ABSTRACT
Influenza, caused by the influenza virus, is a contagious acute viral respiratory disease with a high incidence rate and wide and rapid spread. Influenza-related morbidity, mortality, and hospitalization rates remain high and are increasing continuously in high-risk groups, with a significant impact on human health and the economy. In order to evaluate the immunogenicity of 3 seasonal trivalent influenza vaccines in Chinese military, we conducted this field trial. We assessed the safety and immunogenicity of 3 seasonal trivalent influenza vaccines(TIVs)manufactured by GlaxoSmithKline(GSK), Beijing Sinovac Biotech (Sinovac), and Shenzhen Sanofi Pasteur (Pasteur) in healthy Chinese servicemen. We used theimported GSKTIV as the control, comparing it with the 2 domestic TIVs in a 1:1:1randomized, double-blind, controlled trial in a military command in Beijing. Healthy individuals, aged between 18 and 34 years, who had not received any influenza vaccine in the preceding3 years were enrolled and administered one dose of a TIV. Safety data were collected throughout the whole study (day 0 to day 30). Blood samples were collected to assess the subjects' immunogenicity before vaccination and 21 d after vaccination. In total, 292 subjects enrolled in the study. Twelve participants (4.1%) reported 12 adverse events. The incidence of adverse events was 1%, 5%, and7% for the GSK, Sinovac, and Pasteur TIVs, respectively. The reported injection-site reaction frequencies were similar for all 3 TIVs (p = 0.217). However, the proportion of systemic reactions was higher after the GSKTIV than after the Pasteur or Sinovac TIV (7.1% vs 3.1% or1%, respectively; p = 0.020). Three TIVs satisfied both the European and US Food and Drug Administration criteria for H1N1–179, H1N1–74, H3N2, and B strains based on the post vaccination sero-protection, the sero-conversion rate, and the geometric mean titer ratio. The Sinovac TIV, Pasteur TIV, and GSK TIV were well tolerated and immunogenic in healthy servicemen in the military. There was no significant difference in the immunogenicity of these 3 vaccines.
Introduction
Influenza, caused by the influenza virus, is a contagious acute viral respiratory disease with a high incidence rate and wide and rapid spread. Influenza-related morbidity, mortality, and hospitalization rates remain high and are increasing continuously in high-risk groups, with a significant impact on human health and the economy.Citation1 An influenza vaccine is known to be the most effective way to prevent influenza. The influenza virus can be classified into 3 types, A, B, or C, with easy changing of influenza A virus. Seasonal trivalent influenza vaccines(TIVs), consisting of 3 common strains A (H1N1), A (H3N2), and B strains, have been used in many countries.Citation2 Every year, the World Health Organization (WHO) announces the exact strains that are included in the seasonal influenza vaccines, based on the influenza disease surveillance data from the previous year.Citation2,3 In 1947, the USA approved an inactivated influenza vaccine for the first time. China began to introduce imported split influenza virus vaccines after 1996, and many domestic influenza vaccines have been approved for marketing since 2000.Currently, vaccination is recommended as an important measure against influenza virus in many countries. To achieve mass vaccination in the future, it is crucial to ensure that a vaccines both safe and effective.
The military is a special society with a highly concentrated and multi-ethnic population. In situations of group living, once an influenza virus infection occurs, it can readily cause an outbreak or pandemic, which can affect both the health of servicemen and their daily training. However the influenza vaccine is not a planned vaccine for all recruits. The Beijing military region has predominantly used imported influenza vaccines in the past, lacking experience in both the use of a domestic influenza vaccine and mass vaccination. Importantly, there have been no comparative studies of influenza vaccines in the military. Therefore, it is necessary to conduct clinical trials to assess the safety and immunogenicity of imported and domestic influenza vaccines in servicemen, and to explore the need for mass vaccination in the military. Here, we report the results of a clinical trial in which we assessed the safety and immunogenicity of one imported and 2 domestic TIVs in the military. The purpose of the trial is to compare the immunogenicity of 3 influenza vaccines. Our aim was to provide scientific evidence to establish immunization strategies and choose the appropriate vaccines for the military.
