ABSTRACT
Heat shock protein 70, (Hsp70) constitutes a powerful system of cytoprotection in all organisms studied to date. Exerting such activity, Hsp70 rescues cancer cells from antitumor therapy, posing a great challenge for oncologists. In contrast to its protective action, Hsp70 was found to be released from cancer cells, prompting cytotoxic lymphocytes to target and kill the tumor. A great number of vaccines have been developed on the basis of the ability of Hsp70 to present tumor antigen or to elevate the sensitivity of cancer cells to cytotoxic lymphocytes. In this commentary, we consider novel data on the employment of pure Hsp70 in the therapy of glioma and melanoma malignancies. We show that intratumorally delivered Hsp70 penetrates cancer cells and pulls its intracellular analog outside of the cell. This displacement may activate cells, constituting both innate and adaptive immunity. In vivo delivery of Hsp70 was found to inhibit tumor growth and to extend survival. The technology of intratumoral injection of pure Hsp70 passed through preclinical trials and was investigated in clinics for children with brain cancer; the results show the safety and feasibility of a new approach.
Immunotolerance of tumors and heat shock response
Malignant tumors evade immune attack of the host, although cancer cells themselves have all features necessary for the activation of host immune response: they often bear mutated or aberrant proteins, which can trigger the immune reaction of the body by displaying tumor-associated antigens (TAA) and by stimulation of inflammation at the tumor site.Citation1 According to a well-accepted opinion, immune-suppressive mechanisms are provided by cells inhabiting the tumor microenvironment, including T-cells, macrophages, dendritic cells (DC), myeloid derived suppressive cells (MDSC) and some other representatives of the immune system. Together with tumors, these cells supply the microenvironment with immunosuppressive cytokines, such as IL-10 and TGF-β, which leads to a concentration of MDSC at the tumor location, activation of FoxP3+ regulatory T-cells (Tregs), polarization of tumor-associated macrophages to the M2 phenotype and inhibition of T-cell proliferation.Citation2 The paradox is that, instead of protecting the organism against hyperplasia, the above cells defend the tumor by constantly making the latter more aggressive, actively metastasizing.
To overcome the above-mentioned tolerance, immunotherapeutic technologies based on strong adjuvants and powerful triggers of both innate and adaptive antitumor immunity are warranted. Molecular chaperones or heat shock proteins exert both activities in numerous simulations and vaccine constructs. The heat-shock response is the universal system of cellular reaction to a variety of stressful or toxic factors; the reaction is triggered by a special transcription factor (HSF1) and causes the synthesis of a large group of heat shock proteins belonging to different families commonly designated by the molecular mass of a typical representative, e.g., Hsp27, Hsp40, Hsp70, Hsp90, Hsp110, and some others. Most Hsps were found to be molecular chaperones that formed a system of protein quality control and participated in almost all functions of a cell. It is important that the heat shock response triggered by HSF1 is most effective in stem-like cancer cells; in these cells, it controls the expression of hundreds of genes necessary for cell survival in extremely toxic surroundings or in conditions of an anti-cancer therapy.Citation3 Moreover, stroma cells of the tumor microenvironment demonstrate strong activation of Hsf1, a process induced by highly tumorigenic cells and indicating that the heat-shock response is a significant factor of reprogramming the cells inhabiting a tumor niche.Citation4 Many Hsps possess cytoprotective activity and their enhanced expression typical of cancer cells poses a substantial problem for anticancer therapy (for review see ref. Citation5). Fortunately, this obstacle can be circumvented by employing the specific properties of different Hsps in the design of anticancer vaccines.
