ABSTRACT
This is an observational epidemiological study to describe causes of bacterial meningitis among persons between 1 month and 18 y of age who are hospitalized with suspected bacterial meningitis in 7 Turkish regions. covering 32% of the entire population of Turkey. We present here the results from 2013 and 2014. A clinical case with meningitis was defined according to followings: any sign of meningitis including fever, vomiting, headache, and meningeal irritation in children above one year of age and fever without any documented source, impaired consciousness, prostration and seizures in those < 1 y of age. Single tube multiplex PCR assay was performed for the simultaneous identification of bacterial agents. The specific gene targets were ctrA, bex, and ply for N. meningitidis, Hib, and S. pneumoniae, respectively. PCR positive samples were recorded as laboratory-confirmed acute bacterial meningitis. A total of 665 children were hospitalized for suspected acute meningitis. The annual incidences of acute laboratory-confirmed bacterial meningitis were 0.3 cases / 100,000 population in 2013 and 0.9 cases/100,000 in 2014. Of the 94 diagnosed cases of bacterial meningitis by PCR, 85 (90.4%) were meningococcal and 9 (9.6%) were pneumococcal. Hib was not detected in any of the patients. Among meningococcal meningitis, cases of serogroup Y, A, B and W-135 were 2.4% (n = 2), 3.5% (n = 3), 32.9% (n = 28), and 42.4% (n = 36). No serogroup C was detected among meningococcal cases. Successful vaccination policies for protection from bacterial meningitis are dependent on accurate determination of the etiology of bacterial meningitis. Additionally, the epidemiology of meningococcal disease is dynamic and close monitoring of serogroup distribution is comprehensively needed to assess the benefit of adding meningococcal vaccines to the routine immunization program.
Introduction
Acute bacterial meningitis is an important condition associated with a high fatality rate and disability worldwide, particularly in infancy and childhood.Citation1-3 Currently, although the improvements in antimicrobial treatments as well as intensive care support, mortality rates in children with bacterial meningitis range between 5 to 10 %, even in developed countries.Citation4,5 Among the survivors a proportion up to 20 % suffer of permanent sequelae, including neurologic disabilities, seizures, mental retardation, or sensorineural deafness.Citation6-8 Another aspect is linked to the high medical cost resulted from admission to intensive care unit and long hospital stay in more than 40% of children diagnosed with bacterial meningitis.Citation9 In pre-vaccine era, the most common pathogens that caused acute bacterial meningitis in infants and children are Haemophilus influenzae type b (Hib), Streptococcus pneumoniae (S. pneumoniae), and Neisseria meningitidis (N. meningitidis).Citation5,10
With increasing use in infants and children of efficacious conjugate vaccines against Hib and S. pneumoniae the incidence of meningitis caused by either HibCitation11 or S. pneumoniaeCitation12 in those age groups has drastically decreased.
As a consequence, N. meningitidis has become the leading cause of meningitis and septicemia in many regions of the world.Citation13-15 There are 12 recognized serogroups of N. meningitidis, however, 6 meningococcal serogroups including (Men) A, B, C, W-135, X and Y are associated with the majority of invasive disease with serogroups B and C are causing most cases in industrialized countries and elsewhere.Citation16-19 Before the widespread use of a conjugate meningoccoccal group A vaccine, seasonal meningitis epidemics caused by group A caused high toll of morbidity and mortality in the African meningitis belt countries.Citation20
Serogroup W-135 causes outbreaks in some parts of the world, primarily in Saudi Arabia and Africa.Citation14,15,21 Since the 1990s, isolates of serogroup X have been reported in several countries within the meningitis belt.Citation22 Recently, an increase in serogroup Y invasive meningococcal disease in some European countries was reported based on the epidemiological data.Citation23
As the epidemiology and distribution of these disease-causing pathogens as well as serogroups varies widely by geographic region and show time patterns since 2005 we have been performing a hospital-based meningitis surveillance study across several regions of Turkey. We present here the results from 2013 to 2014.
Results
During 2013–2014 a total of 665 children were hospitalized for suspected acute meningitis in the 12 hospitals participating in the study. Cerebrospinal fluid (CSF) sample was obtained from all patients. The median age of the cases was 3.5 y (interquartile range [IQR], 2–7) and the boy-to-girl ratio was 1.29:1.