Results
Characteristics of research objects
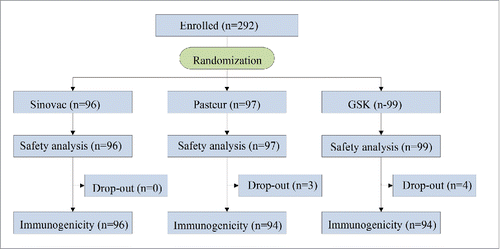
A total of 292 subjects were enrolled in the study (), all of who completed the safety analysis, and 285 completed the study. Seven subjects discontinued the study because they did not provide a blood sample after vaccination (day 21), but no discontinuation was in response to an adverse reaction associated with the vaccine. The mean age of the subjects was 18.9 y (standard deviation, 1.7; range, 18–31), and they were all male. Most of the subjects were from the Han population (96.8%) and the rest were from the Hui(1.4%), Man(0.4%), or Mongolian population (1.4%) (). The 3 groups had similar baseline characteristics in terms of age (P = 0.227) and ethnicity (P = 0.246).
Table 1. Demographic characteristic of the subjects.
Immunogenicity
Baseline antibodies
Before vaccination, the baseline seroprotection rates in the young servicemen were 46.6%, 26.7%, 65.4%, and 59.6% for H1N1-179A, H1N1-74, H3N2, and B strains, respectively. The seroprotection rates for these 4 strains did not differ significantly among the 3 groups, whereas the prevaccination GMT for H3N2 was higher in the Pasteur group than in the Sinovac or GSKgroup(P = 0.025; ).
Table 2. Summary of pre- and postvaccination antibody-mediated immunogenicity.
Immunogenicity assessments
The immunogenicity of the TIVs was assessed from the HI titer against the 4 common influenza virus strains ().In the Sinovac group, the GMT ratios for H1N1-179, H1N1-74, H3N2, and B strains were 15.7(95% CI: 11.7–21.0), 14.8(95% CI: 11.0–19.9), 7.8(95% CI: 6.1–10.1), and 11.1(95% CI: 8.7–14.1), respectively; the post vaccination seroprotection rates were 100.0% (95% CI: 95.2–100.0),100.0% (95% CI: 95.2–100.0), 93.8% (95% CI: 86.4–97.8), and 100.0% (95% CI: 95.2–100.0), respectively; and the seroconversion rates were 86.5% (95% CI: 77.6–92.6), 87.5% (95% CI: 78.8–93.3), 75.0% (95% CI: 64.9–83.2), and 85.4(95% CI: 76.4–91.7), respectively. Therefore, the Sinovac TIV met all the European and FDA criteria. The Pasteur TIV also satisfied both the European and FDA criteria for the following reasons: postvaccination seroprotection rates were ≥ 97.9%, seroconversion rates were > 72.3%, and GMT ratios were ≥ 5.9 for all 4 vaccine strains. The results for the imported vaccine, GSK TIV, were similar to those cited above for the 2 domestic vaccines. The seroprotection rates were ≥ 95.8%, the seroconversion rates were >74.7%, and the GMT ratios were ≥5.5, which met both the European and FDA criteria. The GMT ratios for H1N1-74 were significantly higher (P < 0.001) in the GSK group (36.5; 95% CI: 25.7–51.8) than in the Pasteur group (24.2; 95% CI:17.0–34.4) or Sinovac group (14.8; 95% CI:11.0–19.9). However, as for the seroprotection and seroconversion rates for all the strains, the differences among the 3 groups were not statistically significant ().
Cross-reactivity
The cross-reactivity of the induced antibodies was assessed against 2 similar H1N1 strains, A/California/7/2009NYMC X-179A (H1N1-179) and A/Christchurch/16/2010 NIB-74xp(H1N1-74). The post vaccination seroprotection and seroconversion rates and GMT ratios for both strains satisfied the European and FDA criteria for the 3 TIVs. Although there was no significant difference between the Sinovac TIV and Pasteur TIV in the 3 immunogenicity indicators described above for the 2 similar H1N1 strains, the GMT ratio induced by the H1N1-74 strain was substantially higher than that induced by the H1N1–179 strain for the GSK TIV(36.5 vs 23.9, respectively; P < 0.001) ().
