Abstract
In Italy, no specific recommendation toward maternal pertussis immunization during pregnancy has been issued. However, vaccination during pregnancy will be likely integrated in the Italian immunization program in the future. In order to identify barriers to achieving a sufficient vaccination coverage during pregnancy, we investigated knowledge, attitude and practice toward pertussis vaccination during pregnancy through a web-based survey. A total of 343 Italian pregnant women (N = 164) and women in the postpartum period (N = 183) completed the online questionnaire.
More than a half of the study population was uncertain regarding the benefits of the vaccination during pregnancy. Only 1.7% of women in the postpartum had received the vaccination during pregnancy, and 21% of pregnant women declared the intention to be vaccinated in pregnancy. Only 34% would accept the vaccination in the current or in a future pregnancy, if recommended by a physician, and a half would remain uncertain. Perceiving the vaccine as harmful for the fetus’ development is associated to a decreased willingness to be vaccinated if recommended by a HCP, both in pregnant women (OR 0.25 p = 0.010 95% CI 0.09-0.72) and in women in the postpartum period (OR 0.32 p = 0.006 95% CI 0.15-0.72). Our study suggests that the vaccination recommendation by physicians might not be sufficient to adequately raise vaccination coverage against pertussis among Italian pregnant women. A combination of educational interventions and tailored communi-cation campaigns could be implemented to promote maternal immunization.
Introduction
A reemergence of pertussis has been observed in several countries, due to multiple factors, including waning immunity after primary immunization, limited duration of protection after acellular booster doses, and increase of awareness, diagnosis and reporting.Citation1-4 Newborns are at a higher risk for pertussis-related complications, hospitalization, and death, compared with the other age groups, as the primary vaccination cycle starts at 6-8 weeks of age and is completed only a few months later, according to country schedules.Citation5,6
Strategies aimed at maintaining the protection after the primary immunization include the administration of booster doses in preschool age, adolescence and adulthood. However, reaching high immunization coverage in these age groups is challenging and the acellular pertussis vaccine seems to fail to prevent colonization and transmission.Citation7
The immunization of mothers and households immediately after delivery (cocooning strategy) has been recommended to protect infants who are too young to be immunized.Citation5 This approach, however, seems to achieve high coverage rates in selected settings only.Citation5,8-10
Since evidence exists that a tetanus-diphtheria-acellular pertussis immunization (Tdap) is safe and efficacious in protecting the newborn when administered during pregnancy,Citation5,11 several countries introduced the immunization of pregnant women, between the 27th and the 36th weeks of gestation.Citation9,12-19 The vaccination is recommended in every pregnancy.Citation6
In Italy, pertussis incidence decreased after the achievement of a high immunization coverage with acellular vaccines in the Nineties, even though regular epidemic cycles seem to persist.Citation20 Although the Italian immunization program does not offer pertussis vaccination during pregnancy,Citation21 this strategy will be likely integrated in the Italian program in the future.
Before the introduction of new vaccination strategies, knowledge, attitude, and practice of a target population should be investigated, in order to prevent and overcome barriers to achieving a high immunization coverage. To this aim, we conducted a web-based survey among Italian pregnant women before the 27th week of gestation (before the period when immunization in pregnancy is recommended) and postpartum women within 30 d after the delivery. We investigated their knowledge, attitude of pregnant women and practice of postpartum women toward Tdap vaccination in pregnancy. In particular, we focused on the role of the physician's recommendation on the attitude toward Tdap vaccination in the current or future pregnancy.
Results
A total of 775 women accessed the survey webpage and answered the questions regarding the eligibility criteria. A total of 343 women (44.3%) did not meet the eligibility criteria.
Twelve individuals (1.6%) did not accept the informed consent; of those deemed eligible, 347 (44.8%) completed the web survey and were included in the analysis. Among the enrolled participants, 164 (47.3%) were pregnant and 183 (52.7%) were in the postpartum period.
