ABSTRACT
Melanoma remains a leading cause of death among young adults. Evidence that melanoma tumor cells are highly immunogenic and a better understanding of T-cell immune checkpoints have changed the therapeutic approach to advanced melanoma. Instead of targeting the tumor directly, immunotherapy targets and activates the immune response using checkpoint inhibitors, monoclonal antibodies, vaccines, and adoptive T cell therapy. This review focuses on the immune signaling and biological mechanisms of action of recent immune-based melanoma therapies as well as their clinical benefits.
Introduction
According to the National Cancer Institute, the incidence of invasive melanoma in the United States was estimated to be about 73,870 cases in 2015, and one American dies of melanoma every hour .Citation1 Melanoma treatment depends on the stage of the cancer. Early lesions (Stage 0 melanoma) are often cured by surgical excision alone. Stage II and stage III resectable melanoma are managed with surgery and lymph node resection. Stage III unresectable and stage IV are aggressively treated with chemotherapy, targeted therapy, and recently immunotherapy.Citation2 The 10-year overall survival rate for advanced melanoma is improving, but is still only 10–15%.Citation3
Advances in understanding T-cell immune checkpoints and the immune recognition of melanoma as highly immunogenic opened the way for melanoma immunotherapies.Citation4 Novel immunotherapy approaches showed improved survival, resulting in the FDA approval of 3 immunotherapy agents for advanced melanoma: Ipilimumab, Nivolumab, and Pembrolizumab.Citation5 This review focuses on the biological mechanisms of action and clinical benefits of the recent developed immunotherapies for the management of melanoma.
The biology of immune checkpoints
Melanoma cells display several antigens that are targetable by the host immune system and that stimulate both antibody and cell mediated pathways. The host immune system naturally provides regulatory immune checkpoints to any immune response triggered against antigenic melanoma cells, and melanoma cells have utilized those checkpoints to evade adequate immune recognition and destruction.
For T-cell activation to occur, both primary and secondary signals are required. Primary signals represent the interaction between T-cell receptors (TCR) and tumor specific antigens, whereas secondary signals include the interaction between costimulatory T-cell surface molecule CD28 and its target cell ligand B7.Citation6 Expression of B7 on tumor cells can lead to the generation of tumor cell-specific T cell immunity. Co-inhibitory signals that allow for T-cell down regulation, include the interaction between T-cell inhibitory receptors such as programmed death-1 receptor (PD-1) or CTLA-4 and their ligand on tumor cells, PD-L1 or B7 respectively. outlines the immune checkpoints for T cell activation or downregulation. Tumor infiltrating T-cells can trigger their own inhibition by binding to PD-L1 or CTLA-4. Cancer cells constitutively upregulate the expression of PD-L1 allowing them to evade immune cell-mediated killing. This is known as the adoptive immune resistance mechanism.Citation7,8
Figure 1. T-cell checkpoint co-stimulatory signals. A T-cell can either be activated or down-regulated depending on co-stimulatory signals. The primary event is the binding of TCR on T-cells to the tumor specific antigen. T-cells are activated if CD28 binds to B7-1/B7-2; however, T-cells are downregulated if B7-1/B7-2 binds to CTLA-4 or if PD-L1/PD-L2 on tumor cells binds to PD-1 receptor on T-cells. Thus, antibodies that can activate B7-1/B7-2 or inhibit CTLA-4 or PD-1 or PD-L1 result in T-cell activation and anti-tumor effects.
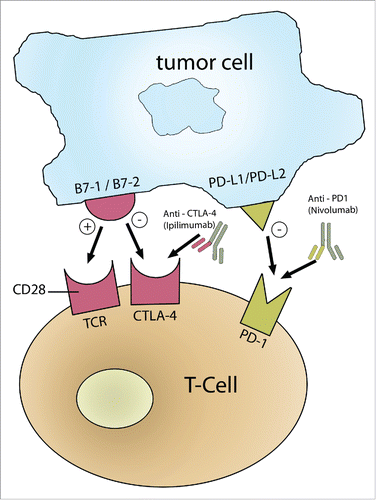
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death-1 receptor (PD-1), both being T-cell inhibitory receptors, are considered targets in the treatment of melanoma. The rationale is to prevent the suppression of T cells and improve their anti-tumor effects. CTLA-4 is expressed on activated T-cells and counters the co-stimulatory signal triggered by CD28, thus downregulating T-cell activation.Citation9 Any mechanism leading to the blockage of CTLA-4 will lead to upregulation of T-cell activation. Ipilimumab, a monoclonal antibody targeting CTLA-4, was approved in March 2011 for treatment of patients with newly diagnosed or previously treated unresectable or metastatic melanoma. PD-1 is expressed on T cells, B cells, and activated myeloid cells, along with its ligand PDL-1.Citation10 Monoclonal antibodies targeting the PD-1/PDL-1 pathway such as Nivolumab and Pembrolizumab have proven effective in melanoma.