Heat shock proteins in anti-cancer vaccines
One of the most frequently used in Hsp-based vaccine proteins is Grp96 (glucose-regulated protein), which forms tight complexes with TAA from colorectal cancer, pancreatic adenocarcinoma and melanoma.Citation6-8 Other candidates for chaperonic vaccine are the high-molecular weight heat shock proteins, Hsp110 and Hsp170 (Grp170). These two chaperones were shown to be effective when combined in an immunization complex with known tumor antigens, gp100 and intracellular domain of human epidermal growth factor-2 (HER-2). The appropriate fusion constructs suppressed B16 melanoma growthCitation9 and mammary tumor development in FBV-neo transgenic mice.Citation10 Another Hsp, Hsp60, was also shown to exert immunomodulatory capacity when its gene was fused into a DNA vaccine construct with papilloma virus tumor antigens.Citation11 Interestingly, the mixture of Hsps purified from mouse sarcoma containing Hsp60, Hsp70, Grp96, and Hsp110, also demonstrated a profound anticancer immune response in autologous tumors.Citation12
In this Commentary, we pay special attention to Hsp70-based vaccines. The Hsp70 family of closely related genes encodes 11 to 14 proteins in mammalian cells, of which 2 may be induced by a great number of harmful and/or therapeutic factors. Elevated synthesis of Hsp70 causes more efficient function of the chaperonic machinery and leads to a reduction of cell sensitivity to repeated action of the same or of other stressful agents, e.g., to the development of a tolerant state. The majority of cancer cells explored to date contain a high amount of Hsp70 and therefore, are protected from environmental stressors; most typical of the latter is oxidative stress, whose deleterious effect was found to depend on the chaperone content.Citation13
Thus, Hsp70 as do many other molecular chaperones, plays a dual role in cancer cells: it protects the latter from natural stressors and antitumor drugs and, when transported to the exterior of a cell, it can represent its antigenic structures to natural killer cells or to dendritic cells. Activation of both types of immune systems by Hsp70 was convincingly proven in studies by several groups (for review see ref. Citation14). P. Srivastava was the first to discover the development of the individual Hsp70-mediated response in animals immunized with preparations of their own chaperone.Citation15 In these works, Hsp70 was isolated from the malignant tissue using the protocol omitting the step of chromatography on adenosine-triphosphate-agarose. The release of the TAA bound to ATP made the preparation immunologically ineffective, thus suggesting the importance of keeping the intact complex Hsp70-TAA. These studies provoked a great number of anticancer vaccines based on the property of Hsp70 to bind TAA and to process these in APC; the samples of individual cancers were used to immunize the patients.Citation16
The technology of personal immunotherapy was subjected to clinical trials to cure high-risk breast cancer and chronic myelogenous leukemia from 2001 to 2008, and though the results were not perfect (www.clinicaltrials.gov), the development of more efficient vaccine constructions continues to date, focusing on newer methodological aspects (see ). First, instead of Hsp70, another stress protein, Grp96 purified from cancerous tissue, was used. The latter vaccine, named Oncophage, was also subjected to clinical trials in which the protein alone or coupled with Gleevec™ was administered to oncological patients (Novartis, Agenus, Inc. 2012). Secondly, in many settings, Hsp70 coupled to a certain peptide antigen derived, for instance, from papilloma virus (EP7) was employed to immunize the animals in a therapeutic setting; the efficacy of such vaccines strongly depends on a tumor origin and antigen presented by the chaperone (for a review see ref. Citation27). One of the recent reports describes the application of mesothelin in complex with mycobacterial Hsp70 in a mouse model of ovarian cancer and mesothelioma.Citation28 Particularly high anticancer activity was found when the animals were immunized with the Hsp70-TAA complex isolated from fusions of dendritic and tumor cells; this immunization resulted in a T cell-mediated immune response including a significant increase in CD8+ T cells and induction of effector and memory T cells.Citation26 Thirdly, DNA vaccines