Sociodemographic characteristics and information regarding health and pregnancy are described in . Median age was 34 y. Three quarters of participants were employed and almost 60% were graduated. Less than a half of the enrolled population had received a preconception visit; 10% received at least one vaccination in the 12 months before the pregnancy. Among them, 16 (4.6% of the total participants) received influenza immunization, 12 (3.5%) a MMR dose and 5 (1.4%) a Tdap booster dose. The group of pregnant women were slightly but significantly younger compared to the group of women in the postpartum period.
Table 1. Sociodemographic characteristics and information regarding health and pregnancy.
We found that 63.6% (N =218) of respondents strongly considered healthcare professionals as a trustable source of vaccine information, followed by national Public Health Organizations and Scientific Societies (40.2%, N = 133).
Regarding knowledge about pertussis risks, nearly 35% of respondents did not know that infants < 1 y of age represent the age group with the highest risk of infection.
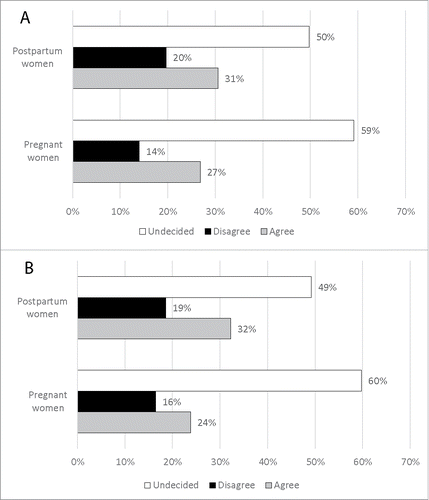
Regarding knowledge of pertussis vaccination during pregnancy, more than a half of the study population answered “undecided” to the specific questions (). Among the enrolled population, 29% considered the vaccine as harmful for the fetus’ development () and almost 18% believed that the vaccination did not protect the infants against pertussis during the early months of life ().
Figure 1. Knowledge of pertussis vaccination during pregnancy. Panel 1A_ Percentage of the pregnant women and of the women in the postpartum who agreed, disagreed or undecided regarding the following statement of the web-based survey: “Pertussis vaccine during pregnancy is harmful for the development of the fetus." Panel 1B_ Percentage of the pregnant women and of the women in the postpartum who agreed, disagreed or undecided regarding the following statement of the web-based survey: “Pertussis vaccine during pregnancy can protect the newborn toward the infection."
Only 12 (3.5%) respondents received a recommendation by their HCP to receive pertussis immunization during pregnancy.
In the population of pregnant women, 34 (21%) expressed their willingness to get vaccinated for pertussis during pregnancy. In the postpartum population, 3 (1.7%) had actually received the pertussis vaccination during pregnancy. reports the reasons for not willing to receive the vaccination (for pregnant women) and not having received the vaccination (for women in the postpartum period). The absence of recommendation by a physician was the main reason of the refusal of the Tdap immunization in pregnancy by postpartum women. In addition, pregnant women did not intend to be vaccinated against pertussis during the third trimester due to an absence of recommendation by a physician. Twenty-three percent were scared by side effects for the fetus’ development (more frequently among pregnant women). Twelve percent declared to refuse the vaccination during pregnancy as they refused vaccinations in general. Seven participants (2%) was advised against vaccination by their HCP.
Table 2. Reasons for not willing to receive the vaccination (for pregnant women) and not having received the vaccination (for women in the postpartum period).
When asking if they would receive pertussis immunization during the current or a future pregnancy if recommended by a HCP almost 34% stated the intention to get the vaccine. Almost 48% declared to be uncertain and more than 18% stated that they would not get the vaccination, even if they had received a recommendation by a HCP. No significant difference was detected between both the populations regarding this item.