Anti-CTLA-4 therapy
The discovery of CTLA-4 and its biological significance in immune regulation provided a rationale for the development of anti-CTLA-4 antibodies.Citation11 Ipilimumab, an IgG1κ monoclonal antibody, inhibits CTLA-4 and is commercially known as Yervoy.Citation12 Ipilimumab received FDA approval in 2011 for the treatment of late stage melanoma.Citation13
Ipilimumab's mechanism of tumor suppression
CTLA-4 downregulates T cell activation and proliferation.Citation14 This inhibitory function on the immune system occurs after the direct binding of CTLA-4 to B7 receptor on antigen presenting cells. The B7 receptor usually binds to CD28 as a co-stimulatory signal for T cell activation, however CTLA-4 can bind B7 with greater avidity than CD28, resulting in the generation of an inhibitory signal to T cells to downregulate the immune response.Citation14-16
Ipilimumab antagonizes CTLA-4′s inhibitory effect on T cells, and thus it is defined as an immune checkpoint inhibitor.Citation17 In the context of tumor biology, Ipilimumab stimulated an increase in the number of T-effector cells which mobilize to mount a direct immune attack against tumor cells.Citation18 Another mechanism for ipilimumab-induced tumor regression is its ability to deplete T-regulatory cells at the tumor site. This results in an increase in the intra-tumoral T-effector/ T-regulatory cell ratio which allows for more tumor cell death.Citation19
Clinical trials involving CTLA-4 inhibitor
The discovery of Ipilimumab was considered a breakthrough as it was the first treatment to improve median and long-term survival rates in metastatic melanoma.Citation20,15,21One of the first reports of the use of Ipilimumab in melanoma treatment described the effect of infusing 17 melanoma patients with 3 mg/kg of Ipilimumab.Citation21,22 The results showed tumor regression in 2 out of the 17 patients enrolled. Such early evidence of response to CTLA-4 blockade led to more extensive investigation of the effect of Ipilimumab on patients with melanoma especially those with metastasis.Citation23,24
Two major phase III clinical trials provided evidence supporting the use of Ipilimumab in the treatment of metastatic melanoma.Citation11 In the first trial in 2010, researchers studied the overall survival of patients with unresectable stage III or IV melanoma treated with Ipilimumab alone (3mg/kg) with or without a gp100 vaccine. Out of the 676 HLA-A*0201–positive melanoma patients, 403 patients received ipilimumab plus gp100, 137 patients received Ipilimumab alone (3 mg/kg), and 136 patients received gp100 vaccine as a monotherapy. Patients underwent induction with Ipilimumab every 3 weeks for up to 4 treatments (with or without gp100). The study found that Ipilimumab with or without the gp100 vaccine improved the overall survival of patients with metastatic melanoma as compared with the use of gp100 alone.Citation15,21 The median overall survival in patients receiving ipilimumab alone or ipilimumab with gp100 was 3.7 months higher than that of patients receiving gp100 alone.Citation22
In the second Phase III trial in 2011, Ipilimumab was compared to Dacarbazine in patients with previously untreated metastatic melanoma.Citation25 250 patients were randomly assigned to receive Ipilimumab (10 mg/kg) plus Dacarbazine (850 mg per square meter of body-surface area) and 252 were randomly assigned to receive Dacarbazine plus placebo. Again, the combination of Ipilimumab with Dacarbazine proved to be superior in terms of overall survival (11.2 months) as compared to the use of Dacarbazine alone (9.1 months).Citation25
Both of the mentioned trials demonstrated a plateau in the survival curves after follow up.Citation15 In addition, a meta-analysis with a total of 1861 patients from 8 Phase II, 2 Phase III, and 2 monocentric observational studies showed that survival curves plateaued after 3 y of treatment.Citation26 This suggests that Ipilimumab has a long term anti-tumor effect.
Safety and toxicity of ipilimumab
The adverse events caused by Ipilimumab are related to its mechanism of action, i.e., increasing the immune response. Thus, scientists refer to the side effects as immune-related adverse events (irAE).Citation11,15,Citation27 Even though rates of irAEs differ in various trials, the phase III trial reported by Hodi, et al in 2010 showed that irAEs most commonly affected the skin (rash/pruritis: 43.5%), liver (hepatitis: 3.8%), the bowel (colitis diarrhea: 29.0%), and the endocrine system (thyroiditis: 7.6%).Citation29
The patients from the MDX010-20 trial were followed up and a detailed analysis of the time of onset and resolution of irAEs found that irAEs from Ipilimumab were of grade 3–4, developed within 12 weeks of initial dosing, and resolved, either alone or with corticosteroid therapy, within 12 weeks of onset.Citation28 Since dosing is an important factor for the development of irAEs, an intra-patient dose escalation study examined whether higher doses of anti–CTLA-4 antibody would induce increased autoimmunity.Citation30 The study concluded that there is a tendency toward a greater incidence of grade III/IV autoimmune toxicity than previously reported with higher doses, but did not seem to increase objective response rates.Citation30
Evaluation of the response to ipilimumab
Due to the mechanism of action of Ipilimumab, clinical response patterns differ from those seen with the use of regular cytotoxic agents that target the tumor itself.Citation31,32 Some patients might take many weeks to 12 months before they show signs of disease regression.Citation31 Other patients might exhibit tumor progression or pseudo-progression due to immune cell infiltration and inflammation.Citation17 Tumor progression does not indicate ineffective therapy because some melanoma patients showed progression according to the traditional Response Evaluation Criteria in Solid Tumors (RECIST) yet they still benefitted from Ipilimumab later on.Citation28,31 RECIST criteria are usually used to evaluate antitumor responses to chemotherapeutic agents. However, in the context of immunotherapeutic agents, the observed responses may extend beyond those of cytotoxic agents to encompass responses after disease progression that are not captured by RECIST. Thus, irRC is proposed to better capture the patterns in response to immunotherapeutic agents and can be used as a tool for clinical investigation of immune therapy in cancer patients.Citation28,32
New criteria termed as immune-related response criteria (irRC) were proposed to evaluate the pattern of response to Ipilimumab. irRC take into account the total tumor burden regardless of the growth of new disease. As such, irRC may allow more comprehensive evaluation of the patterns of response of Ipilimumab that are as follows: (a) shrinkage in baseline lesions, without new lesions; (b) durable stable disease (in some patients followed by a slow, steady decline in total tumor burden); (c) response after an increase in total tumor burden; and (d) response in the presence of new lesions.Citation31,32
Combining anti-CTLA4 therapy with other strategies improves melanoma response rates
Immunotherapies that target distinct immune pathways are being tested in combination in several clinical trials. This is expected to allow for the achievement of higher response rates in the treatment of melanoma and further augments the antitumor response over single agents.Citation33 Ipilimumab was tested in combination with interleukin (IL)-2, VEGF inhibitor (Bevacizumab), and BRAF inhibitor (Vemurafenib).
A phase I/II study in 2005 evaluated the antitumor activity and autoimmune toxicity of anti-CTLA-4 (Ipilimumab) in combination with an immune-activating stimulus, interleukin (IL)-2, in patients with metastatic melanoma.Citation34 This cohort study included 36 patients who all received Ipilimumab and IL-2 therapy (720,000 IU/kg every 8 hours to a maximum of 15 doses). Three of the patients received escalating doses of Ipilimumab (0.1, 0.3, 1.0, and 2.0 mg/kg) while 24 patients received 3mg/kg. An overall response rate of 22 % with 3 complete responses was achieved.Citation11,34 However, there was no evidence to support a synergistic effect of CTLA-4 blockade with IL-2 administration, but this combination did not seem to cause an increase in toxicity.Citation11,34
The combination of CTLA4 blockade using Ipilimumab and VEGF inhibition using Bevacizumab was examined in a phase II study that included 46 patients. In addition to its anti-angiogenic effects, Bevacizumab plays a role in suppressing dendritic cell maturation and modulates lymphocyte endothelial trafficking.Citation22,35 A disease-control rate of 67.4% was obtained. Few irAEs including giant cell arteritis (n = 1), hepatitis (n = 2), and uveitis (n = 2) were reported. It was concluded that the combination of VEGF blockade and CTLA-4 blockade can be safely administered with VEGF blockade having an impact on inflammation, lymphocyte trafficking, and immune regulation.Citation22,27 This study provided the basis for continuing the development of immune checkpoint and antiangiogenic combination therapies for the treatment of melanoma.