can be employed instead of protein-peptide complexes. In these constructs, the Hsp70 gene is fused with a certain sequence of known TAA and expected to be produced in a host tissue (see ). Another group of immunotherapeutic tools is based on the discovery of the surface-bound form of Hsp70; this part of Hsp70 molecules appears on the outer membrane of cancer cells treated with certain factors, one of which was shown to be mild heat shock.Citation29 The membrane-bound Hsp70 exposes its 14-mer TKD peptide at the cell surface, which serves also as an effective transport-promoting domain for the whole Hsp70 molecule.Citation30 One of the important features of the TKD peptide is its specific recognition by cytotoxic NK cells and several technologies were developed employing this kind of tumor cell recognition and eradication (see ). Among these approaches are administration of certain anticancer drugs, photodynamic therapy, X-ray therapy, and others.Citation31,32 Because most cancer cells express TKD peptide on their surface, even under normal conditions, the claim was made that the peptide was a potent tumorigenicity marker, and specific antibody to the peptide was generated and employed in therapeutic modalities.Citation32 Because most Hsp70-based vaccines use the activity of extracellular chaperone or of its domain exposed on a cell surface, it seemed worthwhile to test whether pure Hsp70 delivered to the tumor might induce an anti-cancer immune response. In one such study, recombinant Hsp70 was delivered inside B16 mouse melanoma, causing a delay in tumor growth. The therapeutic effect was enhanced if the protein was injected together with magnetic nanoparticles to heat the tumor and this combinative therapy led to a strong inhibition of tumor growth that was presumably due to an elevation of the specific immune response as follows from the data on interferon-gamma production.Citation33
Table 1. Immunomodulatory activities of Hsp70.
Anti-tumor activity of pure recombinant Hsp70: possible mechanism and therapeutic application
The formulation of anti-cancer vaccine offered by Srivastava in 1990 has a decisive advantage as a personalized approach. However, the method has at least 2 major disadvantages: firstly, the difficulty in obtaining sufficient tumor material for Hsp70-TAA complex isolation and secondly, the high cost of the latter procedure. These problems made the results of phase III clinical trials of personalized Hsp-based vaccine for patients with melanoma upsetting.Citation34
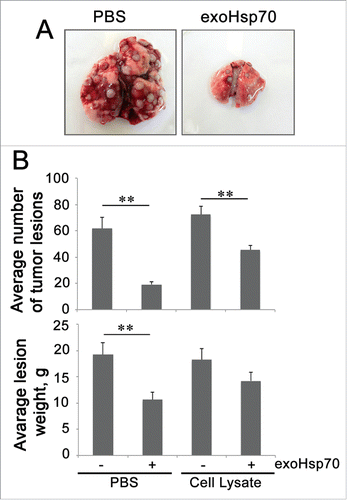
In our experiments demonstrating high metastatic activity with rat RA-2 rhabdomyosarcoma,Citation35,36 we immunized the animals with a preparation of Hsp70 purified from bovine muscle and the latter mixture with tumor extract; a second preparation was administered because it might give a more pronounced immune response to TAA of RA-2. Earlier, this mixture was found to give a profound antitumor response when employed as a therapeutic modality. The unexpected result was that the most efficient reduction in tumor lesion number and size was found in rats injected with pure Hsp70Citation37 (). These data prompted us to study in more detail the immunomodulatory effect of pure Hsp70 delivered into tumors of other origins. Pure human recombinant Hsp70 was injected singly or via Alzet micropump into brains of rats with intracranial C6 glioma tumor. Such injections, particularly those done using an osmotic pump, caused a significant delay in tumor growth and increase the survival of tumor-bearing animals. Importantly, the therapeutic effect was accompanied by the growth of specific CD4+ and CD8+ T lymphocytes and activation of cytotoxic NK cells.Citation38 In the other setting, Hsp70 in a hydrogel composition was applied on the skin surface on top of B16 mouse melanoma. In this case, we also observed substantial inhibition of tumor growth as well as an elevation of the survival rate.Citation39,40
Figure 1. Administration of pure Hsp70 demonstrates a more profound anti-tumor prophylactic effect in a model of RA-2 rat rhabdomyosarcoma as compared with an Hsp70 mixture with tumor antigens. A. Lungs of experimental animals preliminary injected i.v. with PBS or Hsp70 alone or mixed with tumor extract were excised on the 21st day after tumor cell administration. B. Average number and weight of tumor lesions in experimental groups.