At the multivariate analysis, we found that perceiving the vaccine as harmful for the fetus’ development is associated with a decreased willingness to get vaccinated if recommended by a HCP in both populations (pregnant women: OR 0.25 p = 0.010 95% CI 0.09- 0.72; women in the postpartum period: OR 0.32 p = 0.006 95% CI 0.15- 0.72). Moreover, we found that, among women in the postpartum period, willingness to get vaccinated in a future pregnancy was associated with having received a vaccine booster dose in the 12 months before pregnancy (OR 4.6 p = 0.016 95% CI 1.32- 15.98) and with perceiving the vaccine as effective in protecting the newborn against pertussis (OR 3.4 p = 0.001 95% CI 1.65- 7.09).
Discussion
Our study shows a very low rate of pertussis vaccination during pregnancy among women in the postpartum period and a poor attitude toward pertussis immunization among pregnant women. A very low proportion of participants had received a recommendation toward vaccination during pregnancy by their HCP. Only one third of participants would accept the vaccination if recommended by their HCP, and almost half would remain uncertain about the vaccination despite their HCP's recommendation. Moreover, the study population was scarcely informed regarding the benefits of pertussis vaccination during pregnancy.
In Italy, as in most countries, no specific recommendation for maternal immunization against pertussis during pregnancy has been issued by the public health agency. This, together with the general decrease of trust toward vaccinations,Citation22 may explain the substantial lack of culture regarding maternal immunization and the poor attitude and practice toward vaccination during pregnancy, both among women and HCPs. On the other hand, although the Italian Ministry of Health recommends flu vaccination to all women in the 2nd and the 3rd trimester of pregnancy,Citation21 the vaccine uptake during pregnancy is extremely low (less than 4%).Citation23,24
Previous studies have investigated knowledge about pertussis severity, showing that pertussis is considered a serious threat to infants.Citation25,26 In countries where the recommendation for maternal pertussis vaccination is already implemented, several authors have measured a high awareness of the vaccination program (65%-85%), nonetheless, in the same countries, the vaccination uptake during pregnancy is still poor.Citation14-19 A review of the literature showed that vaccine uptake during pregnancy differs by geographical area, context and target population.Citation27
In the literature, studies on the determinants of vaccine refusal indicated that low perception of immunization safety, poor information and lack of professional encouragement represent the main barriers to vaccine uptake.Citation16,17,Citation25 To our knowledge, financial consequences were reported as no important and significant factor in the acceptability of vaccination by women.Citation17 On the other hand, in Italy, booster doses of pertussis vaccinations are currently offered by the National health care system to the population up to 18 y of age, therefore it is likely that the women's decision to accept the vaccination might be influenced by any financial issue.Citation21 A high proportion of participants considered their HCP as a reliable source of information for vaccinations. In line with this observation, we show an improvement of the attitude toward immunization during pregnancy if advised by a HCP (from 21% to 34%). Nevertheless, such improvement is limited. As suggested by others, information received by HCPs has a positive effect on vaccine uptake in pregnant women.Citation1,14,Citation17,28 However, we speculate that HCP recommendation is not sufficient to achieve a remarkable improvement of immunization coverage in this specific population: even if recommended by their physicians, 18% would refuse the immunization and a half would still be uncertain about it. Our finding is in contrast with the results of other studies, which showed a better attitude toward maternal vaccination during pregnancy, when recommended by a HCP (50%-87%).Citation14,15,Citation17,25 Two studies, showing a vaccination acceptance over 80% as an effect of physician's recommendation, were conducted in countries where the vaccination is not yet recommended by the public health agency.Citation12
In our context, the insufficient effect of physician's recommendation could be explained by a number of reasons. First, in Italy, no pertussis outbreaks have been reported recently, and this may contribute to the participants’ skepticism, as they remain unaware of the disease and its consequences. Secondarily, we are observing a worrisome increase and spreading of anti-vaccine sentiments, with a subsequent decrease of vaccine coverage.Citation22 In our study population, 12% declared to be against vaccinations in general. This high percentage might also be explained by the fact that our population has been enrolled on the Internet, and therefore might have been frequently exposed to anti-vaccine movements, which mainly operate through the web.Citation29 The anonymity of this web survey could also partially account for this result. Finally, no information campaign regarding maternal vaccination has ever be implemented in Italy.
