ABSTRACT
Backgrounds: Patients with chronic kidney disease (CKD) are at an increased risk of morbidity and mortality from influenza. However, the immunogenicity of influenza vaccine is known to be attenuated in these patients. In this study, the immunogenicity of MF59-adjuvanted and non-adjuvanted trivalent influenza vaccines was compared in CKD patients undergoing hemodialysis (HD).
Methods: During 2013–2014, 179 CKD patients undergoing HD participated in the study. The patients were randomized into either MF59-adjuvanted vaccine group or non-adjuvanted vaccine group and were immunized with the respective vaccine. Sera were collected prior to vaccination and at 1 month (88 patients in MF59-adjuvanted vaccine group and 86 patients in non-adjuvanted vaccine group) and 6 months post vaccination. Levels of hemagglutination inhibition antibodies were measured.
Results: The seroconversion rate of all 3 vaccine strains at 1 month post-vaccination was significantly higher in the MF59-adjuvanted group than in the non-adjuvanted group (47.7% vs. 17.4%, A/H1N1; 42.0% vs. 16.3%, A/H3N2; 31.8% vs. 7.0%, B, P < 0.01). One month post-vaccination, the fold increase in geometric mean titer from pre-vaccination for A/H1N1, A/H3N2 and B viruses was significantly greater in the MF59-adjuvanted group than in the non-adjuvanted group. In elderly patients (≥65 years), the seroconversion rate at 1 month post-vaccination against influenza B strain was higher in the MF59-adjuvanted group than in the non-adjuvanted group (33.3% vs. 7.1%, P = 0.03).
Conclusion: The MF59-adjuvanted influenza vaccine showed better immunogenicity than the non-adjuvanted influenza vaccine in CKD patients undergoing HD.
Introduction
Annual influenza epidemics occur during the winter and early spring months in the Northern Hemisphere. Most cases of seasonal influenza are mild and self-limiting. However, complications and hospitalizations are more frequent among individuals aged 65 y or more, children under 2 years, and individuals with underlying medical conditions.Citation1 Patients with chronic kidney disease (CKD) are also at an increased risk of morbidity, complications and mortality from influenza.Citation2 Therefore, CKD patients are recommended to get seasonal influenza vaccine annually. However, a suboptimal immune response to the inactivated influenza vaccine (IIV) has been observed in CKD patients relative to that of the healthy population. Defects in complement activation, neutrophil function, and B-cell and T-cell functions cause immune dysfunction in patients undergoing hemodialysis (HD).Citation3 In addition, the loss of immune cells is more rapid in patients undergoing HD than in healthy controls.Citation4 Thus, a strategy to enhance the efficacy and effectiveness of the influenza vaccine in CKD patients undergoing HD is required.
The key role of an adjuvant is in dose sparing, enabling a broad immune response and enhancing of the immunogenicity of vaccine. The MF59-adjuvanted inactivated influenza vaccine (aIIV) was first licensed in Europe in 1997 and also was approved by the U.S. Food and Drug Administration in people 65 y of age and older. The MF59-aIIV has shown enhanced immunogenicity in populations with chronic underlying diseases and older adults.Citation5-7 It was introduced in 2009 and has been used in the population aged 65 y and above in South Korea. The MF59-aIIV induced superior long-term immunogenicity in the elderly when compared to a non-adjuvanted IIV.Citation8 In this study, the immunogenicity of MF59-adjuvanted and non-adjuvanted trivalent influenza vaccines were compared in various age group of CKD patients undergoing HD during 2013–2014 season.
Results
Demographic characteristics
A total of 179 CKD patients undergoing HD participated in the study. Ninety one patients were vaccinated with the MF59-aIIV and 88 patients received the non-adjuvanted IIV. Among them, 174 patients were followed-up at 1 month post-vaccination (88 in the MF59-aIIV group and 86 in the non-adjuvanted IIV group) (). The sex ratio and mean age of the patients were not significantly different between the 2 groups (). The proportion of the elderly (≥65 years) was 23.9% in the MF59-aIIV group and 32.6% in the non-adjuvanted IIV group (P = 0.20).
Figure 1. Flow chart of the study. CKD, chronic kidney disease; aIIV, adjuvanted inactivated influenza vaccine; IIV, inactivated influenza vaccine; TIV, trivalent influenza vaccine; HD, hemodialysis.