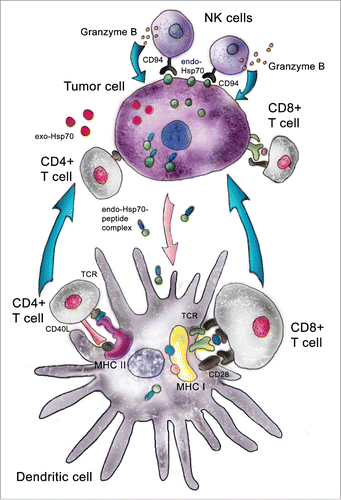
To study the mechanism of Hsp70 immunomodulatory effects, in vitro experiments were carried out in which C6 glioma or B16 melanoma cells incubated with fluorescently labeled Hsp70 were stained with antibody specifically recognizing the TKD-peptide of Hsp70. To our surprise, we observed that only formerly endogenous Hsp70 was presented on the cancer cell surface while exogenous chaperone passed through the cell body and was released without delay on the plasma membrane. The most interesting observation was that exogenous Hsp70, by extrusion of its cellular analog, increased the sensitivity of tumor cells to cytotoxic lymphocytes in the appropriate assay.Citation41 Using affinity chromatography, we found that besides the effect of displacement of its endogenous counterpart, exogenous Hsp70 causes the former to be released into the extracellular milieu. Thus, the hypothetical mechanism of pure Hsp70 added to a cancer cell culture or injected intratumorally may function along 2 pathways. First, exposure of Hsp70 on the exterior side of the plasma membrane makes cancer cells accessible to cytotoxic lymphocytes, NK cells (, upper part). This recognition may be performed by CD94 receptors of NK cells and leads to a release of Granzyme B molecules that attack a target tumor cell. This view completely agrees with data from the Multhoff lab.Citation42 Another pathway is activated by the efflux of Hsp70 molecules, presumably carrying TAA from cells affected by exogenous Hsp70; this flow can also be a result of tumor cell disruption due to the attack of cytotoxic cells (, lower part). According to widely spread opinion, Hsp70 released from tumor cells penetrates inside DC where TAA can be presented in context with MHC Class I or Class II antigen complexes. MHC class II receptor-mediated complexes bind to T-cell receptors on CD4+ cells, whereas MHC class I interacts with CD8+ cells, giving rise to the expansion of the cytotoxic cell population.Citation43 Both components of the general anticancer immune response, innate and adaptive, are presented in the technology of intratumoral delivery of pure Hsp70. This technology was recently passed through preclinical trials and was subjected to limited investigation in the Children's Brain Cancer Clinics of the Polenov's Russian Research Institute of Neurosurgery in St. Petersburg. The study demonstrated the safety of recombinant Hsp70 and feasibility of its intratumoral delivery in patients with brain cancers.Citation44 The experiments were initiated in 2011 and the follow-up period was 12 months; in 2012, experimental clinical investigations were stopped in Russia according to new Federal law. The information as of March, 2016 shows that 11 of the 12 patients who received intratumoral injections of the chaperone are alive, and this is the best argument in favor of Hsp70-based anti-tumor therapy.
Figure 2. Pathways used by intratumorally delivered Hsp70. Pure Hsp70 penetrates inside a tumor cell and withdraws its intracellular analog to the outer membrane; this surface-attached Hsp70 is targeted by cytotoxic lymphocytes, NK cells. Exogenous Hsp70 occurring inside a tumor cell pulls out its endogenous counterpart, which transfers tumor antigens to dendritic cells, which present these in complex with MHC class I or class II antigens. Using different receptor structures, mature DCs activate CD8+ and CD4+ lymphocytes and trigger this specific cytotoxic effect.