Out of 665 subjects with CSF samples taken 94 had bacterial meningitis confirmed by PCR (14.1%), 85 (90.4%) were N. meningitidis spp. and 9 (9.6%) were S. pneumoniae. Hib was not detected in any of the CSF sample. Among meningococcal meningitis, N. meningitidis serogroup Y were 2.4% (n = 2), serogroup A 3.5% (n = 3), of serogroup B 32.9% (n = 28), and of serogroup W-135 42.4% (n = 36. No serogroup C was detected among meningococcal cases ().
Table 1. Distribution of causative agents of bacterial meningitis and meningococcal serogroups during 2013–2014 in Turkey.
There were 3 deaths, all caused by N. meningitidis serogroup B making the case fatality rate for the pathogen 10.7% (3/28) and for all N. meningitidis isolates 3.2% (3/98). Two of the fatal cases isolated serogroup B were ≤ 6 months of age.
Age distribution
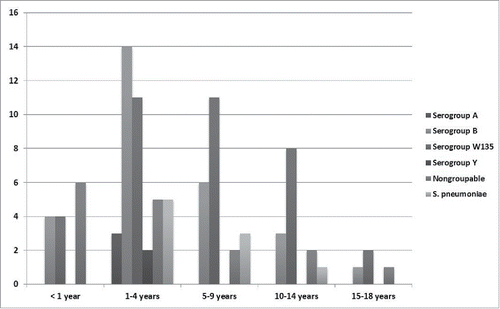
Among meningococcal meningitis, cases in subjects ≤ 1 year, 1–4 years, 5–9 years, 10–14 years, and 15–18 y old were 16.5%, 41.2%, 22.4%, 15.3%, and 4.6%, respectively. Pneumococcal meningitis cases were reported in subjects 1–4 years, 5–9 years, and 10–14 y old as 55.5%, 33.3%, and 12.2%, respectively ().
Incidence and regional distribution
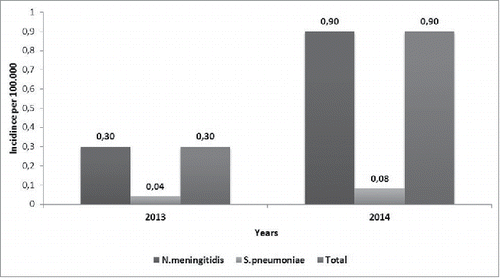
The annual incidence of acute laboratory-confirmed bacterial meningitis was 0.3 cases / 100,000 population in 2013 and 0.9 cases/100,000 in 2014 ().
The regional distribution of etiologic pathogens during 2013–2014 showed that bacterial meningitis was most prevalent in the Central Anatolia (n = 48, 51.1%) followed by Mediterranean (n = 15, 16.0%) and Marmara regions (n = 10, 10.6%). The prevalence of regional distribution of etiologic pathogens in other regions of Turkey was Aegean (n = 7, 7.4%), East Anatolia (n = 6, 6.4%), South East Anatolia (n = 5, 5.3%), and Black Sea (n = 3, 3.2%), in decreasing order.
Discussion
Monitoring epidemiological trends of vaccine-preventable diseases is critical for each country to efficiently implement and evaluate regional and national immunization programs. Therefore, since 2005 the incidence and pathogens causing bacterial meningitis in Turkey have been monitored. Initially, the annual incidence of acute bacterial meningitis in Turkey was 3.5 cases/100,000 population.Citation24 This annual incidence decreased to 0.9 cases/100,000 in 2014 in the present study. Hib vaccination for infants was implemented in the Turkish National Immunization Program (NIP) in 2006 leading to a dramatic decrease of Hib meningitis. In fact, we did not observed any meningitis cases caused by Hib in the present period. Pneumococcal conjugate 7-valent vaccine was included in the NIP in 2009, and replaced by PCV-13 in 2011. At the beginning of the period 2005–2012, the annual incidence of acute bacterial meningitis caused by S. pneumoniae in Turkey ranged from 0.7 to 0.8 cases/100,000 population and then the annual incidence decreased to 0.2 cases/100,000 in 2012.Citation24,25 There has been a major decrease in pneumococcal meningitis incidence with the lowest recorded in 2014 with 0.08 cases/100,000. Therefore, the main contribution to the overall decrease in annual incidence in Turkey can be attributed to the vaccination-induced decrease in Hib and S. pneumoniae meningitis introduced in 2006 and 2009, respectively, and with coverage of 97% for both (see: http://www.sgk.gov.tr).