Almost half of the population enrolled in our study had a preconception visit with a HCP. This result may suggest that informing about and recommending maternal pertussis vaccination during the preconception period is a further strategy that should be taken into account. Women engaged in specific preconception health behaviors are indeed more likely to receive flu influenza vaccination.Citation30 Finally, HCPs should not neglect to inform women after delivery to get vaccinated during a future pregnancy.
Our study focused on 2 target populations: pregnant and postpartum women. Characteristics of these 2 populations might differ, especially regarding information recall and risk perception. Nevertheless, studying 2 different populations may allow to better programming and tailoring information campaigns.Citation31,32
Our study also has a number of limitations. First, a selection bias may affect the results. Our population was highly educated (58% vs 16% of Italian female population).Citation33 This characteristic has been frequently associated to a general knowledge of vaccine preventable diseases.Citation34,35 Moreover, the visitors of the websites hosting the questionnaire were likely more interested in vaccinations and other health topics compared to the general population. This might have caused a wrong estimation of the uncertainty regarding the vaccination, in comparison to the general population. An additional selection bias regards the use of the Internet, which is often associated with being unvaccinated,Citation36,37 probably because of the exposition to anti-vaccination movement.Citation29 An additional information bias regards the pregnancy or postpartum status, since this information was not verified on medical records. We did not investigate the attitude toward cocooning strategy and vaccination in the postpartum, which could have enriched the profile of our population. One last limit regards the questions on attitude and practice of pertussis immunization during pregnancy, which were introduced by a sentence stating that pertussis immunization is recommended during pregnancy in several countries. This might have created confusion regarding the actual absence of recommendation in the Italian vaccination schedule. Nevertheless, the whole questionnaire was introduced by a paragraph on the etiology and epidemiology of pertussis, explaining all issues regarding the protective strategies through immunization in the life course.
In conclusion, our study shows that, in Italy, despite the availability of scientific evidences, and in lack of a national recommendation, only a negligible proportion of women gets vaccinated against pertussis during pregnancy. A very low proportion of HCP recommends the vaccination during preg-nancy.
Although the recommendation by HCPs is crucial, it might not be sufficient to adequately raise vaccination coverage against pertussis among pregnant women. Therefore, a combination of additional strategies to promote vaccination during pregnancy among Italian women is needed, including educational interventions and tailored communication campaigns, which should be implemented.
Methods
We conducted a cross sectional, web-based survey to investigate, Italian women's knowledge, attitude and practice regarding pertussis vaccinations during pregnancy and in the postpartum period.
The survey was conducted between the 1st of April and the 31st of August 2015 and was performed through the administration of anonymous questionnaires on the Surveymonkey platform (www.surveymonkey.com).
The study was approved by the Bambino Gesù Children's Hospital's Ethical Committee.
The survey targeted 2 populations: a) Italian pregnant women before the 27th week of gestation (i.e. before the time when immunization in pregnancy is recommended in other countries); b) women within 30 d after delivery. In the pregnancy group, we investigated the intention to get vaccinated against pertussis. Among women in the postpartum period, we investigated the vaccine uptake during the recent pregnancy. Finally, in both groups, we investigated both the knowledge and the intention to get vaccinated against pertussis in the present or in a future pregnancy if recommended by any health professional.
The eligibility criteria for enrolment were: consent to participate in the study; Italian language spoken; female gender of respondent; aged 18 y or above; being pregnant before the 27th week of gestation or having delivered less than 30 d before enrolment.
Depending on the pregnancy/postpartum status, participants received a different questionnaire. Both questionnaires included a common section, with questions on the following topics: sociodemographic, health status, source of general and reliable information on vaccinations, general knowledge of pertussis, perceptions regarding safety and efficacy of the Tdap vaccination during pregnancy, having received a recommendation for pertussis vaccination during pregnancy, source of vaccination recommendation.