Abbreviations
| DC | = | dendritic cells |
| HSF1 | = | heat shock factor |
| Hsp | = | heat shock protein |
| Grp | = | glucose regulated protein |
| HER-2 | = | human epidermal growth factor-2 |
| IL-10 | = | interleukin-10 |
| MDSC | = | myeloid derived suppressive cells |
| MHC | = | major histocompatibility complex |
| NK cell | = | natural killer cells |
| TAA | = | tumor-associated antigens |
| TGF-β | = | tumor growth factor–β |
| Tregs | = | regulatory T-cells |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Dr. Elena R. Mikhaylova for her kind help with drawing of .
Funding
This work was supported by the Russian Scientific Foundation (Grant # 14-50-00068).
References
- Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol 2016; 39:1-6; PMID:26609943; http://dx.doi.org/10.1016/j.coi.2015.10.009
- Razavi SM, Lee KE, Jin BE, Aujla PS, Gholamin S, Li G. Immunee strategies of glioblastoma. Front Surg 2016; 3:11; PMID:26973839; http://dx.doi.org/10.3389/fsurg.2016.00011
- Mendillo ML, Santagata S, Koeva M, Bell GW, Hu R, Tamimi RM, Fraenkel E, Ince TA, Whitesell L, Lindquist S. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell 2012; 150:549-62; PMID:22863008; http://dx.doi.org/10.1016/j.cell.2012.06.031
- Scherz-Shouval R, Santagata S, Mendillo ML, Sholl LM, Ben-Aharon I, Beck AH, Dias-Santagata D, Koeva M, Stemmer SM, Whitesell L, et al. The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell 2014; 158:564-78; PMID:25083868; http://dx.doi.org/10.1016/j.cell.2014.05.045
- Guzhova IV, Shevtsov MA, Abkin SV, Pankratova KM, Margulis BA. Intracellular and extracellular Hsp70 chaperone as a target for cancer therapy. Int J Hyperthermia 2013; 29:399-408; PMID:23845032; http://dx.doi.org/10.3109/02656736.2013.807439
- Mazzaferro V, Coppa J, Carrabba MG, Rivoltini L, Schiavo M, Regalia E, Mariani L, Camerini T, Marchian∫ A, Andreola S, et al. Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res 2003; 9:3235-45; PMID:12960108
- Santis G, Senzer NN, Champagne P, Isakov L, Teofilovici F. Phase II feasibility study of autologous vaccine (HSPPC-96) in pacients with resectable lung cancer. J Clin Oncol 2008; 26:7584
- Ampie L, Choy W, Lamano JB, Fakurnejad S, Bloch O, Parsa AT. Heat shock protein vaccines against glioblastoma: from bench to bedside. J Neurooncol 2015; 123:441-8; PMID:26093618; http://dx.doi.org/10.1007/s11060-015-1837-7
- Wang XY, Kazim L, Repasky EA, Subjeck JR. Immunization with tumor-derived ER chaperone grp170 elicits tumor-specific CD8+ T-cell responses and reduces pulmonary metastatic disease. Int J Cancer 2003; 105:226-31; PMID:12673684; http://dx.doi.org/10.1002/ijc.11058
- Manjili MH, Henderson R, Wang XY, Chen X, Li Y, Repasky E, Kazim L, Subjeck JR. Development of a recombinant HSP110-HER-2/neu vaccine using the chaperoning properties of HSP110. Cancer Res 2002; 62:1737-42; PMID:11912148
- Huang CY, Chen CA, Lee CN, Chang MC, Su YN, Lin YC, Hsieh CY, Cheng WF. DNA vaccine encoding heat shock protein 60 co-linked to HPV16 E6 and E7 tumor antigens generates more potent immunotherapeutic effects than respective E6 or E7 tumor antigens. Gynecol Oncol 2007; 107:404-12; PMID:17905417; http://dx.doi.org/10.1016/j.ygyno.2007.06.031