We identified N. meningitidis as the most common cause of bacterial meningitis (90.4%) in Turkey consistently with our previous observations,Citation25 particularly in young children. The annual incidence of meningococcal meningitis was 0.9 cases/100,000 and this incidence was relatively increased because of the dramatic decrease in Hib and S. pneumoniae meningitis. Serogroup W-135 is the dominating agent (42.4%) followed by serogroup B (32.9%) in the study period. Serogroup A has decreased; there were no serogroup C cases observed in this study in spite no vaccination program is implemented in Turkey. These data are in contrast to those from many parts of Europe, where serogroups B and C were the leading cause of bacterial meningitis.Citation15 Although serogroup B was the second most common agent in bacterial meningitis in our previous study, serogroup B has tended to decrease during the last few years. The reason for the fluctuation in serogroup B disease in Turkey is unclear as there is no serogroup B vaccine currently available, but the epidemiology of serogroup B has been observed to vary with time in many countries without any apparent reason.Citation25 Additionally, there is no a possible explanation about rarity of the serogroups C and Y in Turkey, as no vaccine has been used against these serotypes.
Our previously published studiesCitation25,26 illustrated pilgrims attending the Hajj may be a possible source for the acquisition of meningococcal serogroup W-135 disease in Turkey. Similar findings have been reported in some European countries such as UK and France.Citation27 Consequently, the Turkish National Immunization Board has planned to implement a conjugated quadrivalent meningococcal vaccine for pilgrims and Umrah visitors, which may help to reduce carriage rates and decrease the burden of W-135 in Turkey.Citation28 Recently, around 2.7 million Syrians refugees were registered by the Government of Turkey (http://data.unhcr.org/syrianrefugees/country.php?id=224) with an increasing of refugee population from other countries including Afghanistan, Iran, Iraq, and Somalia (http://www.unhcr.org.tr/?content=178). Meningitis cases were reported in this refugee population living in Turkey,Citation29 highlighting the importance of including displaced population in the meningitis surveillance study.
Additionally, N. meningitidis was the leading cause of the death in the present study. The case-fatality rate (3.2%) was similar to our previous ratioCitation25 and still lower than that reported in the literature (between 5.3% and 26.2% %).Citation30 This result may possibly be explained by our improved diagnostic and health care facilities. All the fatal meningitis cases were caused by serogroup B and 2 of them were under 6 months of age. Our findings are consistent with the results of previous studiesCitation31 and confirm that infants are at increased risk of severe meningococcal disease.Citation31
This study has several limitations. First, laboratory confirmation of the etiology of acute bacterial meningitis is critical for providing optimal patient therapy and to ensure appropriate case contact management. In addition, case confirmation provides epidemiological information necessary for making decisions concerning immunization programs, and implementation of new available vaccines against the most common bacterial meningitis pathogens.Citation24,32 Of the non-culture diagnostic tests, PCR is most commonly used and generates sufficiently accurate and reliable results, especially when there is a history of antimicrobial drug use before lumbar puncture.Citation24 Although we used the PCR for the diagnosis of the patients, bacterial meningitis was confirmed by PCR in only 14.1 % of the patients. This might be the reason why our study likely missed, thus underestimates many bacterial meningitis cases, particularly among patients whose illnesses did not meet the case definition. Second, we really do not know the effect of the subpopulations such as refugees to our epidemiological data. Despite the limitations, this project has provided useful insights into the incidence and epidemiology of bacterial meningitis in Turkey.