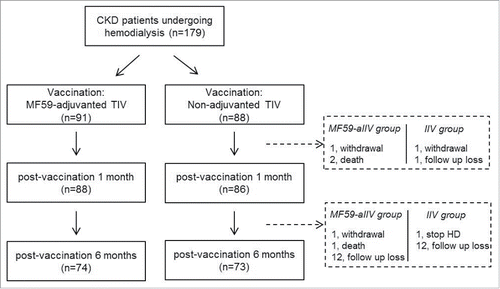
Table 1. Demographic characteristics of the study population (n=174).
Samples at 6 months post-vaccination were collected from 147 patients (74 in the MF59-aIIV group and 73 in the non-adjuvanted IIV group). There were no differences in sex ratio (male, 50.0% vs. 50.7%, P = 0.93) and mean age of the patients (55.5 ± 11.5 y vs. 56.5 ± 11.4 years, P = 0.58) between the MF59-aIIV group and the non-adjuvanted IIV group also.
Immunogenicity of the MF59-aIIV and non-adjuvanted IIV
No significant intergroup differences in pre-vaccination geometric mean titer (GMT) were observed for all 3 influenza strains. Overall, the seroprotection rate at 1 month post-vaccination against the A/H1N1 influenza virus was higher in the MF59-aIIV group than in the non-adjuvanted IIV group (81.8% vs. 65.1%, P = 0.01). There were no significant differences in the seroprotection rate at 1 month post-vaccination against the A/H3N2 and B influenza viruses between the MF59-aIIV group and the non-adjuvanted IIV group (96.6% vs. 90.7%, P = 0.11 for A/H3N2; 89.8% vs. 84.9%, P = 0.33 for B). The seroconversion rates at 1 month post-vaccination for the A/H1N1, A/H3N2 and B viruses were significantly higher in the MF59-aIIV group than in the non-adjuvanted IIV group (47.7% vs. 17.4%, A/H1N1; 42.0% vs. 16.3%, A/H3N2; 31.8% vs. 7.0%, B, P < 0.01) (). At 1 month post-vaccination, the GMT fold increases from pre-vaccination for A/H1N1, A/H3N2 and B were significantly greater in the MF59-aIIV group than in the non-adjuvanted IIV group.
Table 2. A comparison of the immunogenicity of the influenza vaccine in patients undergoing hemodialysis: MF59-adjuvanted versus non-adjuvanted vaccine.
In patients aged from 19 to 64 years, seroprotection rates at pre- and post-vaccination, for all 3 influenza strains, were not significantly different between the MF59-aIIV group and the non-adjuvanted IIV group (). Seroconversion rates at 1 month post-vaccination were higher in the MF59-aIIV recipient group than in the non-adjuvanted IIV group (46.3% vs. 13.8% for A/H1N1; 40.3% vs. 10.3% for A/H3N2; 31.3% vs. 6.9% for B, P < 0.01) ().
Table 3. A comparison of the immunogenicity of MF59-adjuvanted influenza vaccine and non-adjuvanted influenza vaccine in patients undergoing hemodialysis, 19–64 years.
Figure 2. Immunogenicity of trivalent influenza vaccination in chronic kidney disease (CKD) patients undergoing hemodialysis (HD), 1 month after vaccination: (A) 19–64 years; (B) ≥65 y. Statistically significance was indicated with ‘*’.
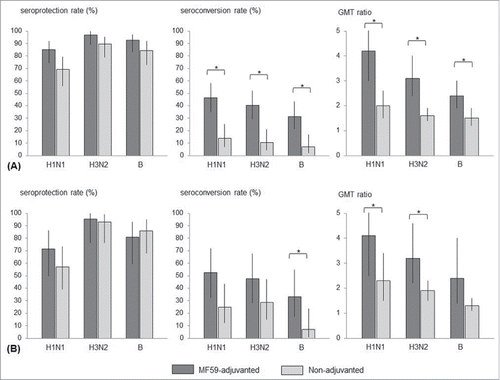
In patients aged ≥65 y, the seroconversion rate at 1 month post-vaccination for influenza B virus was higher in the MF59-aIIV group than in the non-adjuvanted IIV group (33.3% vs. 7.1%, P = 0.03) (). The GMT fold increases at 1 month post-vaccination for influenza A/H1N1 and A/H3N2 viruses were superior in the MF59-aIIV group when compared to the non-adjuvanted IIV group ().
Table 4. A comparison of the immunogenicity of MF59-adjuvanted influenza vaccine and non-adjuvanted influenza vaccine in patients undergoing hemodialysis, ≥65 y.
