, Zaric M, McCrudden C, Scott C, Kissenpfennig A, Donnelly RF. Microneedle-mediated vaccine delivery: harnessing cutaneous immunobiology to improve efficacy. Expert Opin Drug Deliv 2012 May; 9(5):541-50; http://dx.doi.org/10.1517/17425247.2012.676038
- Tozuka M, Oka T, Jounai N, Egawa G, Ishii KJ, Kabashima K, Takeshita F. Efficient antigen delivery to the draining lymph nodes is a key component in the immunogenic pathway of the intradermal vaccine. J Dermatol Sci 2016 Apr; 82(1):38-45; http://dx.doi.org/10.1016/j.jdermsci.2015.11.008
- Depelsenaire AC, Meliga SC, McNeilly CL, Pearson FE, Coffey JW, Haigh OL, Flaim CJ, Frazer IH, Kendall MA. Colocalization of cell death with antigen deposition in skin enhances vaccine immunogenicity. J Invest Dermatol 2014 Sep; 134(9):2361-70; http://dx.doi.org/10.1038/jid.2014.174
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol 1994; 12:991-1045; PMID:8011301; http://dx.doi.org/10.1146/annurev.iy.12.040194.005015
- Matzinger P. The danger model: a renewed sense of self. Science 2002 Apr 12; 296(5566):301-5; http://dx.doi.org/10.1126/science.1071059
- Smith-Norowitz TA, Kusonruksa M, Wong D, Norowitz MM, Joks R, Durkin HG, Bluth MH. Long-term persistence of IgE anti-influenza A HIN1 virus antibodies in serum of children and adults following influenza A vaccination with subsequent H1N1 infection: a case study. J Inflamm Res 2012; 5:111-6; PMID:23097613; http://dx.doi.org/10.2147/JIR.S34152
- Kis EE, Winter G, Myschik J. Devices for intradermal vaccination. Vaccine 2012 Jan 11; 30(3):523-38; http://dx.doi.org/10.1016/j.vaccine.2011.11.020
- Koutsonanos DG, Compans RW, Skountzou I. Targeting the skin for microneedle delivery of influenza vaccine. Adv Exp Med Biol 2013; 785:121-32; PMID:23456844; http://dx.doi.org/10.1007/978-1-4614-6217-0_13
- Pettis RJ, Harvey AJ. Microneedle delivery: clinical studies and emerging medical applications. Ther Deliv 2012 Mar; 3(3):357-71; http://dx.doi.org/10.4155/tde.12.13
- Hultström M, Roxhed N, Nordquist L. Intradermal insulin delivery: a promising future for diabetes management. J Diabetes Sci Technol 2014 May; 8(3):453-7; http://dx.doi.org/10.1177/1932296814530060
- Narayan RJ. Transdermal delivery of insulin via microneedles. J Biomed Nanotechnol 2014 Sep; 10(9):2244-60; http://dx.doi.org/10.1166/jbn.2014.1976
- Senti G, Kündig TM. Novel Delivery Routes for Allergy Immunotherapy: Intralymphatic, Epicutaneous, and Intradermal. Immunol Allergy Clin North Am 2016 Feb; 36(1):25-37; http://dx.doi.org/10.1016/j.iac.2015.08.006
- Spanos S, Booth R, Koenig H, Sikes K, Gracely E, Kim IK. Jet Injection of 1% buffered lidocaine versus topical ELA-Max for anesthesia before peripheral intravenous catheterization in children: a randomized controlled trial. Pediatr Emerg Care 2008; 24(8):511-5; PMID:18645542; http://dx.doi.org/10.1097/PEC.0b013e31816a8d5b
- Ferayorni A, Yniguez R, Bryson M, Bulloch B. Needle-free jet injection of lidocaine for local anesthesia during lumbar puncture: a randomized controlled trial. Pediatr Emerg Care 2012; 28(7):687-90; PMID:22743744; http://dx.doi.org/10.1097/PEC.0b013e31825d210b
- Cázares-Delgadillo J, Ganem-Rondero A, Kalia YN. Human growth hormone: new delivery systems, alternative routes of administration, and their pharmacological relevance. Eur J Pharm Biopharm 2011 Jun; 78(2):278-88; http://dx.doi.org/10.1016/j.ejpb.2011.01.006
- Fabi S, Sundaram H. The potential of topical and injectable growth factors and cytokines for skin rejuvenation. Facial Plast Surg 2014 Apr; 30(2):157-71; http://dx.doi.org/10.1055/s-0034-1372423