In this study we observed a dramatic decrease of Hib and S. pneumoniae meningitis in Turkish children since Hib and PCV vaccines have been introduced in NIP. However, the epidemiology and etiology of bacterial meningitis may change over time and by regions in a way that cannot be predicted. Therefore, ongoing active surveillance is needed to determine the true epidemiological data as well as the correct choice of vaccine type and age for the vaccination to be implemented.Citation33 In our prospective surveillance we showed that 90.4 % of meningitis was caused by N. meningitides with a relative increase over time. In Turkey, the leading cause of the meningococcal meningitis was still serogroup W-135 followed by serogroup B. Consistent with our previous resultsCitation25 and with results from many other countries infants seem to be at highest risk for fatal meningitis caused by serogroup B. Vaccines that provide long-term protection early in life have the potential to reduce the burden of meningococcal disease.Citation16,34 The epidemiology of meningococcal disease is dynamic, and close monitoring of trends is needed to accurately assess the benefit of adding meningococcal vaccines to the routine infant schedule as well as to vaccinate, children, adolescents and displaced populations.
Materials and methods
Study design
Between January 2013 and December 2014 patients attending the pediatric outpatient and inpatient clinics of 12 hospitals across 7 regions of Turkey were included in this hospital-based surveillance study. We estimate that approximately 34% of the entire Turkish population refers to the 12 pediatric hospitals and clinics participating the study. The study protocol was approved by the ethics committee of the Hacettepe University Institutional Ethics Committee as well as the other sites of local ethic committees. Children aged between 1 month and 18 y presenting with meningitis clinical findings during the study period were screened. Newborns were excluded from the study for the different pathogens present in the neonatal period.Citation4
Inclusion criteria of suspected acute meningitis were the following: any sign of meningitis [ fever (≥38°C), vomiting (≥3 episodes in 24 h), headache, signs of meningeal irritation (bulging fontanel, Kernig or Brudzinski signs, or neck stiffness)] in children above 1 y of age; fever without any documented source; impaired consciousness (Blantyre Coma Scale < 4 if < 9 months of age and < 5 if ≥ 9 months of age);Citation35 prostration (inability to sit unassisted if ≥ 9 months of age or breastfeed if < 9 months of age) in those < 1 y of age; and seizures (other than those regarded as simple febrile seizures with full recovery within 1 h). After written parental/legal guardians consent, CSF was collected and processed according pre-defined procedures. Information regarding patient age, sex, clinical signs and symptoms at admission, prior clinical history including hospitalization, underlying diseases and use of antimicrobial agents, laboratory findings at admission, choice of treatment and outcome were recorded on a standardized form for each patient.
Laboratory analyses
The CSF specimens were collected in a sterile tube and leukocyte counts, protein levels and glucose levels were analyzed. The following test were performed and evaluated: 1) > 10 leukocytes/mmCitation3 in the CSF, and/or 2) higher CSF protein levels than normal for the patient's age, and/or 3) lower CSF glucose levels than normal for the patient's age. If positive, CSF culture, polymerase chain reaction (PCR), Gram stain, or antigen detection test were performed. CSF cultures and Gram stain were performed in the local hospitals, but CSF samples (minimum of 0.5 mL) from each patient was stored at −20°C until transportation to the Central Laboratory at Hacettepe University Pediatric Infectious Diseases Unit, Ankara, Turkey for PCR analysis. Available bacterial isolates were also sent to the Central Laboratory and re-cultured on chocolate and blood agars and grown at 37°C in 5% CO2. Suspected meningococcal colonies were identified by Gram stain, oxidase test, and rapid carbohydrate utilization test Api (API NH Ref. 10400, BioMerieux, Germany). The phenotypic determination, based on the antigenic formula (serogroup: serotype: serosubtype) of meningococcal isolates, was performed by standard methods in the Meningococcal Reference Unit, Health Protection Agency, Manchester, United Kingdom.Citation27,36-38
All samples transferred to the Central Laboratory were stored at −80°C and melted immediately before each test. DNA was isolated as previously reported.Citation24 Single tube, multiplex PCR assay was performed for the simultaneous identification of bacterial agents. The specific gene targets were ctrA, bex, and ply for N. meningitidis, Hib, and S. pneumoniae, respectively.Citation24,32 PCR was performed by using a DNA thermal cycler (GeneAmp PCR System model 9700, Applied Biosystems, Foster City, CA, USA). In samples positive for N. meningitidis, serogroup prediction (A, B, C, W-135, and Y) was based on the oligonucleotides in the siaD gene for serogroups B, C, W-135, and Y, and in orf-2 of a gene cassette required for serogroup A.Citation32,39 All amplicons were analyzed by electrophoresis on standard 3% agarose gels and visualized using UV fluorescence. A negative control consisting of distilled water and positive control consisting of an appropriate reference strain (S. pneumoniae ATCC 49613, Hib ATCC 10211, N. meningitidis serogroup C L94 5016 also known as C11 [C:16:P1.7–1.1], serogroup A M99 243594 [A:4,21:P1.20,9], serogroup Y M05 240122 [Y:NT:P1.5], serogroup W-135 M05 240125 [W135:2a: NST], serogroup B M05 240120 [B:NT:NST] were analyzed simultaneously.
