?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: The widely recommended standard schedule of hepatitis B vaccine for adults is months 0, 1 and 6, which takes 6 months to complete. Rapid completion of one vaccination schedule is important to adults because of its low compliance with follow-up doses. A new type of 60 μg Hepatitis B vaccine, made by Shenzhen Kangtai Biological Products Co., LTD., is originally recommended for low or non-responders. The objective of this clinical trial was to test whether this 60 μg hepatitis B vaccine could be used in primary immune population and what is its level of immunogenicity and safety compared with other hepatitis B vaccines.
Methods: This is a 2-center randomized controlled study. A total of 1169 healthy adults aged between 25 and 55 who tested negative for HBsAg, anti-HBs, and anti-HBc were eligible for the study and were enrolled from relatively fixed and stable sites, such as villages, schools and large enterprises et al in Xuanhua county in Hebei province and Huaibei county in Anhui province. They were randomized to group A (20 μg Engerix-B® with 0, 1, 6 month intervals), group B (20 μg Kangtai hepatitis B vaccine with 0, 1, 6 month intervals), group C (60 μg Kangtai hepatitis B vaccine with 0, 2 month intervals) and group D (20 μg Huabei hepatitis B vaccine made by recombinant DNA techniques in CHO cell with 0, 1, 6 month intervals). In group A, B and D, every study object's blood sample was collected in the second month after their final injection to test the anti-HBs levels; while in group C, the blood sample was collected in the second month after the first and the second injection to test the anti-HBs levels. Adverse events were collected after each dose to assess the vaccines' safety.
Results: The seroprotection rates were 93.17%, 97.23%, 93.54% and 98.98% respectively and the geometric mean titers (GMTs) were 1033.38 mIU/ml, 600.75 mIU/ml, 265.69 mIU/ml and 1627.05 mIU/ml in group A,B,C and D respectively. The difference of seroprotection rate among the 4 groups was statistically significant (χ2 = 17.26, P<0.05). The difference of titers of anti-HBs among the 4 groups was statistically significant (H = 162.42, P<0.05). BMI, age (older than 40) and smoking were the influence factors of anti-HBs levels on 60 μg hepatitis B vaccine.
Conclusion: 60 μg hepatitis B vaccine has a satisfactory safety and seroprotection rate in adult, It could be used in rapid adult hepatitis B immunization.
Introduction
Immunization with hepatitis B vaccine is the most effective means of preventing HBV infection and its consequences.Citation1 Hepatitis B vaccine has been used for over 3 decades since the first plasma-derived one was licensed in 1982. The plasma-derived hepatitis B vaccine was used for newborns who were required to be vaccinated according to 0, 1, and 6 month schedule since 1986.Citation2 In 1992, the Chinese Ministry of Health recommended hepatitis B vaccine for routine immunization of infants but parents had to pay for the vaccine, therefore vaccine coverage was higher in urban areas and those with a booming economy than in rural and poverty-stricken areas.Citation3 In 2002, China integrated hepatitis B vaccine into EPI, but vaccine administration fees were still charged to parents.Citation3 Until 2005, the government required that all infant vaccinations be given at no charge to parents.Citation3 Over 20 years, HBsAg prevalence rate among children aged 1–4 has dramatically reduced from 9.7% in 1992 to 1.0% in 2006.Citation3 But, the prevalence of HBsAg among adults aged 20 and older was 8–12% and still at a very high level.Citation3 Therefore, expanded vaccination from children to adults is in urgent need for HBV infection control among all population.