- Laurent PE, Bonnet S, Alchas P, Regolini P, Mikszta JA, Pettis R, Harvey NG. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine 2007 Dec 17; 25(52):8833-42; http://dx.doi.org/10.1016/j.vaccine.2007.10.020
- Alarcon JB, Hartley AW, Harvey NG, Mikszta JA. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin Vaccine Immunol 2007 Apr; 14(4):375-81; http://dx.doi.org/10.1128/CVI.00387-06
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Del Rev 2012; 64:1547-68; http://dx.doi.org/10.1016/j.addr.2012.04.005
- Bariya SH, Gohel MC, Mehta TA, Sharma OP. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol 2012; 64(1):11-29; PMID:22150668; http://dx.doi.org/10.1111/j.2042-7158.2011.01369.x
- Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol 2009; 333:369-93; PMID:19768415
- Camilloni B, Basileo M, Valente S, Nunzi E, Iorio AM. Immunogenicity of intramuscular MF59-adjuvanted and intradermal administered influenza enhanced vaccines in subjects aged over 60: A literature review. Hum Vaccin Immunother 2015; 11(3):553-63; PMID:25714138; http://dx.doi.org/10.1080/21645515.2015.1011562
- Banchereau R, Baldwin N, Cepika AM, Athale S, Xue Y, Yu CI, Metang P, Cheruku A, Berthier I, Gayet I, et al. Transcriptional specialization of human dendritic cell subsets in response to microbial vaccines. Nat Commun 2014 Oct 22; 5:5283; http://dx.doi.org/10.1038/ncomms6283
- Frenck RW Jr, Belshe R, Brady RC, Winokur PL, Campbell JD, Treanor J, Hay CM, Dekker CL, Walter EB Jr, Cate TR, et al. Comparison of the immunogenicity and safety of a split-virion, inactivated, trivalent influenza vaccine (Fluzone®) administered by intradermal and intramuscular route in healthy adults. Vaccine 2011 Aug 5; 29(34):5666-74; http://dx.doi.org/10.1016/j.vaccine.2011.06.010
- Coudeville L, Andre P, Bailleux F, Weber F, Plotkin S. A new approach to estimate vaccine efficacy based on immunogenicity data applied to influenza vaccines administered by the intradermal or intramuscular routes. Hum Vaccin 2010 Oct; 6(10):841-8; http://dx.doi.org/10.4161/hv.6.10.12636
- Morelon E, Pouteil Noble C, Daoud S, Cahen R, Goujon-Henry C, Weber F, Laurent PE, Kaiserlian D, Nicolas JF. Immunogenicity and safety of intradermal influenza vaccination in renal transplant patients who were non-responders to conventional influenza vaccination. Vaccine 2010 Oct 4; 28(42):6885-90; http://dx.doi.org/10.1016/j.vaccine.2010.08.015
- Manuel O, Humar A, Berutto C, Ely L, Giulieri S, Lien D, Meylan PR, Weinkauf J, Pascual M, Nador R, et al. Low-dose intradermal versus intramuscular trivalent inactivated seasonal influenza vaccine in lung transplant recipients. J Heart Lung Transplant 2011 Jun; 30(6):679-84; http://dx.doi.org/10.1016/j.healun.2011.01.705
- Ansaldi F, Valle L, de Florentiis D, Parodi V, Murdaca G, Bruzzone B, Durando P, Setti M, Icardi G. Phase 4 randomized trial of intradermal low-antigen-content inactivated influenza vaccine versus standard-dose intramuscular vaccine in HIV-1-infected adults. Hum Vaccin Immunother 2012 Aug; 8(8):1048-52; http://dx.doi.org/10.4161/hv.20347
- Jo YM, Song JY, Hwang IS, Lee J, Oh SC, Kim JS, Kim SR, Kim WJ, Cheong HJ. Dose sparing strategy with intradermal influenza vaccination in patients with solid cancer. J Med Virol 2009 Apr; 81(4):722-7; http://dx.doi.org/10.1002/jmv.21186
- Marra F, Young F, Richardson K, Marra CA. A meta-analysis of intradermal versus intramuscular influenza vaccines: immunogenicity and adverse events. Influenza Other Resp Viruses 2013; 7:584-603; http://dx.doi.org/10.1111/irv.12000