In both questionnaires, the statement “vaccination against pertussis is recommended between the 27th and 36th week of gestation in several countries” introduced the following population-specific items on attitude and practice:
pregnant women were asked if they were planning to receive the vaccination between the 27th and 36th week of the current gestation. Only the women who answered “No” to the previous question were asked to choose a reason among predefined motivations (see ); those who had not been recommended to receive the vaccination during the present pregnancy were asked if they would get the vaccination if recommended by any HCP.
women in the postpartum period were asked if they had received the vaccination during pregnancy. Those who answered “No” to the previous question were asked to choose a reason among predefined motivations (see ); those who had not been recommended the vaccination during the past pregnancy were asked if they would get vaccinated in a future pregnancy if recommended by any HCP.
The questionnaires were validated through a pilot study conducted among 20 pregnant and postpartum women.
We promoted the study through articles published on the web pages of the Bambino Gesù Children's Hospital and of 2 of the main Italian web-sites dedicated to women's health, preconception, pregnancy and family care (www.nostrofiglio.it; www.periodofertile.it). The web sites account for nearly 3 million visits per year. Articles included a direct link to the Surveymonkey platform. Initially, informed consent was obtained through an online form and eligibility criteria were reviewed through specific questions. If eligibility was met, a specific anonymous questionnaire was administered to the participants, according to their pregnancy/postpartum status. If eligibility criteria were not met, the questionnaires were not accessible. The system did not allow to fill in the questionnaire more than once from the same IP web address to avoid duplicates. The recruitment was conducted until the calculated sample size was reached. In order to measure a prevalence of 30% of pregnant women and postpartum women willing to accept immunization if recommended by any HCP with a precision of ±5% and a confidence level of 95%, a population of 323 individuals was required.
The study was designed according to the current pregnancy healthcare management as recommended in the Italian Ministry of Health guidelines.Citation38 Gynecologists, obstetricians, midwives and general practitioners represent the healthcare professionals involved in the routine and continuous assistance of pregnant women. They operate in the local hospitals and healthcare centers, where specialized physicians and nurses are allowed to administer vaccinations. According to the National Prevention Program, vaccination against rubella, varicella and influenza are offered free of charge to all women in the preconception period and during pregnancy.Citation21,39 The Italian Healthcare System also offers free visits, diagnostic analysis and ultrasounds to all women in the preconception and pregnancy period.Citation39 As measured by the annual survey related to delivery in Italy, Italian women usually attend the hospital for the delivery, while only 0.1% choose to deliver at home.Citation40
We described the proportion of women with different levels of knowledge of pertussis-related topics. Among women in the postpartum period, we reported the proportion of those who received pertussis immunization during pregnancy. In pregnant women only, we described the percentage of women willing to get vaccinated against pertussis during pregnancy. Moreover, we described the proportion of women who would be willing to receive pertussis immunization during the current pregnancy or in a future pregnancy, if recommended by any HCP.
We described the variables as means and standard deviations (SD) or proportions and 95% confidence intervals (CI), as appropriate. The difference between the means of the 2 samples (pregnant/postpartum) was studied by Student's t-test, while the difference between proportions was studied by Pearson's Chi-squared test or Fisher's exact test.
At the univariate analysis, through logistic regression, we explored the association between the attitude to get vaccinated during the present (for pregnant women) or a future pregnancy (women in the postpartum period) if recommended by any HCP, with the following independent variables: age, education level, employment, parity, health status, knowledge regarding the age group at risk for pertussis; knowledge on properties of pertussis vaccination during pregnancy (risk for fetus, protection of the newborn if mother gets vaccinated). Only women who had not been recommended to receive the vaccination and answered “No” to the question on attitude/practice of Tdap vaccination during pregnancy were included in the analysis. A separate analysis was performed in women during pregnancy and in those in the postpartum period. A multivariable analysis was performed, including as independent variables only those which were associated to the outcome with a p-value <0.2 at the univariate analysis. Results were considered statistically significant if the p-value was <0.05.