The widely recommended standard schedule of hepatitis B vaccine for adults in China is months 0, 1 and 6 which takes 6 months to complete. According to feedback from community clinics, adults show poor adherence to the immunization schedule because of the long interval between injections and their urgent needs for rapid protection caused by high risk exposures. Hence, rapid immunization becomes important for adults. The 60 μg Hepatitis B vaccine, Shenzhen Kangtai Biological Products Co., LTD., made by recombinant DNA techniques in yeast, is mainly applied to immunize the low or non-responders who fail to respond to the standard primary vaccination and it has become commercially available since 2010 in China.Citation4 The objective of this clinical trial was to test whether this 60 μg hepatitis B vaccine could be used in primary immune population and what is its level of immunogenicity and safety compared with other hepatitis B vaccines. We thus performed a prospective, randomized 2-center clinical study to assess the immunogenicity and safety of hepatitis B vaccine of different types (Engerix-B®, GlaxoSmithKline Biologicals, Rixensart, Belgium; Kangtai hepatitis B vaccine, Shenzhen Kangtai Biological Products Co., LTD. and Huabei hepatitis B vaccine, North China Pharmaceutical, Jintan biological products Co., LTD.), dose (60 μg and 20 μg) and schedule (0, 1, 6 month and 0, 2 month) in primary immune adults to explore the new approaches for hepatitis B immunization in adults. The experimental group were immunized by the 60 μg Kangtai recombinant hepatitis B vaccine in yeast with 0, 2 month intervals. The control groups were immunized by the 20 μg Engerix-B® with 0, 1, 6 month intervals, 20 μg Kangtai recombinant hepatitis B vaccine in yeast with 0, 1, 6 month intervals and 20 μg Huabei recombinant hepatitis B vaccine in CHO cell with 0, 1, 6 month intervals.
The study hypothesis is whether the seroprotection rate is difference between 60 μg and 20 μg group. The sample size calculation is according to 2 rates comparison.
Result
Demographics analysis
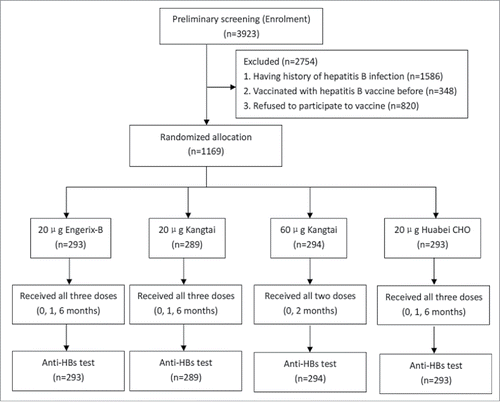
A total of 3923 volunteers participated in preliminary screening, 1586 had history of hepatitis B infection, 348 vaccinated with hepatitis B vaccine or hepatitis A and B combined vaccine before, 820 refused to complete follow-up vaccination and blood sampling. The 820 populations didn't fit for entry criteria and didn't join the grouping. So, demographic characteristics between 820 non-responders and 1169 responders didn't affect our final result. No significant difference was found in gender (χ2 = 2.709, P < 0.05) between 820 non-responders and 1169 responders. At last, 1169 were included in our study. The study process was shown in . The demographics of 1169 study subjects were shown in . No significant differences were found either in gender, age, BMI, marital status, smoking and drinking among 4 groups.
Table 1. Study Subject's demographics data between different groups.
Adverse events
There were 5 adverse events reported in our study (2 adverse events in group B, 2 adverse events in group C and 1 adverse event in group A). Swelling and indurations in injection-site were the most common adverse events. In group B, 2 objects reported swelling and indurations in injection-site. In group C, one objects reported swelling and indurations and another one reported pain in injection-site. In group D, one objects reported fever that lasted for 2 d. There were no serious adverse events reported in the study. All the reactions were mild or moderate and relieved within 24 h without special treatment.
Immunogenicity analysis
The seroprotection rate and titers of anti-HBs after full immunization were shown in . The difference of seroprotection rate among the 4 groups was statistically significantly different (χ2 = 17.26, P < 0.05). The seroprotection rate was the highest in group D and the lowest in group A. The difference of titers of anti-HBs among the 4 groups was statistically significantly different (H = 162.42, P < 0.05).The GMTs of anti-HBs is the highest in group D, followed by group A, B and C in the sequence.
Table 2. Seroprotection rates and titers of anti-HBs after full immunization in 4 groups.
In group C, Seroprotection rates and titers of anti-HBs were also tested after the first dose. The seroprotection rate was 51.70% and the P25, P75, Median and GMTs of titers of anti-HBs were 2.98 mIU/ml, 66.92 mIU/ml, 10.61 mIU/ml and 17.16 mIU/ml after the first dose.
