ABSTRACT
As the main etiologic bacterium of dental caries, Streptococcus mutans (S. mutans) has been considered as the primary object of vaccine research. We previously constructed a recombinant flagellin-rPAc fusion protein (KF-rPAc) that consists of an alanine-rich region to proline-rich region fragment of PAc (rPAc) from S. mutans and flagellin KF from E.coli K12 strain. Intranasal (i.n) immunization of KF-rPAc could induce high level of rPAc-specific antibody responses and offer robust protection against dental caries. In caries development, biofilm formation was considered as the necessary process involved. As PAc possesses other activities besides affecting adherence of S. mutans to salivary glycoproteins, we wondered whether rPAc-specific antibody responses induced by KF-rPAc could inhibit biofilm formation. Hence, in the present study, a simple and convenient in vitro biofilm model of S. mutans was constructed without saliva pre-coated. Both serum and saliva from KF-rPAc immunized rats significantly inhibited biofilm formation. Moreover, with the presence of serum or saliva, the biofilm formation is negatively correlated with the level of rPAc-specific antibody, and positively correlated with caries scores in rat. Moreover, in immunized mice, the level of rPAc-specific antibody also negatively correlated with the biofilm formation. Unlike ampicillin, serum of KF-rPAc immunized mice only inhibited biofilm formation but not proliferation. All together, we discovered that besides the well known blocking adherence of S. mutans to salivary glycoproteins by rPAc-specific antibody, flagellin-rPAc vaccine could also protects tooth from caries by inhibiting biofilm structure formation in between bacteria.
Introduction
Streptococcus mutans (S. mutans) has been demonstrated as the primary etiologic bacterium of human dental caries.Citation1,2 The dental lesion of caries is usually resulted from localized dissolution and destruction of teeth by colonized S. mutans after cariogenic biofilm formation on tooth surfaces.Citation2 PAc is a cell surface fibrillar protein of S. mutans, playing an important role in the initial adherence of S. mutans to tooth surface.Citation3,4 To inhibit biofilm associated dental caries, PAc has long been utilized as a most effective immunogen in many forms such as protein, recombinant or synthetic peptide,Citation5,6 protein-carbohydrate conjugate,Citation7 DNA-based active vaccines,Citation8 or DNA vaccine adjuvanted with recombinant flagellin protein.Citation9
TLR5 agonist flagellin could act as the mucosal adjuvant in vaccines against pathogens.Citation10-12 We previously demonstrated that a recombinant fusion protein (KF-rPAc) consisting of flagellin and alanine-rich region (A-region) to proline-rich region (P-region) fragment of PAc as anti-caries mucosal vaccine enhanced rPAc-specific antibody response and conferred better protection than other anti-caries vaccines.Citation13 Furthermore, KF-rPAc could also inhibit the progression of established caries.Citation14 Although the caries inhibition effect of our vaccine candidate KF-rPAc was solidly demonstrated, and the rPAc-specific antibody response showed negative correlation with caries lesions, how the antibody response might inhibit the development of dental caries was still unknown.
A-P regions of PAc are important in the adherence and colonization of S. mutans.Citation15,16 Thus, antibody directed to the intact PAc or to its salivary-binding domain is presumed to be able to block adherence of S. mutans to saliva-coated tooth surface.Citation17 PAc shows multifunctional activities, such as binding to soluble extracellular matrix glycoproteins and host cell receptors, interacting with salivary glycoproteins, coaggregating with other bacteria.Citation18 Therefore, we wondered whether the antibody response induced by KF-rPAc could inhibit biofilm formation besides the adherence of S. mutans to salivary glycoproteins.
Without saliva coating, PAc could not be involved in the adhesion to hydroxyapatite beads.Citation19 Thus, in present study, a simple and convenient in vitro biofilm model of S. mutans without pre-coated with saliva was constructed and utilized to test whether the KF-rPAc induced immunity by intranasal (i.n.) immunization might interfere with biofilm formation of S. mutans. Furthermore, the characteristics of KF-rPAc induced immunity in inhibiting biofilm formation of S. mutans were also analyzed and compared with that of ampicillin.