Long-term immunogenicity of the MF59-aIIV and the non-adjuvanted IIV
There was no significant difference in the seroprotection and seroconversion rates at 6 months post-vaccination between MF59-aIIV and non-adjuvanted IIV groups. However, GMT fold changes at 6 months post-vaccination were greater in the MF59-aIIV group when compared to the conventional IIV group for the influenza A/H3N2 and B viruses (1.7 vs 1.2, A/H3N2; 1.5 vs. 1.1, influenza B, P < 0.01).
Discussion
The number of CKD patients has markedly increased over the last 30 y. More than 400,000 patients with end-stage renal disease (ESRD) underwent HD in the United States in 2012.Citation9. In South Korea, the number of patients undergoing HD was 42,595 by the end of 2011.Citation10 CKD patients are at a great risk of influenza and influenza-related complications. Immune deficiencies in CKD patients are responsible for not only weakened protective ability against influenza, but also attenuated immune response to the influenza vaccination. A decrease in the phagocytic activity of neutrophils, monocytes, and macrophages is observed in CKD patients.Citation11 Furthermore, the depletion and dysfunction of dendritic cells cause immunodeficiency in CKD patients also.Citation12 Affected T-cells and B-cells lead to a decrease in the response to infection in HD patients.Citation3,13
Vaccination is the primary strategy used to prevent and control influenza. CKD patients are recommended to be vaccinated annually against influenza. In a systematic review, the influenza vaccine effectiveness was 12% to prevent influenza-like illness, 14% to prevent hospitalization due to influenza or pneumonia, 81% for protection of ICU admission and 32% to prevent all-cause mortality in patients with ESRD receiving dialysis, although quality of evidence was very low.Citation14 In Taiwan, an analysis of the insurance claims data for the previous 12 y showed that ESRD patients undergoing HD receiving influenza vaccination had reduced hazard ratio of hospitalization (0.81). Additionally, they had a reduced hazard ratio of heart disease (0.85), ICU admission (0.20) and mortality (0.30) in comparison to unvaccinated patients.Citation15 Compared with the healthy population, the immune response to the influenza vaccine is attenuated in CKD patients undergoing HD. Thus, strategies to enhance the immunogenicity and effectiveness of the influenza vaccination are required in CKD patients undergoing HD.
MF59-, a microfluidized, oil-in-water emulsion containing squalene, adjuvanted IIV was licensed in Europe in 1997 for the first time. The oil is stabilized by adding a water-soluble surfactant (polysorbate 80, Tween 80) and an oil-soluble surfactant (sorbitan trioleate, Span 85).Citation16 MF59 stimulates an influx of inflammatory cells and establishes a localized immunostimulatory environment.Citation17 Proliferation of antigen presenting cells and helper T cells increase the B cell response and enhance antibody production.Citation17 The MF59-aIIV showed a greater immune response than the non-adjuvanted IIV in the elderly.Citation5-7 However, there are limited data for the comparison of the immunogenicity of MF59-aIIV and non-adjuvanted IIV in CKD patients undergoing HD.
In this study, seroconversion rates of all 3 strains, at 1 month post-vaccination, were significantly higher in the MF59-aIIV group than in the non-adjuvanted IIV group in HD patients aged 19–64 y. In addition, the GMT fold increase of A/H1N1, A/H3N2 and B were significantly greater in the MF59-adjuvanted group than in non-adjuvanted group. In elderly patients (≥65 years), the MF59-aIIV showed enhanced immunogenicity in the GMT increase at 1 month post-vaccination for influenza A/H1N1 and A/H3N2. The MF59-aIIV containing 15 μg of HA from each of 3 influenza vaccine strains was used in this study. In the 2009 pandemic influenza, the MF59-aIIV containing 3.75 μg of HA was approved in South Korea. A low-dose (3.75 μg of HA) MF59-adjuvanted monovalent (H1N1pdm09) influenza vaccine showed suboptimal immunogenicity in 48 HD patients. The seroconversion rate at day 28 post-vaccination was 17.2% in patients aged 18–60 y and 26.3% in patients aged over 60 y.Citation18
The MF59-aIIV has been approved in the elderly aged ≥65 years. However, its use in younger adults with underlying medical conditions or in the immunocompromised conditions is dependent on the clinician's decision. The MF59-aIIV was safe and well tolerated in kidney transplant recipients aged 18–64 y.Citation19 Furthermore, the safety of the MF59-aIIV has been well described in children.Citation20 However, the safety profiles of 2 trivalent influenza vaccines were not assessed in this study.