- Wang Y, Liu SY, Yuan M, Tang Y, Guo QY, Cui XM, Sui X, Peng J. Prophylactic antitumor Effect of Mixed Heat Shock Proteins/Peptides in Mouse Sarcoma. Chin Med J (Engl) 2015; 128:2234-41; PMID:26265619; http://dx.doi.org/10.4103/0366-6999.162516
- Lazarev VF, Nikotina AD, Mikhaylova ER, Nudler E, Polonik SG, Guzhova IV, Margulis BA. Hsp70 chaperone rescues C6 rat glioblastoma cells from oxidative stress by sequestration of aggregating GAPDH. Biochem Biophys Res Commun 2016; 470:766-71; PMID:26713364; http://dx.doi.org/10.1016/j.bbrc.2015.12.076
- Murshid A, Gong J, Stevenson MA, Calderwood SK. Heat shock proteins and cancer vaccines: developments in the past decade and chaperoning in the decade to come. Expert Rev Vaccines 2011; 10:1553-68; PMID:22043955; http://dx.doi.org/10.1586/erv.11.124
- Blachere NE, Udono H, Janetzki S, Li Z, Heike M, Srivastava PK. Heat shock protein vaccines against cancer. J Immunother Emphasis Tumor Immunol 1993; 14:352-6; PMID:8280719; http://dx.doi.org/10.1097/00002371-199311000-00016
- Gong J, Zhang Y, Durfee J, Weng D, Liu C, Koido S, Song B, Apostolopoulos V, Calderwood SK. A heat shock protein 70-based vaccine with enhanced immunogenicity for clinical use. J Immunol 2010; 184:488-96; PMID:19949080; http://dx.doi.org/10.4049/jimmunol.0902255
- Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med 1997; 186:1315-22; PMID:9334371; http://dx.doi.org/10.1084/jem.186.8.1315
- Binder RJ, Blachere NE, Srivastava PK. Heat shock protein-chaperoned peptides but not free peptides introduced into the cytosol are presented efficiently by major histocompatibility complex I molecules. J Biol Chem 2001; 276:17163-71; PMID:11278929; http://dx.doi.org/10.1074/jbc.M011547200
- Schmid TE, Multhoff G. Radiation-induced stress proteins - the role of heat shock proteins (HSP) in anti- tumor responses. Curr Med Chem 2012; 19:1765-70; PMID:22414085; http://dx.doi.org/10.2174/092986712800099767
- Etminan N, Peters C, Lakbir D, Bünemann E, Börger V, Sabel MC, Hänggi D, Steiger HJ, Stummer W, Sorg RV. Heat-shock protein 70-dependent dendritic cell activation by 5-aminolevulinic acid-mediated photodynamic treatment of human glioblastoma spheroids in vitro. Br J Cancer 2011; 105:961-9; PMID:21863026; http://dx.doi.org/10.1038/bjc.2011.327
- Zong J, Wang C, Liu B, Liu M, Cao Y, Sun X, Yao Y, Sun G. Human hsp70 and HPV16 oE7 fusion protein vaccine induces an effective antitumor efficacy. Oncol Rep 2013; 30:407-12; PMID:23660931
- Xu C, Sun Y, Wang Y, Yan Y, Shi Z, Chen L, Lin H, Lü S, Zhu M, Su C, et al. CEA promoter-regulated oncolytic adenovirus-mediated Hsp70 expression in immune gene therapy for pancreatic cancer. Cancer Lett 2012; 319:154-63; PMID:22261331; http://dx.doi.org/10.1016/j.canlet.2012.01.009