Results
KF-rPAc induced humoral immunity in S. mutans challenged rats inhibits biofilm formation of S. mutans in vitro
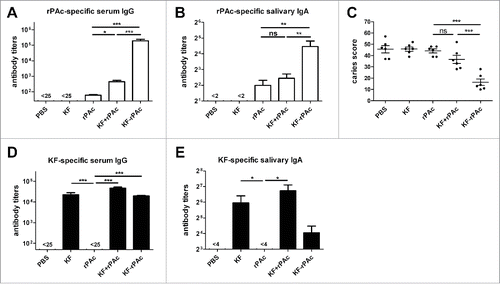
The intranasal (i.n) immunization in rats was repeated as described previously.Citation13 Briefly, after bacterial challenge, we immunized the rats with PBS, 3.5 μg KF, 5 μg rPAc, 3.5 μg KF + 5 μg rPAc, and 8.5 μg KF-rPAc 3 times at 4 weeks interval. Antibody titers of serum and saliva, and the degree of caries lesions in enamel (E), slight dentinal (Ds) and moderate dentinal (Dm) were as similar as previously reported.Citation13 Significantly elevated level of serum rPAc-specific IgG (), serum rPAc-specific IgA (data not shown) and salivary rPAc-specific IgA antibodies () were induced by fusion protein KF-rPAc. E, Ds and Dm in rats immunized with KF-rPAc were all significant fewer than in rats immunized with PBS, KF alone and rPAc alone (data not shown). Significant fewer total caries score (E + Ds + Dm)—were observed in the rats immunized with fusion protein KF-rPAc than in the rats immunized with PBS, KF alone, rPAc alone, and KF + rPAc (). Besides rPAc-specific antibody response, serum KF-specific IgG () and salivary KF-specific IgA antibodies () were also induced by KF, KF+rPAc and KF-rPAc.
Figure 1. Antibody responses and protection against dental caries in S. mutans challenged rat after immunization. Rats were challenged with S. mutans and then immunized with PBS, 3.5 μg KF, 5 μg rPAc, 3.5 μg KF plus 5 μg rPAc, 8.5 μg KF-rPAc at 4 weeks interval. (A and B), rPAc-specific serum IgG and salivary IgA at 2 weeks after the second boost. (C), Total caries score 4 weeks after the second boost. (D and E), KF-specific serum IgG and salivary IgA at 2 weeks after the second boost. (*, p <0.05; **, p < 0.01; ***, p < 0.001)
A simple and convenient in vitro biofilm model of S. mutans was constructed as described in Materials and Methods to test whether the KF-rPAc induced immunity might interfere with biofilm formation of S. mutans. Sera from KF-rPAc or KF+rPAc immunized rats inhibited biofilm formation significantly, but sera from either rPAc alone, or KF alone immunized rats did not (). Sera and saliva of KF-rPAc immunized rats inhibited biofilm formation more significantly than that of KF+rPAc or rPAc alone (). Thus, in S. mutans challenged rats, humoral immunity induced by KF-rPAc could inhibit biofilm formation of S. mutans efficiently.
Figure 2. Biofilm formation inhibition of immunized rats' serum or saliva and its correlation with rPAc-specific antibody and total caries scores. 100 µl BHI diluted rat serum or saliva were mixed with 100 µl BHI diluted S. mutans and incubated for 16 h. The biofilm formation was quantified by measuring the extracted crystal violet stained to plate adherent bacteria and derivatives at 570 nm. The inhibitory effects of 20-fold diluted rat serums (A) and 5-fold diluted saliva (B) form immunized rats that challenged with S. mutans were shown. Data are represented as mean ± SE for 6 samples of one representative experiment that repeated 3 times (*, p < 0.05; **, p < 0.01; ***, p < 0.001). (C and D), Correlation between biofilm formation and rPAc-specific rat serum IgG or saliva IgA. (E and F), Correlation between caries scores and biofilm formation with the presence of rat serum or saliva. Data are analyzed by Graphpad Prism 5. Dotted lines represent the 95% confidence intervals. The correlation coefficients (r) and p values are also shown.
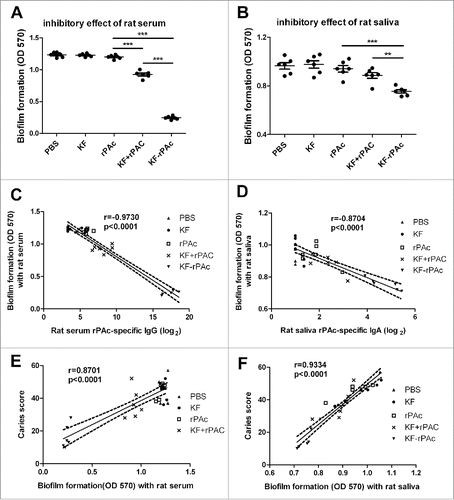
Biofilm formation negatively correlated with rPAc-specific antibody titer in rats, and positively correlated with dental caries score
The inhibition of biofilm formation could not be due to the KF-specific antibody response, as KF group induced comparable level KF specific antibody response as KF-rPAc group, but showed no interference with biofilm formation. The correlation analysis results showed that biofilm formation negatively correlated with both rPAc-specific titers of rat serum IgG (r = −0.9730) () and salivary IgA (r = −0.8704) (). In S.mutans challenged rats, serum rPAc-specific IgG titer if higher than 256, could offer protection in inhibiting biofilm formation (); at the same time, salivary rPAc-specific IgA titer if higher than 32, could offer protection in inhibiting biofilm formation ().