Vemurafenib selectively targets melanoma cells carrying the BRAF V600E gene mutation.Citation36 The concurrent administration of Vemurafenib (BRAF inhibitor) and Ipilimumab was examined in a phase I study consisting of 2 cohorts each recruiting 6 patients. The goal was to evaluate the safety of such a combination. However, this study was discontinued early due to the development of Grade III liver toxic effects characterized by elevation of aminotransferase levels.Citation37 The findings of this study highlighted the importance of conducting trials carefully when combining 2 approved agents together with distinct mehcanisms of action.
Anti-PD-1 therapy
Mechanism of tumor suppression of anti-PD-1 therapy
Given the success of immune checkpoint inhibitors in cancer immunotherapy, the next aim was to explore the validity of PD-1 as the next immune modulatory target. PD-1, also known as Programmed Death- 1 receptor, is a surface receptor expressed on activated T cells, natural killer cells and B cells.Citation38 It is a member of B7-CD28 superfamily.Citation39 PD1 signals through its ligands PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273) to result in the modulation of T cell function.Citation40 PD-1 signaling inhibits T-cell proliferation, halts cytokine production, and induces T cell cytolysis.Citation41
The inhibition of T cell function through the activity of PD-1 is therefore a critical therapeutic target for the activation of T cells. This rationale has led to the development of monoclonal antibodies that block the activity of PD-1 receptors. These antibodies block the PD-1 receptors on T cells, making them unable to respond to PD-L1 or PDL-2 expressed on tumor cells. This leads to the activation of T-cell activity and a decrease in tumor growth. In preclinical and some clinical trials, anti-PD-1 monoclonal antibodies have proven to be efficacious for melanoma treatment and are now FDA approved. outlines the available anti-PD-1 monoclonal antibodies.
Table 1. Clinically relevant anti-PD-1 monoclonal antibodies.
Pembrolizumab (previously known as MK3475 or Lambrolizumab) is a high affinity humanized anti-PD-1 IgG4 isotype antibody. It was the first anti-PD1 antibody to be approved by the FDA for the treatment of metastatic melanoma in patients with disease progression following Ipilimumab.Citation42 The success of the clinical trial involving Pembrolizumab led to its approval by the FDA in September 2014 for the treatment of patients with unresectable or metastatic melanoma with disease progression following Ipilimumab and a BRAF inhibitor if they were positive for BRAFV600 mutation.Citation42
Nivolumab is an IgG4-blocking monoclonal antibody that has been shown to block PD-1 activities.Citation43 Nivolumab first showed promising results in melanoma treatment in a phase 1 trial that was carried out in 2010 and the drug was first approved in Japan for the treatment of patients with unresectable melanoma in July 2014.Citation44 Following the success of Nivolumab in the clinical trials carried out in the US, the FDA approved it in December 2014 for melanoma treatment. Nivolumab has also been associated with significant improvements in overall survival and progression free survival in patients who had metastatic melanoma without a BRAF mutation.Citation45
Pidilizumab (CT-011) is another anti-PD1 blocking antibody. It is a humanized IgG1 monoclonal antibody that has been tested in phase 1 dose-escalation study in patients with hematological malignancies,Citation46 but has not been FDA approved yet,unlike pembrolizumab, which was approved by FDA in September 2014 for the treatment of patients with unresectable or metastatic melanoma. Pidilizumab is also being tested in patients with metastatic melanoma. The results so far show a low overall response rate (6%) but a high overall survival at 12 month of 64.5%. Unike pidilizumab, pembrolizumab in the keynote002 trial was associated with superior progression-free survival (PFS) and better tolerability which lead to it FDA approval.
In addition to monoclonal antibodies targeting the PD-1 receptor, there are monoclonal antibodies that have been developed to target the ligands PD-L1. One of these agents is BMS-936559 (MDX-1105). BMS-936559 is a human IgG4 monoclonal antibody that targets PD-L1 and disrupts the interaction of PD-L1 on tumor cells with PD-1 on effector T cells. In a phase 1 clinical trial using anti-PD-L1 antibody BMS-936559, objective responses to therapy were noted in 9 out of 52 (17%) patients with melanoma.Citation47 Clinicians in this trial also observed prolonged stabilization of the disease in 14 out of 52 (27%) patients assessed 24 weeks after beginning therapy. Another antibody blocking PD-L1 is MPDL3280A which also works by binding PD-L1 and blocking its interaction with PD-1.Citation48 Further studies on PD-L1 and PD-L2 therapies are ongoing and seem to be promising in the treatment of melanoma and other solid tumors.
Clinical response
A phase I clinical trial using anti PD-1 antibody Pembrolizumab included 173 melanoma patients, who either received pembrolizumab 2 mg/kg (n = 89) or 10 mg/kg (n = 84). Treatment was well tolerated with similar safety profiles in the 2 mg/kg and 10 mg/kg groups. The most common drug-related adverse events were fatigue (33 or 37%), pruritus (26 or 19%), and rash (18% in both groups).
A phase III study compared the efficacy of Ipilimumab versus Pembrolizumab. In this study, 834 patients with advanced melanoma were divided into 3 groups; 10 mg/kg of Pembrolizumab every 2 weeks, 10 mg/kg of Pembrolizumab every 3 weeks, or 4 doses of Ipilimumab at 3 mg/kg every 3 weeks. The results highlighted the efficacy of Pembrolizumab compared with Ipilimumb. In addition, the immune-related adverse events were less severe in patients receiving Pembrolizumab than in patients receiving Ipilimumab.Citation50
In a phase I clinical trial, investigators administered Nivolumab to patients with different types of solid tumors. In this study, they found that 26 out of 94 (27%) patients who had advanced melanoma and who had melanoma progression while on previous tumor therapies, showed objective response to treatment after receiving Nivolumab at a dose of 0.1 to 10.0 mg/Kg every 2 weeks over an 8 week cycle period.Citation47 Interestingly, 72% of the melanoma patients that responded to Nivolumab treatment with adequate follow up had responses lasting a year or longer post treatment.
In a phase III clinical trial involving patients with previously untreated melanoma without BRAF mutation, patients who received Nivolumab had a 1-year survival rate of 72.9% compared to 42.1% in patients who received Dacarbazine chemotherapeutic agent.Citation45
Overall, the promising responses to anti-PD-1 immunotherapy from the clinical trials indicate that PD-1 antibodies do have the potential to improve melanoma treatment. The other PD-1 and PD-L1 directed agents are currently in phase I-III clinical trials in multiple tumor types.