- Xie Y, Bai O, Zhang H, Yuan J, Zong S, Chibbar R, Slattery K, Qureshi M, Wei Y, Deng Y, et al. Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8(+) CTL- and NK-mediated antitumour immunity than exosomes released from heat-shocked tumour cells expressing cytoplasmic HSP70. J Cell Mol Med 2010; 14:2655-66; PMID:19627400; http://dx.doi.org/10.1111/j.1582-4934.2009.00851.x
- Wang XP, Lin HP, Wang QX, Gu Y. Specific antitumor immunity induced by cross-linking complex heat shock protein 72 and alpha-fetoprotein. Cancer Biother Radiopharm 2012; 27:189-97; PMID:22372558; http://dx.doi.org/10.1089/cbr.2011.1135
- Dodd K, Nance S, Quezada M, Janke L, Morrison JB, Williams RT, Beere HM. Tumor-derived inducible heat-shock protein 70 (HSP70) is an essential component of anti-tumor immunity. Oncogene 2015; 34:1312-22; PMID:24662819; http://dx.doi.org/10.1038/onc.2014.63
- Enomoto Y, Bharti A, Khaleque AA, Song B, Liu C, Apostolopoulos V, Xing PX, Calderwood SK, Gong J. Enhanced immunogenicity of heat shock protein 70 peptide complexes from dendritic cell-tumor fusion cells. J Immunol 2006; 177:5946-55; PMID:17056519; http://dx.doi.org/10.4049/jimmunol.177.9.5946
- Calderwood SK, Gong J, Stevenson MA, Murshid A. Cellular and molecular chaperone fusion vaccines: targeting resistant cancer cell populations. Int J Hyperthermia 2013; 29:376-9; PMID:23682824; http://dx.doi.org/10.3109/02656736.2013.792126
- Yuan J, Kashiwagi S, Reeves P, Nezivar J, Yang Y, Arrifin NH, Nguyen M, Jean-Mary G, Tong X, Uppal P, et al. A novel mycobacterial Hsp70-containing fusion protein targeting mesothelin augments antitumor immunity and prolongs survival in murine models of ovarian cancer and mesothelioma. J Hematol Oncol 2014; 7:15; PMID:24565018; http://dx.doi.org/10.1186/1756-8722-7-15
- Gastpar R, Gross C, Rossbacher L, Ellwart J, Riegger J, Multhoff G. The cell surface-localized heat shock protein 70 epitope TKD induces migration and cytolytic activity selectively in human NK cells. J Immunol 2004; 172:972-80; PMID:14707070; http://dx.doi.org/10.4049/jimmunol.172.2.972
- Moser C, Schmidbauer C, Gürtler U, Gross C, Gehrmann M, Thonigs G, Pfister K, Multhoff G. Inhibition of tumor growth in mice with severe combined immunodeficiency is mediated by heat shock protein 70 (Hsp70)-peptide-activated, CD94 positive natural killer cells. Cell Stress Chaperones 2002; 7:365-73; PMID:12653481; http://dx.doi.org/10.1379/1466-1268(2002)007%3c0365:IOTGIM%3e2.0.CO;2
- Mitra S, Giesselman BR, Jesus-Andrino FJ, Foster TH. Tumor response to mTHPC-mediated photodynamic therapy exhibits strong correlation with extracellular release of Hsp70. Lasers Surg Med 2011; 43:632-43; PMID:22057491; http://dx.doi.org/10.1002/lsm.21108
- Specht HM, Ahrens N, Blankenstein C, Duell T, Fietkau R, Gaipl US, Günther C, Gunther S, Habl G, Hautmann H, et al. Heat Shock Protein 70 (Hsp70) Peptide activated natural killer (NK) cells for the treatment of patients with non-small cell lung cancer (NSCLC) after radiochemotherapy (RCTx) - from preclinical studies to a clinical Phase II trial. Front Immunol 2015; 6:162; PMID:25926832; http://dx.doi.org/10.3389/fimmu.2015.00162