On the other hand, associations of the caries lesion with the biofilm formation were also tested by correlation analysis. Results showed that caries score in rat positively correlated with biofilm formation tested with both rat sera (r = 0.8701) () and rat saliva (r = 0.9334) (). These results suggested that the inhibition of biofilm formation by rPAc-specific antibody response likely confer protection against dental caries.
KF-rPAc induced rPAc-specific antibody response in mice can also effectively inhibit biofilm formation
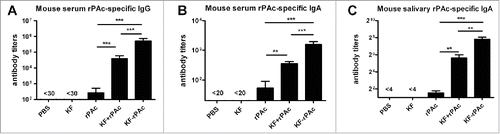
As rats have been challenged by S.mutans, there was a concern that humoral response against other antigens of S.mutans may also be induced and contribute to inhibiting biofilm formation. Mouse model without challenge was adopted, to exclude the disturbance of antibody response against other antigens of S.mutans. We further tested KF-rPAc induced humoral immunity in mice for its inhibitive effect on biofilm formation of S. mutans. KF-rPAc induced the highest antibody responses in serum IgG () and IgA () and saliva IgA () with antibody titers of 8 × 106, 2 × 103, and 1 × 28 respectively among the 5 groups. KF + rPAc induced the second highest serum rPAc-specific IgG and IgA and saliva rPAc-specific IgA, but rPAc alone group induced only marginal antibody responses compared to the KF alone or PBS ().
Figure 3. Immune responses to different vaccine candidates in mice. Mice were intranasally immunized immunized with different vaccine candidates at 4 weeks interval. Serum and saliva samples collected at 2 weeks after the second boost were detected by ELISA for rPAc-specific antibody titer. Serum rPAc-specific IgG (A), serum rPAc-specific IgA (B) and salivary rPAc-specific IgA (C) of mice immunized with different vaccine candidates. Data are represented as mean ± SE for 6 samples of one representative experiment that repeated 3 times (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
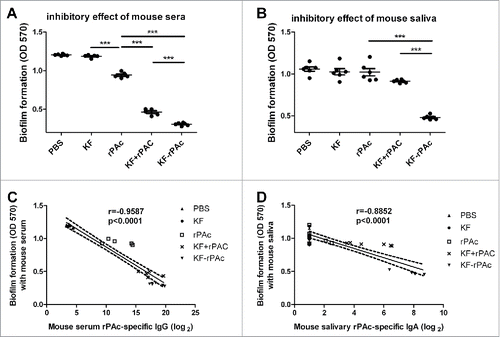
Accordingly, sera of KF-rPAc group showed the highest inhibition on biofilm formation of S. mutans. KF+rPAc group also displayed significant inhibition on biofilm formation but significantly less efficient than KF-rPAc. Even rPAc alone showed significant inhibition effect compared to that of KF or PBS group though much less efficient than that of KF-rPAc or KF+rPAc (p < 0.001) (). In parallel, saliva of KF-rPAc immunized group also showed the highest efficiency in inhibiting biofilm formation, which is significant higher than both the rPAc immunized group (p < 0.001) and KF+rPAc group (p < 0.01) (). Strong inverse correlations showed between biofilm formation with both mouse serum rPAc-specific IgG (r = −0.9587) () and salivary rPAc-specific IgA (r = −0.8852) (), which further supported that rPAc-specific antibodies inhibit biofilm formation. In vaccinated mice, serum rPAc-specific IgG titer if higher than 256, could offer protection in inhibiting biofilm formation (); at the same time, salivary rPAc-specific IgA titer if higher than 64, could offer protection in inhibiting biofilm formation ().
Figure 4. The inhibitory of mice serum or saliva on S. mutans biofilm formation and the correlation with rPAc-specific antibody. 100 µl BHI diluted mice serum or saliva were mixed with 100 µl BHI diluted S. mutans and incubated for 16 h. The biofilm formation was quantified by measuring the extracted crystal violet stained to plate adherent bacteria and derivatives at 570 nm. (A and B), Inhibitory effects of 20-fold diluted rat serums and 5-fold diluted saliva form immunized mice. Data are represented as mean ± SE for 6 samples of one representative experiment that repeated 3 times (***, p <0.001). (C and D), Correlation between biofilm formation and serum rPAc-specific IgG or saliva rPAc-specific IgA. Data are analyzed by Graphpad Prism 5. Dotted lines represent the 95% confidence intervals. The correlation coefficients (r) and p values are also shown.