In South Korea, influenza vaccination coverage rates are up to 80% in the elderly.Citation21 However, influenza vaccination rate in population of a younger age is relatively low, even in patients with underlying medical conditions. Among CKD patients, influenza vaccination rate in the elderly (≥65 years) was 85.3%, whereas 51.5% of CKD patients aged 19–64 y received the influenza vaccine.Citation22 In this study, a remarkable enhancement of the immunogenicity of the MF59-aIIV in HD patients aged 19–64 y was measured. For this reason, an extension of target group of the aIIV as well as improvement of influenza vaccination coverage rate in young patients with CKD would be advantageous.
There are several limitations of this study. The duration of dialysis and uremic status were not considered when patients were enrolled. However, CKD patients undergoing HD were enrolled according to the nephrologist's opinion. Patients undergoing HD are classified to stage 5 of CKD. Other comorbidities of the patients were not investigated, however, CKD requiring HD is considered as a high risk condition of influenza infection. In addition, experience of influenza-like illness (ILI) or influenza infection during study period was not evaluated. Because this study was conducted over 6 months, there were opportunities that influenza infection during study period could induce the increase of GMT in study population. However, influenza vaccine was administered before the start of influenza epidemic and 1 month post vaccination samples were collected before the proportion of patients with ILI exceeded the epidemic threshold in 154 CKD patients (88.5%). Antibody titer was measured by the HI assay and experiment for neutralizing antibody was not conducted. However, this study showed that the MF59-aIIV induced better immunogenicity than non-adjuvanted IIV in CKD patients undergoing HD. The MF59-aIIV could be considered as a feasible strategy to improve immunogenicity in HD patients, especially in those under 65 y of age. Further prospective study on influenza vaccine effectiveness in CKD patients undergoing HD is required.
Methods
Study design and vaccine
From October 2013 to April 2014, a multi-center, open-label, randomized trial was conducted at 3 tertiary hospitals and 2 local clinics with HD units in Korea. CKD patients undergoing HD (≥19 years old) were eligible to take part in the study and recipients of IIV of 2013–2014 season before the study were excluded. Patients were randomized into 2 groups and were vaccinated with the MF59-aIIV (Fluad®, Novartis Vaccines and Diagnostics, S.R.L., Siena, Italy) or non-adjuvanted IIV (Agrippal®, Novartis Vaccines and Diagnostics), respectively. Both vaccines were trivalent, subunit vaccines and contained 15 μg of hemagglutinin (HA) from each of 3 influenza vaccine strains of the 2013–2014 Northern Hemisphere influenza season according to the World Health Organization recommendation: an A/California/7/2009 (H1N1)pdm09-like virus, an A(H3N2) virus antigenically like the cell-propagated prototype virus A/Victoria/361/2011, and a B/Massachusetts/2/2012-like virus.
The MF59-aIIV (Fluad®) was approved for use in the elderly (≥65 years old) in Korea. However, CKD patients were considered as a representative group in which the immunogenicity of the influenza vaccine is weakened. For this reason off-label use of the MF59-aIIV was applied in CKD patients aged 19–64 y in this study.
Immunogenicity assessment
Venous blood samples were collected from each subject prior to vaccination and at 1 month (28 ± 7 days) and 6 months (180 ± 7 days) post-vaccination. The levels of hemagglutination inhibition (HI) antibodies were measured by a standard microtiter assay.Citation23 Sera were pretreated with a receptor-destroying enzyme (RDE II, Denka Seiken Co., LTD., Tokyo, Japan) for 18 h at 37°C and then inactivated at 56°C for 30 min. Serum dilutions ranged from 1:10 to 1:1,280. Serum HI antibody levels were determined using test antigens at a concentration of 4 hemagglutination units per 25 μl of virus per assay in a 0.5% (v/v) suspension of washed chicken erythrocytes. The microtiter plates were maintained at room temperature until sedimentation was visible. The serum dilution at which complete inhibition of hemagglutination was achieved was considered as the serum antibody titer. Titers of <1:10 were considered negative and arbitrarily assigned a titer value of 1:5. Titers of >1:1,280 were assigned a titer value of 1:1,280.
The GMT, seroprotection rate, seroconversion rate, and pre-/post-vaccination GMT fold increase were calculated. The seroprotection rate was defined as the proportion of participants with a HI titer level of ≥1:40. The seroconversion rate was defined as the percentage of subjects with either a pre-vaccination HI titer ≥1:10 and a ≥4-fold increase in post-vaccination HI antibody titer from baseline or pre-vaccination HI titer <1:10 and a post-vaccination HI titer of ≥1:40.