- Pileggi C, Lotito F, Bianco A, Nobile CG, Pavia M. Immunogenicity and safety of intradermal influenza vaccine in immunocompromized patients: a meta-analysis of randomized controlled trials. BMC Infect Dis 2015 Oct 14; 15:427; http://dx.doi.org/10.1186/s12879-015-1161-z
- Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, Van Damme P. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine 2009 Dec 9; 27(52):7304-12; http://dx.doi.org/10.1016/j.vaccine.2009.10.033
- Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, McKeirnan M, Salem H, Mills G, Reid J, Weber F, Saville M. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis 2008 Sep 1; 198(5):650-8; http://dx.doi.org/10.1086/590434
- Tsang P, Gorse GJ, Strout CB, Sperling M, Greenberg DP, Ozol-Godfrey A, DiazGranados C, Landolfi V. Immunogenicity and safety of Fluzone(®) intradermal and high-dose influenza vaccines in older adults ≥65 years of age: a randomized, controlled, phase II trial. Vaccine 2014 May 1; 32(21):2507-17; http://dx.doi.org/10.1016/j.vaccine.2013.09.074
- Hoon Han S, Hee Woo J, Weber F, Joo Kim W, Ran Peck K, Il Kim S, Hwa Choi Y, Myung Kim J. Immunogenicity and safety of Intanza(®)/IDflu(®) intradermal influenza vaccine in South Korean adults: a multicenter, randomized trial. Hum Vaccin Immunother 2013 Sep; 9(9):1971-7; http://dx.doi.org/10.4161/hv.25295
- Ansaldi F, Orsi A, de Florentiis D, Parodi V, Rappazzo E, Coppelli M, Durando P, Icardi G. Head-to-head comparison of an intradermal and a virosome influenza vaccine in patients over the age of 60: evaluation of immunogenicity, cross-protection, safety and tolerability. Hum Vaccin Immunother 2013 Mar; 9(3):591-8; http://dx.doi.org/10.4161/hv.23240
- Ansaldi F, Canepa P, Ceravolo A, Valle L, de Florentiis D, Oomen R, Vogel FR, Denis M, Samson SI, Icardi G. Intanza(®) 15 mg intradermal influenza vaccine elicits cross-reactive antibody responses against heterologous A(H3N2) influenza viruses. Vaccine 2012 Apr 16; 30(18):2908-13; http://dx.doi.org/10.1016/j.vaccine.2012.02.003
- Moro PL, Harrington T, Shimabukuro T, Cano M, Museru OI, Menschik D, Broder K. Adverse events after Fluzone ® Intradermal vaccine reported to the Vaccine Adverse Event Reporting System (VAERS), 2011-2013. Vaccine 2013 Oct 9; 31(43):4984-7; http://dx.doi.org/10.1016/j.vaccine.2013.08.001
- Gorse GJ, Falsey AR, Johnson CM, Morrison D, Fried DL, Ervin JE, Greenberg DP, Ozol-Godfrey A, Landolfi V, Tsang PH. Safety and immunogenicity of revaccination with reduced dose intradermal and standard dose intramuscular influenza vaccines in adults 18-64 years of age. Vaccine 2013 Dec 5; 31(50):6034-40; http://dx.doi.org/10.1016/j.vaccine.2013.09.012
- Gorse GJ, Falsey AR, Fling JA, Poling TL, Strout CB, Tsang PH. Intradermally-administered influenza virus vaccine is safe and immunogenic in healthy adults 18-64 years of age. Vaccine 2013 May 1; 31(19):2358-65; http://dx.doi.org/10.1016/j.vaccine.2013.03.008
- Eizenberg P, Booy R, Naser N, Mason G, Stamboulian D, Weber F. Acceptance of Intanza® 9 μg intradermal influenza vaccine in routine clinical practice in Australia and Argentina. Adv Ther 2011 Aug; 28(8):640-9; http://dx.doi.org/10.1007/s12325-011-0042-0
- Durando P, Alicino C, Alberti M, Sticchi L, Turello V, Marensi L, Caiazzo AL, Panico MG, Giugliano F, Parlato A, Peluso F, Sgricia S, Icardi G; Italian Intradermal Influenza Vaccine Working Group. Acceptance and safety of the intradermal influenza vaccine among the elderly in Italy: an on-field national study. Adv Ther 2012 Apr; 29(4):312-26; http://dx.doi.org/10.1007/s12325-012-0012-1