To visualize the effects of KF-rPAc on inhibiting biofilm formation, S. mutans were incubated into a round cover glass in the presence of the sera from immunized mice and visualized by confocal microscopy. Compared with the sera from PBS immunized mice, sera from KF-rPAc immunized mice significantly decreased the number of bacteria aggregated in the biofilm and reduced the thickness of biofilm (Data not shown).
KF-rPAc induced humoral immunity does not alter proliferation of S. mutans
Biofilm formation is a rather complex process with multiple steps, correlated with not only bacteria cell-cell adhesion but also bacteria proliferation.Citation20 We wondered whether KF-rPAc induced humoral immunity inhibited biofilm formation by either inhibition of cell-cell adhesion, or bacteria proliferation, or both. Thus, during biofilm formation, bacteria growth affected by sera from KF-rPAc immunized mice was tested and compared with ampicillin.
Ampicillin suppressed both biofilm formation and bacteria growth readily in our experiment system likely through retarding growth of bacteria or direct kill of bacteria as previously demonstrated.Citation21 When the concentration of ampicillin was 2 μg/ml, both S. mutans proliferation and biofilm formation were completely inhibited (). When the concentration of ampicillin was 1 μg/ml, both S. mutans proliferation and biofilm formation were partially inhibited. But 16 h to 24 h post bacteria inoculation both biofilm formation and S. mutans propagation were similar as that without 1 μg/ml ampicillin. When the concentration of ampicillin reduced to 0.1 μg/ml, neither S. mutans propagation nor biofilm formation was affected.
Figure 5. Different inhibitiory effect of antibiotics and KF-rPAc immunized serum in S. mutans proliferation and biofilm formation. 100 µl BHI diluted S. mutans were mixed with 100 µl 20-fold BHI diluted mice serum, 5-fold BHI diluted mice saliva, BHI diluted ampicillin or BHI alone and incubated into the wells of 96-well cell culture cluster. For bacteria proliferation quantification, the media in the well were re-suspended with pipette, assessed by measuring the absorbance of suspension at 600 nm. The biofilm formation was quantified by measuring the extracted crystal violet stained to plate adherent bacteria and derivatives at 570 nm. (A and B), Effects of Ampicillin on S. mutans proliferation and biofilm formation. (C and D), Effects of KF-rPAc minimized mice serum on S. mutans proliferation and biofilm formation. Data are represented as mean ± SE for triplicates of one representative experiment.
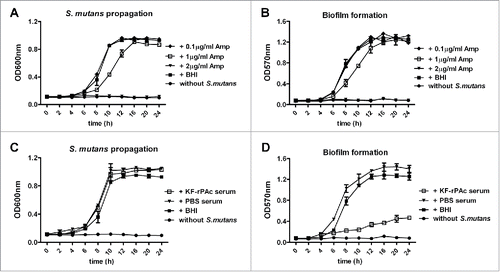
Both sera of PBS immunized mice and serum of KF-rPAc immunized mice slightly promoted the propagation of S. mutans (). Sera of PBS immunized mice also slightly promoted biofilm formation, but sera of KF-rPAc immunized mice significantly inhibited biofilm formation (). These results showed that KF-rPAc induced humoral immunity inhibits biofilm formation likely by inhibition of cell-cell adhesion but not proliferation of S. mutans provided the function of PAc.
Discussion
Biofilm formation is considered as a necessary process involved in caries development, thus draws more and more attentions in anti-caries medicine exploration.Citation22,23 We have found that i.n. immunization of flagellin-rPAc fusion protein significantly reduced the incidence of dental caries in rat model.Citation13,14
Adhesion is the initial step in the biofilm formation of S. mutans.Citation24,25 Several studies suggested that, through binding with salivary glycoproteins, PAc is involved in the adherence of S.mutans to saliva-coated hydroxyapatite (sHA).Citation26,27 But little study aimed the effect of PAc in other steps of biofilm formation besides adherence. As PAc has multifunctional activities,Citation18 we wondered whether KF-rPAc induced antibody response could inhibit biofilm formation besides the adherence of S. mutans to salivary glycoproteins. Without saliva coating, PAc could not be involved in the adhesion to hydroxyapatite beads.Citation19 Thus in present study, a simple and convenient in vitro biofilm model of S. mutans without pre-coated with saliva was constructed.