Statistical analysis
Statistical analyses were performed using the commercial package SPSS for Windows version 19.0 (SPSS, Inc., Chicago, IL, USA). Values for numerical variables were provided as mean standard deviation or median (interquartile ranges), depending on normality of distribution. Categorical variables were given as numbers and total percentages.
Abbreviations
| CSF | = | Cerebrospinal fluid |
| DNA | = | Deoxyribonucleic acid |
| Hib | = | Haemophilus influenzae type b |
| Men A | = | Neisseria meningitidis serogroup A |
| Men B | = | Neisseria meningitidis serogroup B |
| Men C | = | Neisseria meningitidis serogroup C |
| Men X | = | Neisseria meningitidis serogroup X |
| Men Y | = | Neisseria meningitidis serogroup Y |
| NIP | = | National Immunization Program |
| PCR | = | Polymerase chain reaction |
| PCV13 | = | Pneumococcal Conjugate Vaccine 13 valent |
| PCV7 | = | Pneumococcal Conjugate Vaccine 7 valent |
| W-135 | = | Neisseria meningitidis serogroup W-135 |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Authors thank the local ethical committees including Ege University, Izmir, Turkey and Zekai Tahir Burak Maternity Teaching Hospital, Ministry of Health, Ankara, Turkey.
Funding
Funding for this study was provided by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA was provided the opportunity to review a preliminary version of this manuscript for factual accuracy but the authors are solely responsible for final content and interpretation. The authors received no financial support or other form of compensation related to the development of the manuscript.
References
- Shrestha RG, Tandukar S, Ansari S, Subedi A, Shrestha A, Poudel R, Adhikari N, Basnyat SR, Sherchand JB. Bacterial meningitis in children under 15 years of age in Nepal. BMC Pediatrics 2015; 15:94; PMID:26286573; http://dx.doi.org/10.1186/s12887-015-0416-6
- Kostenniemi UJ, Norman D, Borgström M, Silfverdal SA. The clinical presentation of acute bacterial meningitis varies with age, sex and duration of illness. Acta Paediatr 2015; 104(11):1117-24.
- Wee LY, Tanugroho RR, Thoon KC, Chong CY, Choong CT, Krishnamoorthy S, Maiwald M, Tee NW, Tan NW. A 15-year retrospective analysis of prognostic factors in childhood bacterial meningitis. Acta Paediatr 2016; 105(1):e22-9; PMID:26426265; http://dx.doi.org/10.1111/apa.13228
- Feigin RD, Cutrer WB. Bacterial meningitis beyond the neonatal period. In: Feigin & Cherry's Textbook of Pediatric Infectious Diseases, Feigin RD, Cherry JD, Harrison RE, Kaplan SL, eds. (6th ed.). Philadelphia: Saunders Elsevier; 2009; 439–471.