Activities of the 2013–2014 influenza season in Korea
During the 2013–2014 influenza season, according to the surveillance by the Korea Center for Disease Control & Prevention, the proportion of patients with ILI exceeded the system-specific epidemic threshold (12.1/1,000 persons) in week 52 (22 to 28 December, 2013).Citation24 The peak of ILI patients was reported in week 7 (9 to 15 February, 2014) and the rate of ILI patients declined below the epidemic threshold in week 15 (6 to 12 April, 2014). No secondary peak was observed in the 2013–2014 season. Among 2,094 influenza specimens, 1,108 (52.9%) were influenza B viruses and 640 (30.6%) were A/H3N2 influenza viruses. A(H1N1)pdm09 viruses were detected from 346 (16.5%) samples.
Statistical analysis
All statistical analyses were performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). HI antibody titers were expressed as geometric means with 95% confidence intervals (CI). Student t-test was used to analyze continuous variables. For patients aged 65 y and more, Mann-Whitney test was adopted for nonparametric analysis. Categorical variables were analyzed using a chi-square test. The Fisher's exact test was used when any cells had an expected count of less than 5 or when there was mismatch between the chi-square test and continuity correction. Statistical significance was set as P < 0.05.
Ethics approval and study protocol registration
This study was approved by the Institutional Review Board of Korea University Guro Hospital (approval number: KUGH13170). Informed consent was obtained from participants. The study protocol was registered at ClinicalTrials.gov (ClinicalTrials Identifier: NCT02686398).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to thank the members of the Western Dialysis Physician Association of Seoul, Korea and staff in the Asia Pacific Influenza Institute for their support of the study.
Funding
This study was supported by a grant of the TEPIK (Transgovernmental Enterprise for Pandemic Influenza in Korea) which is a part of Korea Healthcare Technology R&D Project by Ministry of Health & Welfare, Republic of Korea (Grant No. : A103001).
References
- Carrillo-Santisteve P, Ciancio BC, Nicoll A, Lopalco PL. The importance of influenza prevention for public health. Hum Vaccin Immunotherap 2012; 8(1):89-95; http://dx.doi.org/10.4161/hv.8.1.19066
- Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices–United States, 2013–2014. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports. Centers for Disease Control 2013; 62(RR-07):1-43
- Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lanc Infect Dis 2009; 9(8):493-504; http://dx.doi.org/10.1016/S1473-3099(09)70175-6
- Sester U, Schmidt T, Kuhlmann MK, Gartner BC, Uhlmann-Schiffler H, Sester M. Serial influenza-vaccination reveals impaired maintenance of specific T-cell memory in patients with end-stage renal failure. Vaccine 2013; 31(38):4111-20; PMID:23845814; http://dx.doi.org/10.1016/j.vaccine.2013.06.076
- Banzhoff A, Nacci P, Podda A. A new MF59-adjuvanted influenza vaccine enhances the immune response in the elderly with chronic diseases: results from an immunogenicity meta-analysis. Gerontology 2003; 49(3):177-84; PMID:12679609; http://dx.doi.org/10.1159/000069172
- Squarcione S, Sgricia S, Biasio LR, Perinetti E. Comparison of the reactogenicity and immunogenicity of a split and a subunit-adjuvanted influenza vaccine in elderly subjects. Vaccine 2003; 21(11-12):1268-74; PMID:12559808; http://dx.doi.org/10.1016/S0264-410X(02)00401-2
- Podda A. The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. Vaccine 2001; 19(17-19):2673-80; PMID:11257408; http://dx.doi.org/10.1016/S0264-410X(00)00499-0
- Song JY, Cheong HJ, Noh JY, Seo YB, Choi WS, Cho GJ, Hwang TG, Kim WJ. Long-term and cross-reactive immunogenicity of inactivated trivalent influenza vaccine in the elderly: MF59-adjuvanted vaccine versus unadjuvanted vaccine. J Med Virol 2013; 85(9):1591-7; PMID:23852684; http://dx.doi.org/10.1002/jmv.23630