Here we found that sera and saliva of KF-rPAc immunized rats display significant higher efficiency in inhibiting biofilm formation. Correlation analysis in the rat challenge experiment showed the higher rPAc-specific IgG or IgA was induced, the lower biofilm formation achieved; meanwhile the lower caries lesion (caries score) detected. Similar results were obtained in the mouse experiments, in which higher rPAc-specific antibody response was induced, the higher inhibition of biofilm formation detected. This significant inverse correlation between biofilm formation and rPAc-specific antibody response in mice further supported rPAc-specific antibody is a specific inhibitor to biofilm formation of S. mutans. Antibodies against PAc might be able to interfere with multiple aspects regarding PAc-mediated binding and adhesion between bacteria. Moreover, this result also suggested that the mouse immunization model and the in vitro biofilm model of S. mutans might be integrated for early screening of caries vaccine candidate before rat challenge and efficacy test. PAc specific antibody could block adherence of S. mutans to salivary proteins on tooth surface. Because teeth or chemical composition was not adopted in this ex vivo model, this blocking effect of PAc specific antibody could not be reflected for screening of caries vaccine candidate.
Taking advantage of the mouse model for most vaccine study, we tried a simple bacteria growth curve assay for detection of mouse anti-sera or anti-saliva for their inhibitory effects on both bacteria proliferation and biofilm formation by comparison with ampicillin. The results showed that in a different way from antibiotics, KF-rPAc induced humoral immunity inhibits biofilm formation but not alters proliferation of S. mutans.
Salivary immunoglobulin A (IgA) has long been considered as a prominent factor for host defense against S. mutans in prevention of dental caries through inhibition of bacterial infection.Citation28 Our study showed that the titer of rPAc-specific salivary IgA induced by i.n. immunization negatively correlated with both biofilm formation ability of S. mutans and severity of caries lesion in challenged rats. Overall, besides blocking adherence of S. mutans to saliva-coated tooth surface,Citation17 KF-rPAc induced rPAc specific humoral immunity can inhibit biofilm structure formation in between bacteria, finally protecting tooth from caries. Our data highlighted the importance of preventive vaccination because PAc-specific antibodies work effectively to inhibit the biofilm formation; thus our next step will be to further optimize PAc-based vaccine for its preventive effect.
Material and methods
Bacteria and animals
Streptococcus mutans Ingbritt (School of stomatology, Wuhan University, China) was used in this study. The strain was grown in Brain and Heart Infusion (BHI) medium in a 5% CO2 anaerobic atmosphere at 37°C.
Female BALB/c mice at 6–8 weeks of age were obtained from Beijing Laboratory Animal Research Center. Female 18-day-old weanling Wistar rats were purchased from Hubei CDC (Wuhan, China). Animals were kept in the Animal Center of Wuhan Institute of Virology (WIV), Chinese Academy of Sciences, under SPF condition. Animal studies were performed according to Regulations for the Administration of Affairs Concerning Experimental Animals in China (1988), the Guideline for Animal Care and Use, WIV, Chinese Academy of Sciences. The protocols were reviewed and approved by the Laboratory Animal Care and Use Committee of WIV, Chinese Academy of Sciences. The investigation complied with ARRIVE guidelines for preclinical animal studies.
The construction and purification of anti-caries vaccine candidates
Anti-caries vaccines of flagellin and rPAc fusion protein were constructed and purified as previously described.Citation13 Briefly, the fliC gene from E. coli K12 strain MG1655 and the A-P fragment, from amino acid residues 219 to 905 of PAc protein encoded by pac gene of S. mutans MT8148 were amplified by PCR, respectively. And then the fragments were cloned into pET28a plasmid vector (Invitrogen) to construct the expression plasmids of pET28a-KF, pET28a-rPAc and pET28a-KF-rPAc. The recombinant flagellin KF, rPAc) and fusion protein KF-rPAc were prepared and purified by affinity chromatography on a Ni-NTA column (Qiagen).
Mouse immunization
Thirty mice were randomly divided into 5 groups (6 per group), and i.n. immunized with PBS, 0.7 μg KF, 1 μg rPAc, 0.7 μg KF plus 1 μg rPAc, 1.7 μg KF-rPAc in a 10 μl aliquot. The immune procedure included primed on day 1, and boosted twice on day 29 and 57. Serum and saliva were collected on day 71 as previously described Citation29 and analyzed.