Biomarkers for treatment
The upregulation of PD-1 on exhausted T cells and PD-L1 on tumor cells or tumor infiltrating lymphocytes may serve as a potential marker for identifying patients likely to respond to the inhibition of PD-1 /PD-L1 pathway. Promising preliminary data show a correlation between PD-L1 expression and responsiveness to anti-PD-1 therapy.Citation51 Another study showed that across multiple cancer types, responsiveness to treatment was determined by the expression of the receptor and its ligands.Citation52 In this study, patients with higher expression of PD-L1 on the tumor infiltrating lymphocytes had better responses to MPDL3280A. In another study, however, patients with high expression levels of PD-1 and PDL1 did not respond any better to a combination of Ipilimumab and Nivolumab as opposed to Nivolumab alone. Interestingly, patients with low levels of these receptors responded better to a combination of these 2 drugs.Citation53 It is important to note that one limitation in such studies is expression measurement as the expression of several genes is affected when tissues are handled. In addition, several studies used different techniques to assess for expression with different antibodies and/or probes.
Toxicity
Clinical trials involving Nivolumab identified low-grade fatigue, diarrhea, nausea, cough, dyspnea, constipation, vomiting, dermatitis, pyrexia, and headache as common side effects.Citation47 Additional reported immune related adverse events included pneumonitis, vitiligo, colitis, hepatitis, hypophysitis and thyroiditis.Citation43 Even though these adverse events can be a limitation for some patients, they occur less frequently and are less severe than those observed with Ipilimumab.
In the phase III Nivolumab trial, grade 3–4 adverse events were detected in 12% of the patients.Citation45 Interestingly, there was no evidence of increasing toxicity with increased drug exposure in patients that received up to 2 y of Nivolumab treatment. This is in contrast to chemotherapy where there is increased drug toxicity associated with increased drug exposure.Citation43 In addition, with both Nivolumab and Pembrolizumab, the maximum-tolerated dose was not reached.Citation47
Clinical trials involving Pembrolizumab indicate that the most common adverse events associated with this antibody treatment are low grade fatigue, rash, pruritus, arthralgia, amylase elevation, and diarrhea.Citation42 These events are also less severe than the adverse events in patients receiving other immune checkpoint inhibitors.Citation50
Combination options
Because of the efficacy shown by both CTLA-4 and PD-1 blocking antibodies, scientists have investigated the possibility of more effective treatment therapy by combining the 2 modes of treatments. One study looked at the combination therapy of Nivolumab and Ipilimumab.Citation54 In this phase 1 clinical trial, patients were given a combination dose of both drugs every 3 weeks for 4 doses then followed by another 4 doses of Nivolumab alone every 3 weeks. It was found that combination therapy provided faster and deep tumor regression suggesting that Nivolumab and Ipilimumab can act synergistically.
In a recent phase III trial, the combination of Nivolumab and Ipilimumab was compared to both Ipilimumab and Nivolumab monotherapy. The study included 945 patients. In patients receiving a combination of Nivolumab and Ipilimumab, the median progression free survival was 11.5 months (95% CI: 8.9 to 16.7). This was compared to the median progression free survival in patients that received Ipilimumab monotherapy (2.9 months; 95% CI: 2.8 to 3.4; p < 0.001) and those that received Nivolumab monotherapy (6.9 months; 95% CI: 4.3 to 9.5; p < 0.001). Although the response to treatment was better in the combination regimen, the immune-related adverse events were also worse in this group compared to the patients in the monotherapy regimen.Citation55 Based on these studies, the U.S FDA approved the combination of Nivolumab with Ipilimumab as a first line treatment for advanced melanoma patients expressing wild type BRAF. compares and contrasts anti-CTLA-4 and anti-PD-1 Mabs when it comes to mechanism of action, overall clinical safety, and efficacy profiles.
Table 2. Anti-CTLA-4 vs. Anti-PD-1.
Table 3. Vaccine approaches for melanoma.
Combination of PD-1 blockage with chemotherapy has also been investigated as some chemotherapeutic agents showed the potential of priming the immune system to respond better to PD-1 inhibitors. For instance, histone deacetylase inhibitors work synergistically with CTLA-4 or PD-1 blockers to kill tumor cells, both primary and metastatic.Citation56 Although this has only been explored in murine models, the results are promising and offer a path for further investigation.
Given the importance of BRAF mutations in how patients respond to immune checkpoint inhibitors, BRAF inhibitors could be combined with PD-1 inhibitors to further increase the response in melanoma patients. Preclinical studies indicate that treating melanoma with BRAF V600E mutation using a combination of PD-1 or PD-L1 blockade and BRAF inhibitor increased the activity of tumor infiltrating lymphocytes and resulted in prolonged survival.Citation57
Tyrosine kinase inhibitors can be combined with immune checkpoint inhibition. Ongoing studies investigating the combination of VEGF-specific monoclonal antibody Bevacizumab and PD-L1 specific monoclonal antibody MPDL3280A demonstrated only moderate toxicity.Citation58 These studies are promising in therapy where VEGF secretion is upregulated.
With the success of PD-1 inhibition, PD-1 inhibitors are now being explored for the treatment of other cancers including non-small cell lung cancer, renal cell carcinoma, and Hodgkin's lymphoma.
OX40 and 4-IBB based melanoma therapy
OX40 (CD134) is a Tumor Necrosis Factor Receptor expressed mainly in lymphoid tissues and plays an important role in immune responses by providing a costimulatory signal for T cell activation.Citation59 Upon antigen stimulation, CD4+ T cells express OX40 within 4 hours. In addition, active T lymphocytes expressing OX40 are found at sites of inflammation rather than peripheral blood.Citation60 OX-40 promotes the division and survival of cytotoxic T cells augmenting the clonal expansion of effector and memory populations as they are being generated.Citation61 In the context of tumor immunology, studies showed the presence of OX40-expressing tumor infiltrating lymphocytes in human melanoma tumors and tumor-draining lymph nodes.Citation61
Given the significance of OX40 expression on tumor infiltrating lymphocytes, several clinical trials tested the antitumor immune properties of OX40 by using OX40 agonists. A phase one trial administered 3 doses of murine anti-human OX40 agonist over 5 d to 30 patients, 7 of which had melanoma. Twelve out of 30 patients exhibited regression in tumor nodules. The toxicity profile was milder than that encountered with Ipilimumab.Citation62,63
Tumor specific properties of OX40 were further tested in peripheral blood mononuclear cells (PBMC) isolated before and after anti-OX40 treatment. PBMCs were co-cultured with autologous tumor, HLA matched, or HLA mismatched melanoma cell lines for up to 5 d. There was a significant increase in IFN-gamma in the supernatants after anti-OX40 treatment in 3 out of 4 patients, suggesting that anti-OX40 induces a melanoma-specific immune response.Citation64
The effect of OX40 agonists on amplifying the immune-stimulatory and anti-tumor effects of T cell checkpoint inhibitors is being explored by several trials. A phase one trial is currently investigating the synergistic effect of using anti-OX40 with anti-CTLA-4 as well as anti-OX40 with anti-PD-L1.