- ESRD in the United States: An Overview of USRDS Annual Data Report Volume 2. Am J Kidney Dis 2015; 66(1):S79–S92
- Jin DC. Current status of dialysis therapy for ESRD patients in Korea. J Korean Med Assoc DE - 2013-07-15 2013; 56(7):562-8
- Mastalerz-Migas A, Gwiazda E, Brydak LB. Effectiveness of influenza vaccine in patients on hemodialysis–a review. Medical science monitor : international medical journal of experimental and clinical research. 2013; 19:1013-8; PMID:24241247
- Agrawal S, Gollapudi P, Elahimehr R, Pahl MV, Vaziri ND. Effects of end-stage renal disease and haemodialysis on dendritic cell subsets and basal and LPS-stimulated cytokine production. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010; 25(3):737-46; PMID:AMBIGUOUS
- Lisowska KA, Debska-Slizien A, Jasiulewicz A, Heleniak Z, Bryl E, Witkowski JM. Hemodialysis affects phenotype and proliferation of CD4-positive T lymphocytes. J Clin Immunol 2012; 32(1):189-200; PMID:21993694; http://dx.doi.org/10.1007/s10875-011-9603-x
- Remschmidt C, Wichmann O, Harder T. Influenza vaccination in patients with end-stage renal disease: systematic review and assessment of quality of evidence related to vaccine efficacy, effectiveness, and safety. BMC Med 2014; 12:244; PMID:25523432; http://dx.doi.org/10.1186/s12916-014-0244-9
- Wang IK, Lin CL, Lin PC, Liang CC, Liu YL, Chang CT, Yen TH, Morisky DE, Huang CC, Sung FC. Effectiveness of influenza vaccination in patients with end-stage renal disease receiving hemodialysis: a population-based study. PloS one 2013; 8(3):e58317; PMID:23516462; http://dx.doi.org/10.1371/journal.pone.0058317
- Martin JT. Development of an adjuvant to enhance the immune response to influenza vaccine in the elderly. Biologicals 1997; 25(2):209-13; PMID:9236054; http://dx.doi.org/10.1006/biol.1997.0086
- Tsai TF. Fluad(R)-MF59(R)-Adjuvanted influenza vaccine in older adults. Infect Chemother 2013; 45(2):159-74; PMID:24265964; http://dx.doi.org/10.3947/ic.2013.45.2.159
- Son J, Lee SB, Lee DW, Kim IY, Lee SJ, Lee SM, Song SH, Seong EY, Kwak IS. Immunogenicity of low-dose MF59-adjuvanted 2009 influenza A/H1N1 vaccine in dialysis patients. Clin Exp Nephrol 2013; 17(2):275-83; PMID:22990301; http://dx.doi.org/10.1007/s10157-012-0696-1
- Kumar D, Campbell P, Hoschler K, Hidalgo L, Al-Dabbagh M, Wilson L, Humar A. Randomized controlled trial of adjuvanted versus nonadjuvanted influenza vaccine in kidney transplant recipients. Transplantation 2016; 100(3):662–669; PMID:26335915
- Knuf M, Leroux-Roels G, Rumke HC, Abarca K, Rivera L, Lattanzi M, Pedotti P, Arora A, Kieninger-Baum D, Della Cioppa G. Safety and immunogenicity of an MF59-adjuvanted A/H1N1 pandemic influenza vaccine in children from three to seventeen years of age. Vaccine 2015; 33(1):174-81; PMID:25444803; http://dx.doi.org/10.1016/j.vaccine.2014.10.085
- Kee SY, Lee JS, Cheong HJ, Chun BC, Song JY, Choi WS, Jo YM, Seo YB, Kim WJ. Influenza vaccine coverage rates and perceptions on vaccination in South Korea. J Infect 2007; 55(3):273-81; PMID:17602750; http://dx.doi.org/10.1016/j.jinf.2007.04.354
- Yang TU, Song JY, Noh JY, Cheong HJ, Kim WJ. Influenza and Pneumococcal Vaccine Coverage Rates among Patients Admitted to a Teaching Hospital in South Korea. Infect Chemother 2015; 47(1):41-8; PMID:25844262; http://dx.doi.org/10.3947/ic.2015.47.1.41
- Cheong HJ, Song JY, Park JW, Yeon JE, Byun KS, Lee CH, Cho HI, Kim TG, Kim WJ. Humoral and cellular immune responses to influenza vaccine in patients with advanced cirrhosis. Vaccine 2006; 24(13):2417-22; PMID:16406176; http://dx.doi.org/10.1016/j.vaccine.2005.11.064
- Korea Influenza Sentinel Surveillance Report, 2013-2014. Korea centers for disease control & prevention. Available at http://www.cdc.go.kr/CDC/info/CdcKrInfo0301.jsp?menuIds=HOME001-MNU1132-MNU1138-MNU0037-MNU1380&cid=60812