Rat immunization and caries model
Rat caries model was performed as previously described.Citation9 Briefly, rats were fed with a cariogenic diet, Keyes 2000, and then were challenged with 2 × 109 CFU of S. mutans Ingbritt on day 24–26. After challenge, the rats were grouped (6 per group) and i.n. immunized on day 28 with PBS, 3.5 μg KF, 5 μg rPAc, 3.5 μg KF plus 5 μg rPAc, 8.5 μg KF-rPAc in a 10 μl aliquot, and boosted on day 56 and 84. Serum and saliva were collected on day 98 and analyzed. On day 112, rats were sacrificed; the mandibles were removed, cleaned and stained with murexide. The teeth were hemisectioned and observed by a stereomicroscope (Zeiss, German), caries level were scored by Keyes method.Citation30
Antibody analysis
Antibody titers in serum and saliva were determined by ELISA as previously described.Citation13,31-33 Briefly, polystyrene 96-well microplates (Greiner, Germany) were coated with 100 μl rPAc or KF (5 μg/ml) at 37°C for 3 h, and then blocked with PBS containing 1% bovine serum albumin (BSA) overnight at 4°C. Serial dilutions of serum or saliva samples were added in each well and incubated at 37°C for 2 h. After 6washes with PBS containing 0.5% Tween-20 (PBST), the plates were incubated with 100 μl of alkaline phosphatase-conjugated goat anti-mouse/rat heavy chain IgG or IgA at 37°C for 1 h. After 6 washes of PBST, 100 μl phosphate substrate (p-nitrophenylphosphate) was added into each well for color development. Endpoint titers were expressed as the reciprocal of the last dilution giving an optical density at 405 nm of 0.1 greater than that of control after 30 min of incubation.
Biofilm assay
Biofilm assays in 96-well microtitre plates were carried out as described with minor modifications.Citation34,35 Briefly, overnight cultures of S. mutans were inoculated into pre-warmed BHI medium and grew to an optical density at 600 nm of about 0.5. The cultures were diluted 1:100 in fresh BHI, and then 100 μl the cell suspension with 100 μl 20-fold BHI diluted serum or 5-fold BHI diluted saliva were incubated into the wells of a 96-well cell culture cluster (Corning, USA). Plates were incubated at 37°C in a 5% CO2 aerobic atmosphere for 16 h. For bacteria proliferation quantification, the media in the well were re-suspended with pipette, assessed by measuring the absorbance of suspension at 600 nm by using Microplate Spectrophotometer (Bio tek, USA). Media and unattached bacterial cells were gently poured out from the wells after lightly oscillation on a horizonal shaker, and then the plates were blotted on paper and air dried, adherent bacteria and derivatives were fixed with Bouin's fixative for 2 h, after 2 times gently wash with PBS to remove the Bouin's fixative, the biofilm on the bottom was stained with 100 μl 0.1% crystal violet for 15 min at room temperature. After two rinses with PBS, the bound dye was extracted from the stained cells by using 200 μl ethanol : acetone (4 : 1, v : v), and the plates were set on a horizonal shaker to allow full release of the dye. Biofilm formation was then quantified by measuring the absorbance of the solution at 570 nm with Microplate Spectrophotometer.
Statistics
All data analysis was performed with 1-way analysis of variance (ANOVA) if not stated. When the p value was significant at the 5% level, further pairwise comparisons were made between the experimental group and control conditions using the Bonferroni multiple-comparison test. Statistical analysis was carried out with GraphPad Instat Software, version 5.0 (GraphPad Software, La Jolla, CA, USA). Data are represented as mean ± standard error (SE) for individual sample of one representative experiment. Statistical significance is indicated by * (P < 0.05), ** (P < 0.01), and *** (P < 0.001). A P value less than 0.05 was considered to be statistically significant.
Abbreviations
| KF | = | E.coli K12 strain-derived flagellin |
| rPAc | = | alanine-rich region to proline-rich region fragment of PAc |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgements
We thank Xuefang An and Fan Zhang in the core facility for technical support and the Wuhan Institute of Virology for their kind help in animal experiments.
Funding
This work was financially supported by grants from the Ministry of Science and Technology of China (973 Program, No. 2012CB518904), and the “One-Three-Five” Strategic Planning Program of Wuhan Institute of Virology, Chinese Academy of Sciences (No. Y206515SA1).