Influence factors analyses
In order to analyze factors affecting seroprotection rate in 60 μg hepatitis B vaccine, whether anti-HBs level ≥ 10 mIU/ml was used as a dependent variable; and independent variables used to conduct logistic regression analyses include: gender, age (older than 40 or not), BMI, smoking, drinking, drug allergy history, allergic rhinitis history, urticaria history, bronchial asthma history, eczema history, diabetes history, whether used hormone within one month, anemia history, whether insomnic within one month, whether parents were hepatitis B patients or virus carriers, whether spouse was hepatitis B patient or virus carrier, whether family members were hepatitis B patient or virus carrier, whether friends were hepatitis B patient or virus carrier, hemodialysis history, whether shared a razor with others, operation history, oral diagnosis and treatment history, endoscope treatment history, blood transfusion history, acupuncture history, whether shared syringe, tattoo history, ears or navel hole history, whether went to barber or shave( more than 1 times/month). Stepwise regression method was used and p-value < 0.05 was considered significant. The final analysis was shown in . BMI, age (older than 40) and smoking were the influence factors for whether anti-HBs level ≥ 10 mIU/ml on 60 μg hepatitis B vaccine.
Table 3. logistic regression analyses of influence factors for whether anti-HBs level ≥ 10 mIU/ml on 60 μg hepatitis B vaccine.
Discussion
Since comprehensive hepatitis B vaccine immunization in children became available in 1992, China has had made great achievement in reducing HBsAg carrier positive rate in young population. The national goal of reducing HBsAg prevalence among children younger than 5 y old to less than 1% has been reached by 2010.Citation3 To further reduce HBsAg positive population, expanded hepatitis B vaccination to adults is needed. Considering the time of Chinese hepatitis B immunization, population younger than 25 y old may have received hepatitis B vaccine although they claimed otherwise. So, adults aged over 25 y old have been focused on in our study. 60 μg hepatitis B vaccine is originally recommended for low or non-responders, not for primary immune population. Considering the dose and accelerated schedule, we first used this 60 μg hepatitis B vaccine on primary immune adults to get accelerated seroprotection.
The results of our study show that 60 μg at 0–2 months can produce a comparable immunogenic response to HBV. The seroprotection rate in the second month after the completion of the vaccination course is 93.54% in 60 μg group compared to Engerix-B®, 20 μg Kangtai hepatitis B vaccine and 20 μg Huabei hepatitis B vaccine groups which are 93.17%, 97.23%, and 98.98% respectively. This seroprotective rate is comparable to previous studies in adults where seroprotection rates between 76.4% and 100% have been described.Citation5-10 The GMTs of 60 μg group is lower than other group because of the rapid completion. In two-dose schedules, a longer interval between the doses also results in higher GMTs.Citation11-12 Although the GMTs is lower in 60 μg group, the seroprotection rate is all higher than 90% in all 4 groups. The rapid completion is important for individuals at high risk of exposure (such as health care workers, patients diagnosed with sexually transmitted infections, injection drug users, and others), individuals with imminent travel to an endemic area, and for those who are known to be less compliant with follow-up doses.Citation13-16 60 μg hepatitis B vaccine could be used because of its high seroprtection rate and shorter course of immunization.
Several factors have been found to affect the seroprotection rate of hepatitis B vaccine, such as age, gender, BMI, smoking, needle length, immunization technique and genetic factors.Citation17-18 In our study, BMI, age (older than 40) and smoking were the influence factors for whether anti-HBs level ≥ 10 mIU/ml on 60 μg hepatitis B vaccine.
No serious adverse event attributable to the hepatitis B vaccines has been observed in our study and the vaccines only caused mild adverse events like swelling, indurations, fever and pain in injection-site. So, we could believe that the 60 μg hepatitis B vaccine used in primary immune adults is safe.
According to the finding of our previous laboratory studies, seroprotection rate and GMTs in the second month after final injections is higher than the first month, so we collected bloods in month-8 (Groups A,B,D) or month 4 (Group C).