We used the STATA 12 statistical package to perform the statistical analysis.
Abbreviations
| Tdap | = | Tetanus-diphtheria-acellular-pertussis |
| HCPs | = | Healthcare professionals |
Disclosure of potential conflicts of interest
All authors declare that they have no financial and personal competing interests.
Authors’ contributions
EA coordinated the study, designed the study and participated in the writing process and data review. FG drafted the manuscript. LA, ADA, EP, IC, LR, BF revised the final version of the manuscript. EC performed the statistical analysis. AET conceived the study, participated in its design and coordination and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors acknowledge Bambino Gesù Children Hospital, nostrofiglio.it and periodofertile.it for promoting and hosting the study. Authors also thank all women whose participation made this study possible.
References
- Vilajeliu A, Garcia-Basteiro AL, Bayas JM. Protecting newborns against pertussis: The value of vaccinating during pregnancy. Expert Rev Vaccines 2015; 14:1051-3; PMID:26028129; http://dx.doi.org/10.1586/14760584.2015.1050386
- Terranella A, Rea V, Griffith M, Manning S, Sears S, Farmer A, Martin S, Patel M. Vaccine effectiveness of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine during a pertussis outbreak in maine. Vaccine 2016; PMID:27038131
- Zepp F, Heininger U, Mertsola J, Bernatowska E, Guiso N, Roord J, Tozzi AE, Van Damme P. Rationale for pertussis booster vaccination throughout life in europe. Lancet Infect Dis 2011; 11:557-70; PMID:21600850; http://dx.doi.org/10.1016/S1473-3099(11)70007-X
- Magpantay FM, Domenech DE, Celles M, Rohani P, King AA. Pertussis immunity and epidemiology: Mode and duration of vaccine-induced immunity. Parasitology 2016; 143(7):1-15; PMID:26337864; http://dx.doi.org/10.1017/S0031182015000979
- Pertussis vaccines: WHO position paper - september 2015. Wkly Epidemiol Rec 2015; 90:433-58; PMID:26320265.
- Marti M. Pertussis vaccines: WHO position paper, august 2015-recommendations. Vaccine 2016; 34(12):1423-5; PMID:26562318
- Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A 2014; 111:787-92; PMID:24277828; http://dx.doi.org/10.1073/pnas.1314688110
- Carcione D, Regan AK, Tracey L, Mak DB, Gibbs R, Dowse GK, Bulsara M, Effler PV. The impact of parental postpartum pertussis vaccination on infection in infants: A population-based study of cocooning in western australia. Vaccine 2015; 33:5654-61; PMID:26320420; http://dx.doi.org/10.1016/j.vaccine.2015.08.066
- Forsyth K, Plotkin S, Tan T, Wirsing von Konig CH. Strategies to decrease pertussis transmission to infants. Pediatrics 2015; 135:e1475-82; PMID:25963002; http://dx.doi.org/10.1542/peds.2014-3925
- Swamy GK, Wheeler SM. Neonatal pertussis, cocooning and maternal immunization. Expert Rev Vaccines 2014; 13:1107-14; PMID:25075629; http://dx.doi.org/10.1586/14760584.2014.944509
- Moro PL, McNeil MM, Sukumaran L, Broder KR. The centers for disease control and prevention's public health response to monitoring tdap safety in pregnant women in the united states. Hum Vaccin Immunother 2015; 34(12):1-8; PMID:26378718.