References
- Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev 1980; 44:331-84; PMID:6446023
- Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev 1986; 50:353-80; PMID:3540569
- Forester H, Hunter N, Knox KW. Characteristics of a high molecular weight extracellular protein of Streptococcus mutans. J Gen Microbiol 1983; 129:2779-88; PMID:6415234
- Russell RR. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol 1979; 114:109-15; PMID:118231; http://dx.doi.org/10.1099/00221287-114-1-109
- Vonderviszt F, Aizawa S, Namba K. Role of the disordered terminal regions of flagellin in filament formation and stability. J Mol Biol 1991; 221:1461-74; PMID:1942062; http://dx.doi.org/10.1016/0022-2836(91)90946-4
- Smith DJ, King WF, Rivero J, Taubman MA. Immunological and protective effects of diepitopic subunit dental caries vaccines. Infect Immun 2005; 73:2797-804; PMID:15845483; http://dx.doi.org/10.1128/IAI.73.5.2797-2804.2005
- Wachsmann D, Klein JP, Scholler M, Ogier J, Ackermans F, Frank RM. Serum and salivary antibody responses in rats orally immunized with Streptococcus mutans carbohydrate protein conjugate associated with liposomes. Infect Immun 1986; 52:408-13; PMID:3699888
- Xu QA, Yu F, Fan MW, Bian Z, Chen Z, Peng B, Jia R, Guo JH. Protective efficacy of a targeted anti-caries DNA plasmid against cariogenic bacteria infections. Vaccine 2007; 25:1191-5; PMID:17095128; http://dx.doi.org/10.1016/j.vaccine.2006.10.013
- Shi W, Li YH, Liu F, Yang JY, Zhou DH, Chen YQ, Zhang Y, Yang Y, He BX, Han C, et al. Flagellin enhances saliva IgA response and protection of anti-caries DNA vaccine. J Dent Res 2012; 91:249-54; PMID:22027714; http://dx.doi.org/10.1177/0022034511424283
- McDonald William F, Huleatt James W, Foellmer Harald G, Hewitt D, Tang J, Desai P, Price A, Jacobs A, Takahashi VN, Huang Y, et al. A West Nile Virus Recombinant Protein Vaccine That Coactivates Innate and Adaptive Immunity. J Infect Dis 2007; 195:1607-17; PMID:17471430; http://dx.doi.org/10.1086/517613
- Mizel SB, Bates JT. Flagellin as an Adjuvant: Cellular Mechanisms and Potential. J Immunol 2010; 185:5677-82; PMID:21048152; http://dx.doi.org/10.4049/jimmunol.1002156
- Honko AN, Sriranganathan N, Lees CJ, Mizel SB. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect Immun 2006; 74:1113-20; PMID:16428759; http://dx.doi.org/10.1128/IAI.74.2.1113-1120.2006
- Sun Y, Shi W, Yang JY, Zhou DH, Chen YQ, Zhang Y, Yang Y, He BX, Zhong MH, Li YM, et al. Flagellin-PAc Fusion Protein is a High-efficacy Anti-caries Mucosal Vaccine. J Dent Res 2012; 91:941-7; PMID:22895510; http://dx.doi.org/10.1177/0022034512457684
- Bao R, Yang JY, Sun Y, Zhou DH, Yang Y, Li YM, Cao Y, Xiao Y, Li W, Yu J, et al. Flagellin-PAc Fusion Protein Inhibits Progression of Established Caries. J Dent Res 2015; 94:955-60; PMID:25883108; http://dx.doi.org/10.1177/0022034515582224
- Brady LJ, Cvitkovitch DG, Geric CM, Addison MN, Joyce JC, Crowley PJ, Bleiweis AS. Deletion of the central proline-rich repeat domain results in altered antigenicity and lack of surface expression of the Streptococcus mutans P1 adhesin molecule. Infect Immun 1998; 66:4274-82; PMID:9712778
- Okahashi N, Sasakawa C, Yoshikawa M, Hamada S, Koga T. Cloning of a surface protein antigen gene from serotype c Streptococcus mutans. Mol Microbiol 1989; 3:221-8; PMID:2503676; http://dx.doi.org/10.1111/j.1365-2958.1989.tb01811.x
- Hajishengallis G, Russell MW, Michalek SM. Comparison of an adherence domain and a structural region of Streptococcus mutans antigen I/II in protective immunity against dental caries in rats after intranasal immunization. Infect Immun 1998; 66:1740-3; PMID:9529105
- Jenkinson HF, Demuth DR. Structure, function and immunogenicity of streptococcal antigen I/II polypeptides. Mol Microbiol 1997; 23:183-90; PMID:9044252; http://dx.doi.org/10.1046/j.1365-2958.1997.2021577.x
- Bowen WH, Schilling K, Giertsen E, Pearson S, Lee SF, Bleiweis A, Beeman D. Role of a cell surface-associated protein in adherence and dental caries. Infect Immun 1991; 59:4606-9; PMID:1937820