This study has a few limitations. First, the bloods samples have been collected in the second month after the third injections, so they are lacking of antibody seroprotection data in the long term such as in a whole year or longer. We will continue to follow up the objects to make this data complete. Second, the blood samples in group A, B and D have not been collected after the second injections and therefore are not comparable to those in group C.
Although GMTs is lower in 60 μg Kangtai hepatitis B vaccine, its seroprtection rate is high and immunization course is accelerated. Compared with different dose and schedules of hepatitis B vaccine in adults, 60 μg Kangtai hepatitis B vaccine in primary immune population with satisfactory safety and seroprtection rate could be used in rapid immunization and could provide a new strategy in China. Further antibody seroprotection data also needed to be obtained to support the existing conclusions.
Methods
Study design
It was a 2-center randomized controlled study. Xuanhua county in Hebei province and Huaibei county in Anhui province were chosen as our study sites. Relatively fixed and stable sites, such as villages, schools and large enterprises et al, were chosen as our study sites.
The study was first approved by the medical ethics committee in China Centers for Disease Control and Prevention (CDC) and then approved by the medical ethics committee in Xuanhua and Huaibei CDC. The procedure of the trial was in accordance with the Good Clinical Practice Guidelines and the ethical standards of the Helsinki Declaration. All relevant documents were approved by the ethical review committee of Xuanhua and Huaibei CDC.
Each volunteer's blood sample was collected to test HBsAg, anti-HBs, HBeAg, anti-HBe and anti-HBc pre-vaccination. The volunteers who meet the entry conditions and signed informed consent were enrolled in this study. The study subjects were randomly allocated to group A, B, C and D in accordance with a computer-generated randomization code. The randomization codes were created using a random allocation sequence with SPSS software, version 21.0 (SPSS Inc., Chicago,IL, USA) and code numbers were assigned to each subject in chronological order. Questionnaire including demographics, diseases and risk factors of hepatitis B needed to be filled in before the first vaccine.
According to different brands, dose and schedule of recombinant hepatitis B vaccine, our study objects were divided into 4 groups randomly. Group A: 20 μg Engerix-B® (Batch no: YHBVC292AA) with 0, 1, 6 month intervals; Group B: 20 μg Kangtai recombinant hepatitis B vaccine (Batch no: B201202006) in yeast with 0, 1, 6 month intervals; Group C: 60 μg Kangtai recombinant hepatitis B vaccine (Batch no: A201106003) in yeast with 0, 2 month intervals; Group D: 20 μg Huabei recombinant hepatitis B vaccine (Batch no: 201303Y1306) in CHO cell with 0, 1, 6 month intervals. The adjuvant (aluminum salt and quantity) of 20 μg and 60 μg is the same. The volume of 20 μg and 60 μg is both 1 ml per dose.
Study objects were monitored by study staff for 30 min after each injection for immediate reactions. Adverse events were recorded by study objects until 3 d after their injection. Adverse events included general reaction, such as headache, fever, fatigue, muscle pain, cough, nausea, vomiting, diarrhea, local pain, local swelling, local indurations, skin rash and others general reaction, and serious adverse events, such as allergic shock, thrombocytopenic purpura, vascular edema, nervous system reaction and others.
5-mL volumes of blood were collected during month-8 (Groups A,B,D) or month-2 and 4 (Group C). All study objects must complete bloods collection on the day of blood collection.The primary endpoints were seroprotection rates and anti-HBs geometric mean titers (GMTs) in the second month after the final injections in 4 groups and didn't look at the intermediate process. So, we collected blood in the second month after the final injections. Reducing the number of blood to reduce the loss of follow-up and increase the compliance. Blood sampling twice in 60 ug group was used to explore the vaccine program. Serum was separated after centrifugation in each spots and than stored in transfer ice box to laboratory. All serum samples in experimental group and control groups stored in −40°C refrigerator and tested in the same time. Serums were tested for anti-HBs to assess seroprotection rate and titer. All serum samples were tested by Architect i2000 (Chemiluminescence Microparticle Imunoassay, Abbott, Chicago, USA). Laboratory test used blind method. The laboratory staff didn't know the group and relied on the bar code to detect and submit the results.