Safety
During the study (day 0 to day 21), a total of 12participants(4.1%) reported 12 AEs and all were considered to be vaccine related ().The incidence of AEs was higher in the GSK TIV group than in the Pasteur TIVor Sinovac TIV group(7.1% vs 5.2% or 1.0%, respectively; P = 0.024). The reported injection-site reaction frequencies, all of which were indurations, were similar for all 3 TIVs(P = 0.217). However, the proportion of systemic reactions was higher for the GSK TIV than for the Pasteur TIV or Sinovac TIV (7.1% vs 3.1% or 0%, respectively; P = 0.020).The most common systemic reactions were headache, followed by dizziness and nausea, all of which were reported more often in the GSK group than in the Sinovac group or Pasteur group
Table 3. Summary of cross-reactivitybetween H1N1strains.
Table 4. Summary of adverse events.
Discussion
In this clinical study, we selected the imported GSK TIV as the control with which to compare the immunogenicity and safety of the domestic vaccines (Sinovac TIV and Pasteur TIV), and to assess the immunogenicity and safety of these 3 TIVs according to the European and FDA criteria. This study demonstrates that the 3 vaccines satisfied all the European and FDA criteria for H1N1-179, H1N1–74, H3N2, and B strains in terms of the factors post vaccination seroprotection, seroconversion rate, and GMT ratio. All three TIVs were well tolerated and immunogenic in healthy serviceman in the military. In this study, we also found that the domestic and imported vaccines did not differ significantly based on the unified criteria. These results are similar to those of a systematic review conducted among Chinese people,Citation9 suggesting that there is good consistency in the quality of domestic and imported TIVs. As we noted, the GMT ratio for H1N1-74 was significantly higher in the imported vaccine group than in the domestic vaccine groups. A possible reason is that the infection status of the enrolled subjects differed among the 3 groups before the study. However, the GMT ratio for the H1N1strains also met the European and FDA criteria. This had no substantial effect on the evaluation results.
It is noteworthy that the induced antibodies were cross-reactive for 2 similar H1N1 strains: A/California/7/2009 (H1N1-179) and A/Christchurch/16/2010 (H1N1-74).Moreover, the domestic and imported influenza vaccines met all the European and FDA criteria for these 2 strains. However, we also found that the GMT ratio for the H1N1-74 strain was significantly higher for the imported vaccine than for the domestic vaccine (as mentioned above). The GMT ratio for the imported vaccine induced by H1N1-74 was substantially higher than that induced by the H1N1-179 strain. As laboratory results generated by WHO have shown, the difference in cross-reactivity depends on the immunogenicity of the H1N1-74 strain itself.Citation10 Based on our study of similar strains, we suggest that vaccine manufacturers synthesize vaccines based on several parameters, such as the viral yield and production rate, and using laboratory results to choose the viral strain with which to produce a vaccine. However, the person being vaccinated will be effectively protected against the influenza virus, regardless of which manufacturer produced the vaccine.
In this study, the incidence of AEs in healthy servicemen was low for all 3 TIVs. No serious or life-threatening AE was reported. This result is similar to that of a clinical trial conducted in China in 2013,Citation11 which demonstrated that all 3 TIVs were well tolerated. However, we observed a higher incidence of systemic reactions with the imported influenza vaccine (GSK TIV) than with the 2 domestic vaccine (Sinovac TIV and Pasteur TIV). This is probably attributable to the small sample size and the different formulations and manufacturing processes used by them and factures.Citation12 A systematic review of 10studies that compared a single dose of an imported and a domestic influenza vaccine, conducted in Chinese in 1996–2008, showed that there was no statistical difference in the AEs induced by the imported and domestic influenza vaccines.Citation9 Additional studies comparing imported and domestic vaccines, with sufficiently large sample sizes, are required to confirm the results of our study.