- Anwar H, Strap JL, Costerton JW. Establishment of aging biofilms: possible mechanism of bacterial resistance to antimicrobial therapy. Antimicrob Agents Chemother 1992; 36:1347-51; PMID:1510427; http://dx.doi.org/10.1128/AAC.36.7.1347
- Baker CN, Thornsberry C. Antimicrobial susceptibility of Streptococcus mutans isolated from patients with endocarditis. Antimicrob Agents Chemother 1974; 5:268-71; PMID:4840435; http://dx.doi.org/10.1128/AAC.5.3.268
- Huang L, Xu Q-A, Liu C, Fan M-w, Li Y-h. Anti-caries DNA vaccine-induced secretory immunoglobulin A antibodies inhibit formation of Streptococcus mutans biofilms in vitro. Acta Pharmacologica Sinica 2013; 34:239-46; PMID:23274411; http://dx.doi.org/10.1038/aps.2012.145
- Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, Vacca-Smith AM, Bowen WH. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother 2003; 52:782-9; PMID:14563892; http://dx.doi.org/10.1093/jac/dkg449
- Rendueles O, Ghigo J-M. Multi-species biofilms: how to avoid unfriendly neighbors. FEMS Microbiol Reviews 2012; 36:972-89; PMID:22273363; http://dx.doi.org/10.1111/j.1574-6976.2012.00328.x
- Shemesh M, Tam A, Steinberg D. Expression of biofilm-associated genes of Streptococcus mutans in response to glucose and sucrose. J Med Microbiol 2007; 56:1528-35; PMID:17965356; http://dx.doi.org/10.1099/jmm.0.47146-0
- Douglas CW, Russell RR. Effect of specific antisera on adherence properties of the oral bacterium Streptococcus mutans. Arch Oral Biol 1982; 27:1039-45; PMID:6222726; http://dx.doi.org/10.1016/0003-9969(82)90009-7
- Lee SF, Progulske-Fox A, Erdos GW, Piacentini DA, Ayakawa GY, Crowley PJ, Bleiweis AS. Construction and characterization of isogenic mutants of Streptococcus mutans deficient in major surface protein antigen P1 (I/II). Infect Immun 1989; 57:3306-13; PMID:2807526
- Bolton RW, Hlava GL. Evaluation of salivary IgA antibodies to cariogenic microorganisms in children. Correlation with dental caries activity. J Dent Res 1982; 61:1225-8; PMID:6958716; http://dx.doi.org/10.1177/00220345820610110201
- Liu F, Yang J, Zhang Y, Zhou D, Chen Y, Gai W, Shi W, Li Q, Tien P, Yan H. Recombinant flagellins with partial deletions of the hypervariable domain lose antigenicity but not mucosal adjuvancy. Biochem Biophys Res Commun 2010; 392:582-7; PMID:20102702; http://dx.doi.org/10.1016/j.bbrc.2010.01.077
- Keyes PH. Dental caries in the molar teeth of rats. I. Distribution of lesions induced by high-carbohydrate low-fat diets. J Dent Res 1958; 37:1077-87; PMID:13611122; http://dx.doi.org/10.1177/00220345580370060801
- Yang J, Zhong M, Zhang Y, Zhang E, Sun Y, Cao Y, Li Y, Zhou D, He B, Chen Y, et al. Antigen replacement of domains D2 and D3 in flagellin promotes mucosal IgA production and attenuates flagellin-induced inflammatory response after intranasal immunization. Hum Vaccin Immunother 2013; 9:1084-92; PMID:23377752; http://dx.doi.org/10.4161/hv.23809
- Li W, Yang J, Zhang E, Zhong M, Xiao Y, Yu J, Zhou D, Cao Y, Yang Y, Li Y, et al. Activation of NLRC4 downregulates TLR5-mediated antibody immune responses against flagellin. Cell Mol Immunol 2015; 13(4):514-23; PMID: 25914934; http://dx.doi.org/10.1038/cmi.2015.33
- Xiao Y, Liu F, Yang J, Zhong M, Zhang E, Li Y, Zhou D, Cao Y, Li W, Yu J, et al. Over-activation of TLR5 signaling by high-dose flagellin induces liver injury in mice. Cell Mol Immunol 2015; 12:729-42; PMID:25418468; http://dx.doi.org/10.1038/cmi.2014.110
- Beenken KE, Blevins JS, Smeltzer MS. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect Immun 2003; 71:4206-11; PMID:12819120; http://dx.doi.org/10.1128/IAI.71.7.4206-4211.2003
- Pecharki D, Petersen FC, Assev S, Scheie AA. Involvement of antigen I/II surface proteins in Streptococcus mutans and Streptococcus intermedius biofilm formation. Oral Microbiol Immunol 2005; 20:366-71; PMID:16238597; http://dx.doi.org/10.1111/j.1399-302X.2005.00244.x