4-1BB is another Tumor Necrosis Factor Receptor which plays an important role in T cell co-stimulation. 4-1BB and its ligand 4-1BBL are expressed on T cells and antigen-presenting cells respectively.Citation65 Upon trigging of 4-1BB receptor as an activating co-stimulatory signal, several signaling pathways are activated including NF-kB, c-Jun, and p38. This results in cytokine production and secretion. Activation of NF-kappaB also promotes the survival of CD8+ T lymphocytes by increasing expression of anti-apoptotic genes bcl-X L and bfl-l. Because of the immune activating properties of 4-1BB, it would be important to test the anti-tumor propertied of 4-1BB agonists.Citation66
Therapies utilizing the 4-1BB:4-1BBL signaling pathway showed antitumor effects in a number of model systems.Citation33,67,Citation68 Urelumab (BMS-663513) is a fully humanized anti-CD137 agonist mAb that has been tested in a phase I dose-escalation study. Only 3 of 54 melanoma patients included had an objective response to the treatment.Citation69 However, preclinical data demonstrated synergistic activity with Nivolumab.Citation70 As for the toxicity profile, Urelumab induced severe hepatic toxicity in rare cases.Citation71
Vaccines for the treatment of melanoma
Aside from using checkpoint inhibitors, designing vaccines to treat high risk and metastatic melanoma has been an attractive alternative given the highly immunogenic nature of melanoma cells. Experimental clinical trials with “melanoma vaccines” are currently in progress and few have shown significant benefit in the adjuvant setting of high-risk melanoma. On-going trials; however, have been more promising especially with the advances in the immunology of melanoma. One recent study demonstrated higher response rates and longer progression-free survival in advanced melanboma patients when gp100 vaccine was combined with interleukin-2 (IL-2) immune activating agent.Citation72 The median overall survival was also longer in the gp100+IL-2 group than in the IL-2 only group (17.8 months; 95% CI, 11.9 to 25.8 vs. 11.1 months; 95% CI, 8.7 to 16.3; p = 0.06).Citation72
Multiple types of antigen sources have been used in the production of melanoma vaccines including autologous/allogenic peptide antigens, glycolipids, tumor-associated antigens, and dendritic cells.Citation73
Vaccines using tumor cell-derived antigens are divided into 2 categories: autologous and allogeneic vaccines. In autologous vaccines, the patient's tumor cells are used thus providing a narrow antigen spectrum specific to this particular patient. Limitations to its use include limited amount of tumor tissue accessible for vaccine preparation especially after complete resection of clinically evident disease. In a recent phase II clinical trial for metastatic melanoma, an autologous vaccine composed of tumor-derived heat shock protein peptide complexes gp96 was shown to induce an anti-melanoma, class I HLA-restricted T cell-mediated immune reaction in a proportion of treated patients. However, of the 28 patients enrolled, only 2 had a complete response and only 3 had stable disease at the end of follow-up.Citation74
Allogeneic vaccines may be more representative as they are composed of melanoma cells from other patients selected for a variety of shared antigens. Even though they may not contain all of the tumor-associated antigens on the treated patient's tumor, they do allow for large-scale randomized trials. One studied allogenic vaccine is Canvaxin polyvalent cancer vaccineThe cumulative data for Canvaxin therapeutic cancer vaccine represent the largest phase II clinical trial of any cancer vaccine. The vaccine exhibited prognostic significance for patients with stage III and IV melanoma. However, a phase III clinical trial for stage III unresected and stage IV melanoma showed unfavorable results.Citation75
Another category of vaccines is composed of cell surface glycolipids, such as gangliosides GD3 and GM2.Citation76 In a phase III clinical trial for stage II resected melanoma, adjuvant ganglioside GM2 vaccine was not shown to improve clinical outcome.Citation77
In addition to the use of tumor cell-derived antigens and gangliosides, tumor-associated antigens have been integrated into vaccines and often combined with adjuvants such as GM-CSF. Melanoma specific tumor-associated antigens include Melan-A/MART-1, gp100, tyrosinase, tyrosinase-related protein-1 (trp-1), and tyrosinase-related protein-2 (trp-2).Citation78,79
Dendritic cells, being antigen-presenting cells specialized for the induction of a primary T-cell response, have been explored as well for the manufacturing of vaccines in advanced melanoma. Mouse studies have shown that dendritic cells do induce antitumor immunity, and thus multiple studies aimed at demonstrating the clinical effect of such vaccines on the survival of melanoma patients.Citation80 However, one study showed that vaccinating with peptide-loaded dendritic cells can result in long-term clinical response in only a minority of metastatic melanoma patients (2 out of 15 patients).Citation81 In addition, a recent phase I/IIa clinical trial in stage IV melanoma using autologous tumor–dendritic cell fusion (dendritoma) vaccine with low dose interleukin-2 showed that overall survival was significantly higher in the experimental group (23.8 vs. 8.7 months, p = 0.004).Citation82
Moreover, another vaccine tested in melanoma is herpes simplex virus-1 oncolytic vaccine known as Talimogene laherparepvec (T-VEC). T-VEC is designed to induce systemic antitumor immunity and was effective in increasing the response rate and survival (≥6 months) vs GM-CSF in a phase 3 melanoma trial.Citation83 A phase 1 trial studies its toxicity and showed that combining T-VEC with Ipilimumab was tolerable and did not result in DLTs but did result in grade ¾ adverse events in 32% of the patients. The adverse events included hypophysitis, adrenaln insufficiency, and diarrhea. Studies on T-VEC suggest T-VEC+ipilimumab is more effective than ipilimumab alone.Citation84
Advanced techniques using cDNA-expression cloning and autologous antibodies have allowed for the identification of a wide array of antigens and peptides utilized in manufacturing melanoma vaccines. Further trials are imperative at this point to establish the therapeutic benefit of those vaccines in advanced melanoma as evidence so far is lacking.