- Arnou R, Frank M, Hagel T, Prébet A. Willingness to vaccinate or get vaccinated with an intradermal seasonal influenza vaccine: a survey of general practitioners and the general public in France and Germany. Adv Ther 2011 Jul; 28(7):555-65; http://dx.doi.org/10.1007/s12325-011-0035-z
- Ambrose CS, Wu X. The safety and effectiveness of self-administration of intranasal live attenuated influenza vaccine in adults. Vaccine 2013 Jan; 31(6):857-60; http://dx.doi.org/10.1016/j.vaccine.2012.12.028
- Zahn M, Pursiful P, Carrico R, Woods C, Troutman A. Self-immunization with live attenuated influenza vaccine in a mass vaccination clinic. Dis Med Public Health Preparedness 2013 Apr; 7(2):215-7; http://dx.doi.org/10.1017/dmp.2013.25
- Coleman BL, McGeer AJ, Halperin SA, Langley JM, Shamout Y, Taddio A, Shah V, McNeil SA. A randomized control trial comparing immunogenicity, safety, and preference for self- versus nurse-administered intradermal influenza vaccine. Vaccine 2012 Sep 28; 30(44):6287-93; http://dx.doi.org/10.1016/j.vaccine.2012.08.006
- Dhont PA, Albert A, Brenders P, Podwapinska A, Pollet A, Scheveneels D, Tihon F, Verheyden I, Victor J, Samson SI. Acceptability of Intanza® 15 μg intradermal influenza vaccine in Belgium during the 2010-2011 influenza season. Adv Ther 2012 Jun; 29(6):562-77; http://dx.doi.org/10.1007/s12325-012-0025-9
- Coleman BL, McNeil SA, Langley JM, Halperin SA, McGeer AJ. Differences in efficiency, satisfaction and adverse events between self-administered intradermal and nurse-administered intramuscular influenza vaccines in hospital workers. Vaccine 2015 Nov 27; 33(48):6635-40; http://dx.doi.org/10.1016/j.vaccine.2015.10.095
- Richard SA, Viboud C, Miller MA. Evaluation of Southern Hemisphere influenza vaccine recommendations. Vaccine 2010; 28(15):2693-9; PMID:20153352; http://dx.doi.org/10.1016/j.vaccine.2010.01.053
- Mosnier A, Caini S, Daviaud I, Nauleau E, Bui TT, Debost E, Bedouret B, Agius G, van der Werf S, Lina B, et al. Clinical Characteristics Are Similar across Type A and B Influenza Virus Infections. PLoS One 2015 Sep 1; 10(9):e0136186; http://dx.doi.org/10.1371/journal.pone.0136186
- Gorse GJ, Falsey AR, Ozol-Godfrey A, Landolfi V, Tsang PH. Safety and immunogenicity of a quadrivalent intradermal influenza vaccine in adults. Vaccine 2015 Feb 25; 33(9):1151-9; http://dx.doi.org/10.1016/j.vaccine.2015.01.025
- Greenberg DP, Robertson CA, Noss MJ, Blatter MM, Biedenbender R, Decker MD. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine 2013 Jan 21; 31(5):770-6; http://dx.doi.org/10.1016/j.vaccine.2012.11.074
- Chen D, Bowersock T, Weeratna R, Yeoh T. Current opportunities and challenges in intradermal vaccination. Ther Deliv 2015 Sep; 6(9):1101-8; http://dx.doi.org/10.4155/tde.15.65
- Young F, Marra F. A systematic review of intradermal influenza vaccines. Vaccine 2011 Nov 8; 29(48):8788-801; http://dx.doi.org/10.1016/j.vaccine.2011.09.077
- Weniger BG, Papania MJ. Alternative vaccine delivery methods. In: Plotkin SA, Orenstein WA, Offi PA, editors. Vaccines. 6th edition. New York, NY: Elsevier; 2013. pp. 1200-31
- Banchereau J, Klechevsky E, Schmitt N, Morita R, Palucka K, Ueno H. Harnessing human dendritic cell subsets to design novel vaccines. Ann N Y Acad Sci 2009 Sep; 1174:24-32; http://dx.doi.org/10.1111/j.1749-6632.2009.04999.x
- Teunissen MBM. Intradermal immunization. Düsseldorf, Germany: Springer-Verlag; 2012