Study population
Healthy adults aged between 25 and 55 y who tested negative for HBsAg, anti-HBs, and anti-HBc were eligible for the study. The exclusion criteria were: (1) vaccinated with hepatitis B vaccine, hepatitis A and B combined vaccine, and received anti-hepatitis B immunoglobulin before; (2) having a medical history of allergy to any ingredient of vaccine; (3) having medical history of autoimmune disease or immunodeficiency; (4) having received any vaccines one month ago; (5) having acute diseases one week ago; (6) having fever (Axillary temperature more than 38 centigrade) in previous 3 days;(7) refused to participate to vaccine.
The sample size calculation is according to 2 rates comparison principle of statistical arithmetic formula: on the basis that α = 0.05, β = 0.1, seroprotection rate for 20 μg p1 = 96%, seroprotection rate for 60 μg p2 = 90%, and the estimated sample size is 295 in each group.
Statistical analyses
The primary endpoints were seroprotection rates and anti-HBs geometric mean titers (GMTs) in the second month after the final injections. Hypothesis testing was 2-sided with an α value of 0.05.Seroprotection was defined as an anti-HBs level ≥ 10 mIU/ml. Statistics were performed using SPSS 18.0 software. Percentage between different groups was compared using the χ2 or Fisher's exact test, and the titers of anti-HBs were compared using Anova or Kruskal-Wallis tests (H test). A p-value < 0.05 (2-tailed) was considered statistically significant. The influence factors for seroprotection rate of anti-HBs factors used by logistic regression analyses.
Quality control
(1) Field investigation quality control: optimizing investigators, pre-job training, checking and rechecking, reducing the loss of sample. (2) Blood quality control: employing professional staff in blood collection, avoiding haematolysis, regulating blood storage and transportation. (3) Laboratory quality control: Using bar code to avoid confusion. a clear division of labor, using high-quality reagent and advanced equipment, application of blind and parallel sample, quality control products, standard products, negative control and positive control, etc. (4) Statistical analysis quality control: data examine and verify, double-entry in database setup, error correction logically, etc.
Abbreviations
| HBV | = | Hepatitis B Virus |
| HBsAg | = | Hepatitis B Surface Antigen |
| Anti-HBs | = | Hepatitis B Surface Antibody |
| HBeAg | = | Hepatitis B E Antigen |
| Anti-HBe | = | Hepatitis B E Antibody |
| Anti-HBc | = | Hepatitis B Core Antibody |
| Miu | = | Million International Units |
| EPI | = | Expanded Program on Immunization |
| BMI | = | Body Mass Index |
| GMTs | = | Geometric Mean Titers |
| CDC | = | Centers for Disease Control and Prevention |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge all the adults who took part in the study and all the personnel from the Beijing Center Disease Control and Prevention, Xuanhua District Center for Disease Control and Prevention and Huaibei District Center for Disease Control and Prevention who took part in collection of samples.
Funding
Funding for this work was provided by National Key Project on Infections Disease, 2012ZX10002001-003-003, 2012ZX10004-904.
References
- World Health Organization, Hepatitis B. http://www.who.int/mediacentre/factsheets/fs204/en/
- Wang F, Shen L, Cui F, Zhang S, Zheng H, Zhang Y, Liang X, Wang F, Bi S. The long-term efficacy, 13–23 years, of a plasma-derived hepatitis B vaccine in highly endemic areas in China. Vaccine 2015; 33(23):2704-9; PMID:25843269; http://dx.doi.org/10.1016/j.vaccine.2015.03.064
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Epidemiological serosurvey of hepatitis B in China-declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009; 27(47):6550-7; PMID:19729084; http://dx.doi.org/10.1016/j.vaccine.2009.08.048
- Pan HX, Zeng Y, Song XF, Zhang YJ, Xu K, Liang ZL, Zhu FC. Immune response to hepatitis B vaccine with high antigen content in non-responders after standard primary vaccination in Chinese adults. Vaccine 2014; 32(29):3706-12; PMID:24681228
- Bock HL, Löscher T, Scheiermann N, Baumgarten R, Wiese M, Dutz W, Sänger R, Clemens R. Accelerated schedule for hepatitis B immunization. J of Travel Med 1995; 2(4):213-7.