Even though treating melanoma using a cancer vaccine is an ingenious approach, several challenges are arising with this strategy. So far, vaccines have been developed based on tumor antigens that are commonly overexpressed and shared across many patients and tumors. One challenge is to develop vaccines that are personalized to each patient; i.e., vaccines based on the antigens the tumor expresses in a particular patient. This approach will add more cost and time but might be more beneficial compared with the general vaccines. In addition, another challenge is to develop vaccines composed of nucleic acids that encode antigens. Developing vaccines based on these nucleic acids might allow more specific immune responses toward the tumor rather than normal tissue. It also allows for vaccinating against several antigens rather than one because of the ability to administer several nucleic acid sequences encoding different antigens. For example, a recent vaccines was developed composed of a nanoparticle containing a tetravalent RNA sequences, each encoding a separate antigen, for the treatment of patients with malignant melanoma. The approach allows more efficient targeting of antigen-presenting cells.Citation85,86 summarizes the different vaccines for the treatment of melanoma.
Adoptive T cell therapy
Adoptive T cell therapy (ACT) involves the isolation of tumor specific T cells from cancer patients, expanding them ex-vivo, and transfusing them back to the patient for more a effective attack on cancer cells. These T cells could be derived from a single T cell clone or just an assortment of tumor infiltrating lymphocytes. Some T cells are modified to improve their potency against cancer cells.Citation87,88 Although there are currently no FDA approved ACT therapies for cancer, there have been some clinical trials investigating the efficacy of this treatment modality in melanoma and other tumors. ACT has been shown in clinical trials to be especially efficient in treating patients with metastatic melanoma.Citation89,90 A phase I study was conducted to test the feasibility, safety, and survival of adoptively transferred Melan-A–specific cytotoxic T lymphocytes lines in melanoma patients.Citation91The study showed that the adoptive transfer of antigen-specific T cells in melanoma patients can induce clinical tumor-specific immune responses without major adverse effects. Another phase I/II clinical study investigated the effectiveness of adoptive T cell therapy in conjunction with activating cytokine IFN-γ in melanoma, showing promising possibility of this type of combinatorial therapy.Citation92 Compared to other treatment modalities such as chemotherapy, adoptive T cell therapy has fewer side effects. In addition, since the T cells are cancer specific, ACT is more tumor selective with less harm to normal non-cancerous cells.
Conclusion
The last decade has witnessed a dramatic increase in researching immunotherapy for advanced melanoma. This resulted in the development of effective anti-melanoma agents that work by activating the immune system rather than inducing direct cytotoxicity in cancer cells. Future approaches should aim to limit toxicity and maximize the clinical effect. In addition, the field is expanding to include other immunomodulatory targets (such as LAG3 and CD40) and other therapeutic strategies, such as using a herpes simplex virus-1 oncolytic vaccine known as Talimogene laherparepvec (T-VEC). In addition, the current approach to maximize clinical response is testing the combination of 2 or more immunotherapeutic agents, or the combination of an immunomodulatory agent with small molecule inhibitors. Some of the combinations tested previously were very successful as discussed in this article, while some were not due to toxicity. As such, cautious and careful planning for a combination clinical trial is essential.
The field of melanoma immunotherapy has made a lot of progress in a short period of time, yet there is a lot to be done. For instance, even though pembrolizumab efficacy and safety have been established, the optimal treatment duration for pembrolizumab is not clear. Data from ongoing clinical trials may help address the question of the optimal treatment duration. In addition, it is important to detect irAEs early to prevent the severity of such events. For instance, it is recommended that thyroid function be routinely assessed in patients who receive pembrolizumab treatments. Other screening tests can also be utilized for early irAEs detection. Another aspect of immunorhtapy that needs to be explored in the near future is a clear comparison between the efficacy and tolerability among PD-1 inhibitors. Comparing pembrolizumab vs. nivolumab using the current literature is challenging because these treatments have been evaluated in different patient populations. Additional studies will be helpful to compare these 2 agents. Finally, one important aspect of immunotherapy that requires closer attention during treatment is pseudoprogression, i.e., an initial increase in tumor burden followed by disease stabilization. The establishment of certain criteria that help us differentiate between a non-responding versus pseudoprogressing patient is crucial.
Disclosure of potential conflicts of interest
Authors declare no conflict of interest.
References
- Vennepureddy A, Thumallapally N, Nehru VM, Atallah J, Terjanian T. Novel drugs and combination therapies for the treatment of metastatic melanoma. J Clin Med Res 2016; 8:63
- Miller AJ, Mihm Jr MC. Melanoma. N Engl J Med 2006; 355:51-65
- Macdonald JB, Dueck AC, Gray RJ, Wasif N, Swanson DL, Sekulic A, Pockaj BA. Malignant melanoma in the elderly: Different regional disease and poorer prognosis. J Cancer 2011; 2:538-43; PMID:22084644
- Shtivelman E, Davies MQ, Hwu P, Yang J, Lotem M, Oren M, Flaherty KT, Fisher DE. Pathways and therapeutic targets in melanoma. Oncotarget 2014; 5:1701-52; PMID:24743024
- La‐Beck NM, Jean GW, Huynh C, Alzghari SK, Lowe DB. Immune checkpoint inhibitors: New insights and current place in cancer therapy. Pharmacotherapy 2015; 35:963-76