- Mace SE. Acute Bacterial Meningitis. Emerg Med Clin North Am 2008; 26:281-317; PMID:18406976; http://dx.doi.org/10.1016/j.emc.2008.02.002
- Baraff LJ, Lee SI, Schriger DL. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr Infect Dis J 1993; 12(5):389-94; PMID:8327300; http://dx.doi.org/10.1097/00006454-199305000-00008
- Grandgirard D, Leib SL. Strategies to prevent neuronal damage in paediatric bacterial meningitis. Curr Opin Pediatr 2006; 18(2):112-8; PMID:16601488; http://dx.doi.org/10.1097/01.mop.0000193292.09894.b7
- Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10(5):317-28; PMID:20417414; http://dx.doi.org/10.1016/S1473-3099(10)70048-7
- Husain E, Chawla R, Dobson S, Dele Davies H. Canada PICNoli: Epidemiology and outcome of bacterial meningitis in Canadian children: 1998-1999. Clin Invest Med 2006; 29:131-5; PMID:17058430
- Kim KS. Acute Bacterial Meningitis in infants and children. Lancet Infect Dis 2010; 10:32-42; PMID:20129147; http://dx.doi.org/10.1016/S1473-3099(09)70306-8
- Schuchat A, Robinson K, Wenger JD, Harrison LH, Farley M, Reingold AL, et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med 1997; 337:1569-76; PMID:9371853; http://dx.doi.org/10.1056/NEJM199710023371404
- Albrich WC, Baughman W, Schmotzer B, Farley MM. Changing characteristics of invasive pneumococcal disease in metropolitan Atlanta, Georgia, after introduction of a 7-valent pneumococcal conjugate vaccine. Clin Infect Dis 2007; 44:1569-76; PMID:17516400; http://dx.doi.org/10.1086/518149
- Polan GA. Prevention of meningococcal disease: current use of polysaccharide and conjugate vaccines. Clin InfectDis 2010; 50: 545-53; PMID:20144016
- Pathan N, Faust SN, Levin M. Pathophysiology of meningococcal meningitis and septicaemia. Arch Dis Child 2003; 88(7):601-7; PMID:12818907; http://dx.doi.org/10.1136/adc.88.7.601
- Organization. WH. Outbreak news. Meningococcal disease, African meningitis belt, epidemic season 2006. Wkly Epidemiol Rec 2006; 81:119-20; PMID:16673512
- Choudhuri D, Huda T, Theodoratou E, Nair H, Zgaga L, Falconer R, Luksic I, Johnson HL, Zhang JS, El Arifeen S, et al. An evaluation of emerging vaccines for childhood meningococcal disease. BMC Public Health 2011; 11:S29; PMID:21501447; http://dx.doi.org/10.1186/1471-2458-11-S3-S29
- Tan LK, Carlone GM, Borrow R. Advances in the development of vaccines against Neisseria meningitidis. N Eng J Med 2010; 362(16):1511-20; PMID:20410516; http://dx.doi.org/10.1056/NEJMra0906357
- Frota AC, Milagros LG, Harrison LH, Ferreira B, Menna Bareto D, Pereira GS, Cruz AC, Pereira-Manfro W, de Oliveira RH, Abreu TF, et al. Immunogenicity and safety of meningococcal C conjugate vaccine in children and adolescents infected and uninfected with HIV in Rio de Janeiro, Brazil. Pediatr Infect Dis J 2015; 34: e113-8; PMID:25876102; http://dx.doi.org/10.1097/INF.0000000000000630
- Preparedness for outbreaks of meningococcal meningitis due to Neisseria meningitidis serogroup C in Africa: recommendations from a WHO expert consultation. Wkly Epidemiol Rec 2015; 90: 633-6; PMID:26591025
- Diomonde FV, Djingarey MH, Daugla DM, Novak RT, Kristiansen PA, Collard JM, Gamougam K, Kandolo D, Mbakuliyemo N, Mayer L, et al. Public health impact after the introduction of PsA-TT: The first 4 years. Clin Infect Dis 2015; 61: S467-472; PMID:26553676; http://dx.doi.org/10.1093/cid/civ499