- Halperin SA, McNeil S, Langley JM, Smith B, MacKinnon-Cameron D, McCall-Sani R, Heyward WL, Martin JT. Safety and immunogenicity of different two-dose regimens of an investigational hepatitis B vaccine (hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide) in healthy young adults. Vaccine 2012; 30(36):5445-8; PMID:22704926; http://dx.doi.org/10.1016/j.vaccine.2012.05.074
- Velu V, Nandakumar S, Shanmugam S, Shankar EM, Thangavel S, Kulkarni PS, Thyagarajan SP. Comparative Efiicacy of two dosage of recombinant hepatitis B vaccine in healthy adulescents in India. Pediat Infect Dis J 2007; 26(11):1038-41; PMID:17984812; http://dx.doi.org/10.1097/INF.0b013e3181342887
- De Schryver A, Verstrepen K, Vandersmissen L, Vandermeeren N, Vernaillen I, Vranckx R, Van Damme P, van Sprundel M. Comparative immunogenicity of two vaccination schedules of a combined hepatitis A and B vaccine in healthy volunteers. J Viral Hepatitits 2011; 18(4):e5-e10; http://dx.doi.org/10.1111/j.1365-2893.2010.01365.x
- Rendi-Wagner P, Kundi M, Stemberger H, Wiedermann G, Holzmann H, Hofer M, Wiesinger K, Kollaritsch H. Antibody response to three recombinant hepatitis B vaccines: comparative evaluation of multicenter travel-clinic based experience. Vaccine 2001; 19(15–16):2055-60; PMID:11228377; http://dx.doi.org/10.1016/S0264-410X(00)00410-2
- Zhang W, Han L, Lin C, Wang H, Pang X, Li L, Gao P, Lin H, Gong X, Tang Y, et al. Surface antibody and cytokine response to recombinant Chinese hamster ovary cell (CHO) hepatitis B vaccine. Vaccine 2011; 29(2011):6276-82; PMID:21722684
- Herona LG, Chanta KG, Jalaludinb BB. A novel hepatitis B vaccination regimen for adolescents: two doses 12 months apart. Vaccine 2002; 20(29–30):3472-6; PMID:12297392; http://dx.doi.org/10.1016/S0264-410X(02)00346-8
- Burgess MA, McIntyre PB, Hellard M, Ruff TA, Lefevre I, Bock HL. Antibody persistence six years after two doses of combined hepatitis A and B vaccine. Vaccine 2010; 28(10):2222-6; PMID:20056187; http://dx.doi.org/10.1016/j.vaccine.2009.12.053
- Cassidy WM, Watson B, Ioli VA, Williams K, Bird S, West DJ. A randomized trial of alternative two- and three-dose hepatitis B vaccination regimens in adolescents: antibody responses, safety, and immunologic memory. Pediatrics 2001; 107(4):626-31; PMID:11335734; http://dx.doi.org/10.1542/peds.107.4.626
- Kane MA. Global status of hepatitis B immunisation. Lancet 1996; 348:696; PMID:8806283; http://dx.doi.org/10.1016/S0140-6736(05)65598-5
- Advisory Committee on Immunization Practices. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination: recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep 1991; 40(RR-13):1-19
- Centers for Disease Control and Prevention. Hepatitis B vaccination among high-risk adolescents and adults – San Diego, California, 1998–2001. MMWR Morb Mortal Wkly Rep 2002; 51:618-21; PMID:12236303
- Stoffel M, Lievens M, Dieussaet I, Martin I, André F. Immunogenicity of Twinrix in older adults: a critical analysis. Expert Rev Vaccines 2003; 2(1):9-14; PMID:12901592; http://dx.doi.org/10.1586/14760584.2.1.9
- Van der Wielen M, Van Damme P, Chlibek R, Smetana J, von Sonnenburg F. Hepatitis A/B vaccination of adults over 40 years old: comparison of three vaccine regimens and effect of influencing factors. Vaccine 2006; 24(26):5509-15; PMID:16725234; http://dx.doi.org/10.1016/j.vaccine.2006.04.016