- Willemsen RA, Ronteltap C, Chames P, Debets R, Bolhuis RL. T cell retargeting with MHC class I-restricted antibodies: The CD28 costimulatory domain enhances antigen-specific cytotoxicity and cytokine production. J Immunol 2005; 174:7853-8; PMID:15944290
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37; PMID:22461641; http://dx.doi.org/10.1126/scitranslmed.3003689
- Atefi M, Avramis E, Lassen A, Wong DJ, Robert L, Foulad D, Cerniglia M, Titz B, Chodon T, Graeber TG, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res 2014; 20:3446-57; PMID:24812408; http://dx.doi.org/10.1158/1078-0432.CCR-13-2797
- Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 1996; 4:535-43
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192:1027-34; PMID:11015443
- Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest 2015; 125:3377-83; PMID:26325034; http://dx.doi.org/10.1172/JCI80012
- Harvey RD. Immunologic and clinical effects of targeting PD‐1 in lung cancer. Clinical Pharmacology & Therapeutics 2014; 96:214-23
- Delyon J, Maio M, Lebbe C. The ipilimumab lesson in melanoma: Achieving long-term survival. Semin Oncol 2015; 42:387-401; PMID:25965357; http://dx.doi.org/10.1053/j.seminoncol.2015.02.005
- Camacho LH. CTLA-4 blockade with ipilimumab: Biology, safety, efficacy, and future considerations. Cancer Med 2015; 4:661-72; PMID:25619164; http://dx.doi.org/10.1002/cam4.371
- Delyon J, Maio M, Lebbe C. The ipilimumab lesson in melanoma: Achieving long-term survival. Semin Oncol 2015; 42:387-401; PMID:25965357
- Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol 2001; 1:220-8; PMID:11905831; http://dx.doi.org/10.1038/35105024
- Michielin O, Hoeller C. Gaining momentum: New options and opportunities for the treatment of advanced melanoma. Cancer Treat Rev 2015; 41:660-70; PMID:26096079; http://dx.doi.org/10.1016/j.ctrv.2015.05.012
- Trinh VA, Hagen B. Ipilimumab for advanced melanoma: A pharmacologic perspective. J Oncol Pharm Pract 2013; 19:195-201; PMID:23047236; http://dx.doi.org/10.1177/1078155212459100
- Hanaizi Z, van Zwieten-Boot B, Calvo G, Lopez AS, van Dartel M, Camarero J, Abadie E, Pignatti F. The european medicines agency review of ipilimumab (yervoy) for the treatment of advanced (unresectable or metastatic) melanoma in adults who have received prior therapy: Summary of the scientific assessment of the committee for medicinal products for human use. Eur J Cancer 2012; 48:237-42
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27:6199-206; PMID:19917835; http://dx.doi.org/10.1200/JCO.2009.23.4799
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, Zeng W, Giobbie-Hurder A, Atkins MB, Ibrahim N, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2014; 2:632-42; PMID:24838938; http://dx.doi.org/10.1158/2326-6066.CIR-14-0053
- Ansell SM, Hurvitz SA, Koenig PA, LaPlant BR, Kabat BF, Fernando D, Habermann TM, Inwards DJ, Verma M, Yamada R, et al. Phase I study of ipilimumab (MDX-010), an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-hodgkin lymphoma. Clin Cancer Res 2009; 15:6446-53; PMID:19808874; http://dx.doi.org/10.1158/1078-0432.CCR-09-1339
- Ribas A. Anti-CTLA4 antibody clinical trials in melanoma. Update Cancer Ther 2007; 2:133-9; PMID:19543441; http://dx.doi.org/10.1016/j.uct.2007.09.001
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517-26; PMID:21639810; http://dx.doi.org/10.1056/NEJMoa1104621
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015; 33:1889-94; PMID:25667295; http://dx.doi.org/10.1200/JCO.2014.56.2736
- Eggermont AM, Maio M, Robert C. Immune checkpoint inhibitors in melanoma provide the cornerstones for curative therapies. Semin Oncol 2015; 42:429-35; PMID:25965361
- Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS. MDX010-20 Investigators. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: Detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 2013; 119:1675-82; PMID:23400564; http://dx.doi.org/10.1002/cncr.27969
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23
- Maker AV, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Hughes M, Yellin MJ, Haworth LR, Levy C, et al. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother 2006; 29:455-63; PMID:16799341; http://dx.doi.org/10.1097/01.cji.0000208259.73167.58
- Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012; 30:2691-7; PMID:22614989; http://dx.doi.org/10.1200/JCO.2012.41.6750
- Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res 2009; 15:7412-20; PMID:19934295; http://dx.doi.org/10.1158/1078-0432.CCR-09-1624
- Antonia SJ, Larkin J, Ascierto PA. Immuno-oncology combinations: A review of clinical experience and future prospects. Clin Cancer Res 2014; 20:6258-68; PMID:25341541; http://dx.doi.org/10.1158/1078-0432.CCR-14-1457
- Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Haworth LR, Levy C, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: A phase I/II study. Ann Surg Oncol 2005; 12:1005-16; PMID:16283570; http://dx.doi.org/10.1245/ASO.2005.03.536
- Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res 2001; 23:263-72; PMID:11444391
- Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, Ferrone CR, Flaherty KT, Lawrence DP, Fisher DE, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 2010; 70:5213-9; PMID:20551059; http://dx.doi.org/10.1158/0008-5472.CAN-10-0118
- Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med 2013; 368:1365-6; PMID:23550685; http://dx.doi.org/10.1056/NEJMc1302338
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996; 8:765-72; PMID:8671665
- Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992; 11:3887-95; PMID:1396582
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med 2002; 8:793-800
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002; 99:12293-7; PMID:12218188; http://dx.doi.org/10.1073/pnas.192461099
- Khoja L, Butler MO, Kang SP, Ebbinghaus S, Joshua AM. Pembrolizumab. J Immunother Cancer 2015; 3:1-13
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32:1020-30; PMID:24590637; http://dx.doi.org/10.1200/JCO.2013.53.0105
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28:3167-75; PMID:20516446; http://dx.doi.org/10.1200/JCO.2009.26.7609
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320-30
- Tarhini AA. Immunotherapy of melanoma. Curr Mol Pharmacol 2015; PMID:26177647.