- Ito A, Matsuoka F, Honda H, Kobayashi T. Atitumor effects of combined therapy of recombinant heat shock protein 70 and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Immunol Immunother 2004; 53:26-32; PMID:14551746; http://dx.doi.org/10.1007/s00262-003-0416-5
- Berd D. A tale of two pities: autologous melanoma vaccines on the brink. Hum Vaccin Immunother 2012; 8:1146-51; PMID:22854662; http://dx.doi.org/10.4161/hv.20923
- Guzhova IV, Margulis BA, Kaminskaya EV. Antibody against p58 surface antigen of RA-2 rat rhabdomyosarcoma cells inhibits their metastatic activity. Int J Cancer 1992; 52:892-5; PMID:1459731; http://dx.doi.org/10.1002/ijc.2910520611
- Kaminskaia EV, Stepan'ian LI, Vakhtin IuB. Variability in the DNA content in the progeny of the clonogenic cells from transplantable rat rhabdomyosarcoma. Tsitologiia 1981; 23:811-7; PMID:7281230
- Guzhova IV, Komarova EIu, Pimenova AA, Bakhtin IuB, Kaminskaia EV, Margulis BA. The role of extracellular chaperone Hsp70 in creating antitumor immunity in rat rhabdomyosarcoma RA-2 model. Vopr Onkol 2008; 54:611-7; PMID:19069476
- Shevtsov MA, Pozdnyakov AV, Mikhrina AL, Yakovleva LY, Nikolaev BP, Dobrodumov AV, Komarova EY, Meshalkina DA, Ischenko AM, Pitkin E, et al. Effective immunotherapy of rat glioblastoma with prolonged intratumoral delivery of exogenous heat shock protein Hsp70. Int J Cancer 2014; 135:2118-28; PMID:24691976; http://dx.doi.org/10.1002/ijc.28858
- Abkin SV, Pankratova KM, Komarova EY, Guzhova IV, Margulis BA. Hsp70 chaperone-based gel composition as a novel immunotherapeutic anti-tumor tool. Cell Stress Chaperones 2013; 18:391-6; PMID:23233202; http://dx.doi.org/10.1007/s12192-012-0391-x
- Abkin SV, Ostroumova OS, Komarova EY, Meshalkina DA, Shevtsov MA, Margulis BA, Guzhova IV. Phloretin increases the anti-tumor efficacy of intratumorally delivered heat-shock protein 70 kDa (HSP70) in a murine model of melanoma. Cancer Immunol Immunother 2016; 65:83-92; PMID:26646850; http://dx.doi.org/10.1007/s00262-015-1778-1
- Shevtsov MA, Komarova EY, Meshalkina DA, Bychkova NV, Aksenov ND, Abkin SV, Margulis BA, Guzhova IV. Exogenously delivered heat shock protein 70 displaces its endogenous analogue and sensitizes cancer cells to lymphocytes-mediated cytotoxicity. Oncotarget 2014; 5:3101-14; PMID:24797019; http://dx.doi.org/10.18632/oncotarget.1820
- Gross C, Koelch W, DeMaio A, Arispe N, Multhoff G. Cell surface-bound heat shock protein 70 (Hsp70) mediates perforin-independent apoptosis by specific binding and uptake of granzyme B. J Biol Chem 2003; 278:41173-81; PMID:12874291; http://dx.doi.org/10.1074/jbc.M302644200
- Calderwood SK, Murshid A, Gong J. Heat shock proteins: conditional mediators of inflammation in tumor immunity. Front Immunol 2012; 3:75; PMID:22566956; http://dx.doi.org/10.3389/fimmu.2012.00075
- Shevtsov MA, Kim AV, Samochernych KA, Romanova IV, Margulis BA, Guzhova IV, Yakovenko IV, Ischenko AM, Khachatryan WA. Pilot study of intratumoral injection of recombinant heat shock protein 70 in the treatment of malignant brain tumors in children. Onco Targets Ther 2014; 7:1071-81; PMID:24971017; http://dx.doi.org/10.2147/OTT.S62764