According to the baseline seroprotection rates in our study, the young servicemen were generally susceptible to seasonal influenza viruses. Many studies have shown that the influenza vaccine is effective in preventing influenza in the Chinese population. In other countries, studies of serviceman have demonstrated that influenza vaccines protected 75% and 57% of individuals from infection in the USA and Finland military.Citation12,13 Considering that group living is necessary in the military, we recommend that servicemen be vaccinated against influenza before the height of a pandemic.
This study selected recruits as subjects to compare the immune effect of 3 influenza vaccines and intended to provide useful advices for immunization programs of the recruits, and this study met its intended purpose. However, there were also some shortcomings in our study, we should to strive to improve in future studies. The first aspect is the sample of subjects, relative to the total recruits every year, 300 people was smaller than normal, in the next study we should expand the sample size to make it more representative. The second aspect is that all the subjects were male, we should include male and female in our study according to the sex ratio to reduce gender bias. In this study, all the subjects were recruits, 95% aged 18–20 years, the results can't show the picture of other age groups. In the follow-up study, we will enrolled different ages people to show the whole immunogenicity picture of the military people. After intensive training, the recruits were assigned to different positions in different geographical and it was difficult to set together. Therefore, this study was relatively short time, and there was no long-term adverse reactions were observed. In the follow-up study, we will focus on long-term adverse vaccine reaction observe and provide more reliable evidence for development of immunization programs and vaccine selection.
In Chinese military, the influenza vaccine is not the EPI vaccine, but every year many people from the army suffering from flu and do great harmful for the health. Through our study, we believe that the influenza vaccine could provided good protection for the army groups and could reduce the health cost. Each year, we carry out immunization programs for the recruits, include hepatitis B vaccine, MMR triple vaccine, epidemic encephalitis vaccine and so on. These vaccines were used for a long time and the safety is no problem, but the immune effect among the recruits was not clearly, as for the cross-cutting role with influenza vaccine, which has not revenant research. These are what we need further study.
In conclusion, Sinovac TIV, Pasteur TIV, and GSK TIV were well tolerated and immunogenic in healthy servicemen in the military. There was no significant difference in the immunogenicity of the domestic and imported vaccines.
Materials and methods
Study design
This was a randomized, double-blind, controlled trial conducted in a military command in Beijing. In this trial,. study staff and subjects did not know which vaccine was used for each subject. The primary objective was to assess the safety and immunogenicity of 3 TIVs manufactured by GlaxoSmithKline (GSK), Beijing Sinovac Biotech (Sinovac), and Shenzhen Sanofi Pasteur (Pasteur) in healthy Chinese servicemen. Using an imported TIV(GSK) as the control, the secondary objective was to compare it with the 2 domestic TIVs. Healthy servicemen aged 18–34 y were enrolled and randomized1:1:1 to the 3 arms of the trial to receive one dose of a TIV. The 300 subjects belonging to 3 different sub-units and all of them were new recruits, using drawing lots to determine which type of vaccine inoculation of the 3 units, thereby the group of each member has been confirmed. Immediate adverse events (AEs) were recorded within 30 min of vaccination at the clinical sites. All subjects used diary cards to record any solicited injection-site and systemic reactions within3 days of vaccination. Other unsolicited AEs were also recorded until the end of the study (day 21). Blood samples were collected to assess the immunogenicity of the subjects before vaccination and 21 d after vaccination. This study was approved by the Medical Ethics Committee of the Beijing Military Area Command Center for Disease Control and Prevention (Center [2014] no.92). All participants provided their written informed consent before they were included in the study.