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54
- Cha E, Wallin J, Kowanetz M. PD-L1 inhibition with MPDL3280A for solid tumors. 2015; 42:484-7
- Hamid O, Robert C, Daud A, Hodi FS, Hwu W, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS. Safety and tumor responses with lambrolizumab (anti–PD-1) in melanoma. N Engl J Med 2013; 369:134-44
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372(26):2521-32; http://dx.doi.org/10.1056/NEJMoa1503093; Epub 2015 Apr 19
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014; 20:5064-74; PMID:24714771; http://dx.doi.org/10.1158/1078-0432.CCR-13-3271
- Herbst RS, Soria J, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515:563-7
- Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Lao CD. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. The Lancet Oncology 2015; 16:375-84
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon R, Reed K. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122-33
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373:23-34
- Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, Diaz LA Jr, Papadopoulos N, Kinzler KW, Vogelstein B, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A 2014; 111:11774-9; PMID:25071169; http://dx.doi.org/10.1073/pnas.1410626111
- Cooper ZA, Juneja VR, Sage PT, Frederick DT, Piris A, Mitra D, Lo JA, Hodi FS, Freeman GJ, Bosenberg MW, et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol Res 2014; 2:643-54; PMID:24903021; http://dx.doi.org/10.1158/2326-6066.CIR-13-0215
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nature reviews Drug discovery 2015; 14:561-84
- Godfrey WR, Fagnoni FF, Harara MA, Buck D, Engleman EG. Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. J Exp Med 1994; 180:757-62; PMID:7913952
- Weinberg AD, Wegmann KW, Funatake C, Whitham RH. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J Immunol 1999; 162:1818-26; PMID:9973447
- Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: A potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol 1998; 161:6510-7; PMID:9862675
- Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res 2013; 19:997-1008; PMID:23460531; http://dx.doi.org/10.1158/1078-0432.CCR-12-2214
- Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer 2007; 7:95-106
- Imura A, Hori T, Imada K, Ishikawa T, Tanaka Y, Maeda M, Imamura S, Uchiyama T. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J Exp Med 1996; 183:2185-95; PMID:8642328
- Alderson MR, Smith CA, Tough TW, Davis‐Smith T, Armitage RJ, Falk B, Roux E, Baker E, Sutherland GR, Din WS. Moslecular and biological characterization of human 4‐1BB and its ligands. Eur J Immunol 1994; 24:2219-27
- Cheuk AT, Mufti GJ, Guinn B. Role of 4-1BB: 4-1BB ligand in cancer immunotherapy. Cancer Gene Ther 2004; 11:215-26
- Li S, Liu Y. Immunotherapy of melanoma with the immune costimulatory monoclonal antibodies targeting CD137. Clinical pharmacology: advances and applications 2013; 5:47
- Orloff M, Valsecchi ME, Sato T. Successes and setbacks of early investigational drugs for melanoma. Expert Opin Investig Drugs 2015; 24:993-7
- Sznol M, Hodi F, Margolin K, McDermott D, Ernstoff M, Kirkwood J, Wojtaszek C, Feltquate D, Logan T. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA). 2008; 26:3007
- Sanmamed MF, Rodriguez I, Schalper KA, Onate C, Azpilikueta A, Rodriguez-Ruiz ME, Morales-Kastresana A, Labiano S, Perez-Gracia JL, Martin-Algarra S, et al. Nivolumab and urelumab enhance antitumor activity of human T lymphocytes engrafted in Rag2-/-IL2Rgammanull immunodeficient mice. Cancer Res 2015; 75:3466-78; PMID:26113085; http://dx.doi.org/10.1158/0008-5472.CAN-14-3510
- Makkouk A, Chester C, Kohrt HE. Rationale for anti-CD137 cancer immunotherapy. Eur J Cancer 2016; 54:112-9
- Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med 2011; 364:2119-27
- Ozao-Choy J, Lee DJ, Faries MB. Melanoma vaccines: Mixed past, promising future. Surg Clin North Am 2014; 94:1017-30
- Belli F, Testori A, Rivoltini L, Maio M, Andreola G, Sertoli MR, Gallino G, Piris A, Cattelan A, Lazzari I, et al. Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: Clinical and immunologic findings. J Clin Oncol 2002; 20:4169-80; PMID:12377960
- Hsueh EC, Morton DL. Antigen-based immunotherapy of melanoma: Canvaxin therapeutic polyvalent cancer vaccine. 2003; 13:401-7
- Klein O, Schmidt C, Knights A, Davis ID, Chen W, Cebon J. Melanoma vaccines: Developments over the past 10 years. 2011
- Eggermont AM, Suciu S, Rutkowski P, Marsden J, Santinami M, Corrie P, Aamdal S, Ascierto PA, Patel PM, Kruit WH, et al. Adjuvant ganglioside GM2-KLH/QS-21 vaccination versus observation after resection of primary tumor >1.5 mm in patients with stage II melanoma: Results of the EORTC 18961 randomized phase III trial. J Clin Oncol 2013; 31:3831-7; PMID:24019551; http://dx.doi.org/10.1200/JCO.2012.47.9303
- Kawakami Y, Robbins PF, Wang RF, Parkhurst M, Kang X, Rosenberg SA. The use of melanosomal proteins in the immunotherapy of melanoma. J Immunother 1998; 21:237-46
- Zarour HM, Kirkwood JM. Melanoma vaccines: Early progress and future promises. 2003; 22:68-75
- Nesrua FO, Atuaolcl S, GILLIE M, SUNZ Y, Gimasr S. Vaccination of melanoma patients with peptide-or tumor lysate-pulsed dendritic cells. Peptides 1:A2
- Lesterhuis WJ, Schreibelt G, Scharenborg NM, Brouwer HMH, Gerritsen MP, Croockewit S, Coulie PG, Torensma R, Adema GJ, Figdor CG. Wild-type and modified gp100 peptide-pulsed dendritic cell vaccination of advanced melanoma patients can lead to long-term clinical responses independent of the peptide used. Cancer Immunol Immunother 2011; 60:249-60
- Greene JM, Schneble EJ, Jackson DO, Hale DF, Vreeland TJ, Flores M, Martin J, Herbert GS, Hardin MO, Yu X. A phase I/IIa clinical trial in stage IV melanoma of an autologous tumor–dendritic cell fusion (dendritoma) vaccine with low dose interleukin-2. Cancer Immunol Immunother 2016; 65:383-92
- Andtbacka RHI, Collichio FA, Amatruda T, Senzer NN, Chesney J, Delman KA, Spitler LE, Puzanov I, Doleman S, Ye Y. OPTiM: A randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. 2013; 31:LBA9008
- Puzanov I, Milhem MM, Andtbacka RHI, Minor DR, Hamid O, Li A, Chastain M, Gorski K, Anderson A, Vanderwalde AM. Primary analysis of a phase 1b multicenter trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma. 2014; 32:9029
- Jabulowsky RA, Loquai C, Diken M, Kranz LM, Haas H, Attig S, Britten CM, Buck J, Derhovanessian E, Diekmann J. Abstract B041: A novel nanoparticular formulated tetravalent RNA cancer vaccine for treatment of patients with malignant melanoma. Cancer Immunol Res 2016; 4:B041
- Butterfield LH. Lessons learned from cancer vaccine trials and target antigen choice. Cancer Immunol Immunother 2016; 1-8
- June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest 2007; 117:1466-76; PMID:17549249; http://dx.doi.org/10.1172/JCI32446
- Wu R, Forget MA, Chacon J, Bernatchez C, Haymaker C, Chen JQ, Hwu P, Radvanyi LG. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: Current status and future outlook. Cancer J 2012; 18:160-75; PMID:22453018; http://dx.doi.org/10.1097/PPO.0b013e31824d4465
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et al. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008; 26:5233-9; PMID:18809613; http://dx.doi.org/10.1200/JCO.2008.16.5449
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008; 8:299-308
- Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol 2006; 24:5060-9; PMID:17075125
- Khammari A, Nguyen J, Saint-Jean M, Knol A, Pandolfino M, Quereux G, Brocard A, Peuvrel L, Saiagh S, Bataille V. Adoptive T cell therapy combined with intralesional administrations of TG1042 (adenovirus expressing interferon-γ) in metastatic melanoma patients. Cancer Immunol Immunother 2015; 64:805-15