- Varan AK, Esteves-Jaramillo A, Richardson V, Esparza-Aguilar M, Cervantes-Powell P, Omer SB. Intention to accept bordetella pertussis booster vaccine during pregnancy in mexico city. Vaccine 2014; 32:785-92; PMID:24394441; http://dx.doi.org/10.1016/j.vaccine.2013.12.054
- Vizzotti C, Neyro S, Katz N, Juarez MV, Perez Carrega ME, Aquino A, Kaski Fullone F. Maternal immunization in argentina: A storyline from the prospective of a middle income country. Vaccine 2015; 33:6413-9; PMID:26277071; http://dx.doi.org/10.1016/j.vaccine.2015.07.109
- Beel ER, Rench MA, Montesinos DP, Mayes B, Healy CM. Knowledge and attitudes of postpartum women toward immunization during pregnancy and the peripartum period. Hum Vaccin Immunother 2013; 9:1926-31; PMID:23782490; http://dx.doi.org/10.4161/hv.25096
- Dempsey AF, Brewer SE, Sevick C, Pyrzanowski J, Mazzoni S, O'Leary ST. Tdap vaccine attitudes and utilization among pregnant women from a high-risk population. Hum Vaccin Immunother 2016; 12(4):872–8; PMID:26430729
- Donaldson B, Jain P, Holder BS, Lindsay B, Regan L, Kampmann B. What determines uptake of pertussis vaccine in pregnancy? A cross sectional survey in an ethnically diverse population of pregnant women in london. Vaccine 2015; 33(43):5822-8; PMID: 26409139
- Healy CM, Rench MA, Montesinos DP, Ng N, Swaim LS. Knowledge and attitiudes of pregnant women and their providers towards recommendations for immunization during pregnancy. Vaccine 2015; 33:5445-51; PMID:26307234; http://dx.doi.org/10.1016/j.vaccine.2015.08.028
- Laenen J, Roelants M, Devlieger R, Vandermeulen C. Influenza and pertussis vaccination coverage in pregnant women. Vaccine 2015; 33:2125-31; PMID:25796339; http://dx.doi.org/10.1016/j.vaccine.2015.03.020
- Maertens K, Cabore RN, Huygen K, Hens N, Van Damme P, Leuridan E. Pertussis vaccination during pregnancy in belgium: Results of a prospective controlled cohort study. Vaccine 2016; 34:142-50; PMID:26592142; http://dx.doi.org/10.1016/j.vaccine.2015.10.100
- Gonfiantini MV, Carloni E, Gesualdo F, Pandolfi E, Agricola E, Rizzuto E, Iannazzo S, Ciofi Degli Atti ML, Villani A, Tozzi AE. Epidemiology of pertussis in italy: Disease trends over the last century. Euro Surveill 2014; 19:20921; PMID:25323077; http://dx.doi.org/10.2807/1560-7917.ES2014.19.40.20921
- Ministero della salute. piano nazionale prevenzione vaccinale 2012-2014.
- Bonanni P, Ferro A, Guerra R, Iannazzo S, Odone A, Pompa MG, Rizzuto E, Signorelli C. Vaccine coverage in italy and assessment of the 2012-2014 national immunization prevention plan. Epidemiol Prev 2015; 39:146-58; PMID:26499433.