Sample size estimation
The main purpose of the trial was designed to test the non-inferiority of the aimed vaccine to the other vaccines. We through calculating the geometric mean ratio of the 2-side 95% confidence interval (CI) of titer of the HI antibody of each vaccine strain virus after 21 d of vaccination to analyze whether the target influenza vaccine is not inferior to other influenza vaccines. We should obtain the difference of the HI antibody titers after a logarithmic conversion. According to the non-inferiority of FDA“Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines”standards, among the 3 types of strain, if the lower limit of confidence intervals (CI) were not exceed the lower limit of the non-inferiority margin 1.5(log-transformed to 0.6), the non-inferiority was established. So the HI titers mean difference lower CI of the non-inferiority margin value was taken as δ = 0.6. The total power of test of the 3 strain of influenza virus should not be less than 80%, so the power of test of each individual type of the virus strain should be 92.8%, thereby calculating the number of experiments in each group was 94. We considering the loss of follow-up, randomized and other factors, identify each group include 100 people and a total of 300 people enrolled.
Subjects
Healthy individuals aged 18–34 y who had not received any influenza vaccine in the preceding3 years were eligible for the study. Subjects were excluded if they were allergic to egg or to any other vaccine component; were in the active stage of any chronic disease, acutedisease, or malignant tumor; or were immunodeficient or had a history of Guillain-Barré syndrome.
Vaccine
The study vaccines were administered in 0.5 mL per needle containing 15μg of hemagglutinin (HA) for each strain, which was recommended by the WHO for the 2014 seasonal influenza formulation for the northern hemisphere: A/Texas/50/2012(H3N2) and B/Massachusetts/02/2012.Citation4 As for H1N1, the 2 viral strains for the imported vaccine ((A/Christchurch/16/2010)(NIB-74xp)) and domestic vaccines ((A/California/7/2009) (NYMC X-179A) were similar. The imported vaccine (GSK), with the product name Fluarix, was manufactured by GlaxoSmithKlineBiologicals (a stock solution of the vaccine was imported from Germany and packed locally in Shanghai, batch number YFLUA832AA). The domestic vaccines were manufactured by the Beijing Sinovac Biotech Company (product name AnFlu, batch number 201405011) and Shenzhen Sanofi Pasteur Biological Products Co., Ltd (product name Vaxigrip, batch number FL20140408). All the vaccines had passed the tests of the National Institute of the Control of Pharmaceutical and Biological Products, and were uniformly packaged and blindly labeled in this study.
Safety assessments
The safety of the vaccines was assessed according to the State Food and Drug Administration guidelines for the classification of adverse reactions to the prevention of clinical trials of vaccines ([2005]493).Citation5 Adverse events were recorded, including the following signs: solicited injection-site reactions (pain, redness, swelling, itching, induration), systemic reactions (anaphylaxis, headache, fatigue, nausea, vomiting, diarrhea, muscle ache), and vital signs.
Immunogenicity assessments
The hemagglutination inhibition (HI) titers were measured with antigen and standard sera provided by the National Institute for Biological Standards and Control. To calculate the geometric mean titers(GMTs), samples with HI < 100% at the lowest serum dilution tested (1:10) were assigned a titer of 5. Seroconversion in a subject was defined as either a prevaccination HI titer <1:10 and a day 21 titer ≥ 1:40 or by a prevaccination titer ≥1:10 and a minimum 4-fold titer increase at day 21. Seroprotection was defined as a pre- or post vaccination HI titer ≥1:40.Citation6 The first immunogenicity assessment criteria were the guidance on the harmonization of requirements for influenza vaccines by the European Committee(European criteria)Citation7:in adults aged 18–60 years, post vaccination seroprotection rate ≥70%, seroconversion rate >40%, and post vaccination/prevaccination GMT ratios (GMT ratios)≥2.5 for all 4 vaccine strains. Furthermore, according to the Guidance for Industry-Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines of the US Food and Drug Administration (FDA criteria),Citation8 the lower limits of the 95% CIs for seroprotection rates and seroconversion rates were ≥40% and ≥70%,respectively.