- Rizzo C, Rota MC, Bella A, Giannitelli S, De Santis S, Nacca G, Pompa MG, Vellucci L, Salmaso S, Declich S. Response to the 2009 influenza A(H1N1) pandemic in italy. Euro Surveill 2010; 15:19744; PMID:21163178
- Fabiani M, Bella A, Rota MC, Clagnan E, Gallo T, D'Amato M, Pezzotti P, Ferrara L, Demicheli V, Martinelli D, et al. A/H1N1 pandemic influenza vaccination: A retrospective evaluation of adverse maternal, fetal and neonatal outcomes in a cohort of pregnant women in italy. Vaccine 2015; 33:2240-7; PMID:25820060; http://dx.doi.org/10.1016/j.vaccine.2015.03.041
- Chamberlain AT, Seib K, Ault KA, Orenstein WA, Frew PM, Malik F, Cortes M, Cota P, Whitney EA, Flowers LC, et al. Factors associated with intention to receive influenza and tetanus, diphtheria, and acellular pertussis (tdap) vaccines during pregnancy: A focus on vaccine hesitancy and perceptions of disease severity and vaccine safety. PLoS Curr 2015; 7; http://dx.doi.org/10.1371/currents.outbreaks.d37b61bceebae5a7a06d40-a301cfa819; PMID:25789203
- Wiley KE, Cooper SC, Wood N, Leask J. Understanding pregnant women's attitudes and behavior toward influenza and pertussis vaccination. Qual Health Res 2015; 25:360-70; PMID:25246330; http://dx.doi.org/10.1177/1049732314551061
- Wilson RJ, Paterson P, Jarrett C, Larson HJ. Understanding factors influencing vaccination acceptance during pregnancy globally: A literature review. Vaccine 2015; 33(47):6420-9; PMID: 26320417
- Larson HJ. Maternal immunization: The new "normal" (or it should be). Vaccine 2015; 33(47):6374-5; PMID:26320418
- Tafuri S, Gallone MS, Cappelli MG, Martinelli D, Prato R, Germinario C. Addressing the anti-vaccination movement and the role of HCWs. Vaccine 2014; 32:4860-5; PMID:24262311; http://dx.doi.org/10.1016/j.vaccine.2013.11.006
- Scheminske M, Henninger M, Irving SA, Thompson M, Williams J, Shifflett P, Ball SW, Avalos LA, Naleway AL, >Pregnancy and Influenza Project Workgroup. The association between influenza vaccination and other preventative health behaviors in a cohort of pregnant women. Health Educ Behav 2015; 42:402-8; PMID:25504630; http://dx.doi.org/10.1177/1090198114560021
- Goldstein S, MacDonald NE, Guirguis S, SAGE Working Group on Vaccine Hesitancy. Health communication and vaccine hesitancy. Vaccine 2015; 33:4212-4; PMID:25896382; http://dx.doi.org/10.1016/j.vaccine.2015.04.042
- WHO. 2013.
- Ministero del lavoro e delle politiche sociali.
- Bianco A, Pileggi C, Iozzo F, Nobile CG, Pavia M. Vaccination against human papilloma virus infection in male adolescents: Knowledge, attitudes, and acceptability among parents in italy. Hum Vaccin Immunother 2014; 10:2536-42; PMID:25483471; http://dx.doi.org/10.4161/21645515.2014.969614
- Giambi C, D'Ancona F, Del Manso M, De Mei B, Giovannelli I, Cattaneo C, Possenti V, Declich S, Local Representatives for VALORE. Exploring reasons for non-vaccination against human papillomavirus in italy. BMC Infect Dis 2014; 14:545,014-0545-9; PMID:25410754; http://dx.doi.org/10.1186/s12879-014-0545-9
- Restivo V, Napoli G, Marsala MG, Bonanno V, Sciuto V, Amodio E, Calamusa G, Vitale F, Firenze A. Factors associated with poor adherence to MMR vaccination in parents who follow vaccination schedule. Hum Vaccin Immunother 2015; 11:140-5; PMID:25483527; http://dx.doi.org/10.4161/hv.34416
- Stefanelli P, Rezza G. Contrasting the anti-vaccine prejudice: A public health perspective. commentary. Ann Ist Super Sanita 2014; 50:6-9; PMID:24695248
- Ministero della Salute, Sistema Nazionale per le Linee Guida. Linee guida, gravidanza fisiologica. aggiornamento 2011.
- Ministero della Salute. D.M. 10 SETTEMBRE 1998 aggiornamento del d.m. 6 marzo 1995 concernente l'aggiornamento del d.m. 14 aprile 1984 recante protocolli di accesso agli esami di laboratorio e di diagnostica strumentale per le donne in stato di gravidanza ed a tutela della maternità.
- Fulvio Basili, Anita Di Rosa, Valerio Montorio e Cristina Tamburini. Certificato di assistenza al parto (CeDAP). analisi dell’evento nascita - anno 2013.