Statistical analysis
The statistical analysis was performed with SPSS(version17.0). Safety was assessed in all the participants who received a study vaccine. Immunogenicity was analyzed in all the immunized subjects who provided a blood sample on day 21. P < 0.05 was deemed to indicate a statistically significant difference on 2-side statistical tests. The GMTs and GMT ratios of multiple groups were compared with one-way analysis of variance and intragroup comparisons were made with a paired t est. Comparisons of the seroconversion and seroprotection rates among groups were made with a χ2 test or Fisher's exact test.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Yuan-Sheng Hu and Yu-Fei Song for their technical assistance, and they have no conflict of interest for this article.
Funding
This study was supported by the National Natural Science Foundation of China (grant no. 81460520), Projects of the Key Laboratory of Emergency and Disaster Medicine in PLA (JY1408).
References
- Mc Elhaney JE, Zhou X, Talbot HK, Soethout E, Bleackley RC, Granville DJ, Pawelec G. The unmet need in the elderly: how immunosenescence, CMV infection, comorbiditiesand frailty are a challenge for the development of more effectiveinfluenza vaccines. Vaccine 2012; 30(12):2060-7; PMID:22289511; http://dx.doi.org/10.1016/j.vaccine.2012.01.015
- Centers for Disease Control and Prevention. Prevention and control of seasonalinfluenza with vaccines. MMWR Recomm Rep 2013; 62(RR-07):1-43.
- Cadorna-Carlos JB, Nolan T, Borja-Tabora CF, Santos J, Montalban MC, de Looze FJ, Eizenberg P, Hall S, Dupuy M, Hutagalung Y, et al. Safety, immunogenicity, and lot-to-lot consistency of a quadrivalentinactivated influenza vaccine in children, adolescents, and adults:A randomized, controlled, phase III trial. Vaccine 2015; 32:2485-92.
- World Health Organization. Recommended viruses forinfluenza vaccines for use in the 2014 northern hemisphere influenza season. Wkly Epidemiol Rec 2010; 85:81-92; PMID:20210260
- State Food and Drug Administration. Guidelines for the classification of adverse reactions to the prevention of clinical trials of vaccines 2005; 493:35-45.
- Peter T, Geoffrey JG, Cynthia BS, Malcolm S, David PG, Ayca OG, Carlos DG, Victoria L. Immunogenicity and safety of Fluzone intradermal and high-dose influenza vaccines in older adults≥65 years of age: a randomized, controlled, phase II trial. Vaccine 2014; 32:2507-17; PMID:24120672; http://dx.doi.org/10.1016/j.vaccine.2013.09.074
- European Committee for Proprietary Medicinal Products. Note for guidance on harmonisation of requirements for influenza vaccines (CPMP/BWP/214/96). European Agency Evaluation Medicinal Products 1997. p. 26-40.
- FDA. Guidance for industry: clinical data needed to support the licensure ofpandemic influenza vaccines. FDA 2007. p. 1-15.
- Zhao YW, Feng ZJ. The meta analysis on the safety and immunogenicity of domestic and imported split influenza virus vaccines l.Chin. J Vaccines Immun 2014; 15(1):19-26.
- World Health Organization. WHO manual on animalinfluenza diagnosis and surveillance. Geneva: The World Health Organization 2002. p. 1-98.
- Luo FJ, Yang LQ, Ai X, Bai YH, Wu J, Li SM, Zhang Z, Lu M, Li L, Wang ZY, et al. Immunogenicity and safety of three 2010–2011 seasonal trivalent influenza vaccines in Chinese toddlers, children and older adults: A double-blind and randomized trial. Hum Vaccin Immunother 2013 2013-08-01; 9(8):1725-34; PMID:23896581; http://dx.doi.org/10.4161/hv.24832
- Williams MS, Wood JM. A brief history of inactivated influenza virus vaccines. Elsevier Science, 1993; 169-71.
- Pyhala R, Haanpaa M, Kleemola M. Acceptable protective efficacy of influenza vaccination in young military conscripts under circumstances of incomplete antigenic and genetic match. Vaccine 2001; 9(23–24):3253-60; http://dx.doi.org/10.1016/S0264-410X(01)00010-X