- Borrow R. Meningococcal disease and prevention at the Hajj. Travel Med Infect Dis 2009; 7:219-25; PMID:19717104; http://dx.doi.org/10.1016/j.tmaid.2009.05.003
- Agnememel A, Hong E, Giorgini D, Nunez-Samudio V, Deghmane AE, Taha MK. Neisseria meningitidis serogroup X in Sub-Saharan Africa. Emerg Infect Dis 2016; 22: 698-702; PMID:26982628; http://dx.doi.org/10.3201/eid2204.150653
- Bröker M, Emonet S, Fazio C, Jacobsson S, Koliou M, Kuusi M, Pace D, Paragi M, Pysik A, Simões MJ, et al. Meningococcal Serogroup Y disease in Europe: continuation of high importance in some European regions in 2013. Hum Vaccin Immunother 2015; 11: 2281-6; PMID:26036710; http://dx.doi.org/10.1080/21645515.2015.1051276
- Ceyhan M, Yildirim I, Balmer P, Borrow R, Dikici B, Turgut M, Kurt N, Aydogan A, Ecevit C, Anlar Y, et al. A prospective study of etiology of childhood acute bacterial meningitis, Turkey. Emerg Infect Dis 2008; 14(7):1089-96; PMID:18598630; http://dx.doi.org/10.3201/eid1407.070938
- Ceyhan M, Gürler N, Ozsurekci Y, Keser M, Aycan AE, Gurbuz V, Salman N, Camcioglu Y, Dinleyici EC, Ozkan S, et al. Meningitis caused by Neisseria meningitidis, Haemophilus Influenzae Type B and Streptococcus pneumoniae during 2005-2012 in Turkey: A Multicenter Prospective Surveillance Study. Hum Vaccin Immunother 2014; 10:2706-12; PMID:25483487; http://dx.doi.org/10.4161/hv.29678
- Ceyhan M, Celik M, Demir ET, Gurbuz V, Aycan AE, Unal S. Acquisition of meningococcal serogroup W-135 carriage in Turkish Hajj pilgrims who had received the quadrivalent meningococcal polysaccharide vaccine. Clin Vaccine Immunol 2013; 20(1):66-8; PMID:23136117; http://dx.doi.org/10.1128/CVI.00314-12
- Taha MK, Achtman M, Alonso JM, Greenwood B, Ramsay M, Fox A, Gray S, Kaczmarski E. Serogroup W135 meningococcal disease in Hajj pilgrims. Lancet 2000; 356(9248):2159; PMID:11191548; http://dx.doi.org/10.1016/S0140-6736(00)03502-9
- Dinleyici EC, Ceyhan M. The dynamic and changing epidemiology of meningococcal disease at the country-based level: the experience in Turkey. Exp Rev Vaccines 2012; 11(5):515-8; PMID:22827237; http://dx.doi.org/10.1586/erv.12.29
- Tezer H, Ozkaya-Parlakay A, Kanık-Yuksek S, Gülhan B, Güldemir D. A Syrian patient diagnosed with meningococcal meningitis serogroup B. Hum Vaccin Immunother 2014; 10:2482; PMID:25424959; http://dx.doi.org/10.4161/hv.28951
- Luksic I, Mulic R, Falconer R, Orban M, Sidhu S, Rudan I. Estimating global and regional morbidity from acute bacterial meningitis in children: assessment of the evidence. Croat Med J 2013; 54(6):510-8; PMID:24382845; http://dx.doi.org/10.3325/cmj.2013.54.510
- MacNeil JR, Bennett N, Farley MM, Harrison LH, Lynfield R, Nichols M, Petit S, Reingold A, Schaffner W, Thomas A, et al. Epidemiology of infant meningococcal diasease in the United States, 2006-2012. Pediatrics 2015; 135:e305-11; PMID:25583921; http://dx.doi.org/10.1542/peds.2014-2035
- Taha MK. Simultaneous approach for nonculture PCR-based identification and serogroup prediction of Neisseria meningitidis. J Clin Microbiol 2000; 38(2):855-7; PMID:10655397
- Bakir M. Meningococcal serogroup B disease in Turkey: a guess or reality? Hum Vaccin Immunother 2014; 10:1721-4; PMID:24637878; http://dx.doi.org/10.4161/hv.28438
- Crum-Cianflone N, Sullivan E. Meningococcal vaccinations. Infect Dis Ther 2016; 5:89-112; PMID:27086142
- Berkley JA, Versteeg AC, Mwangi I, Lowe BS, Newton CR. Indicators of acute bacterial meningitis in children at a rural Kenyan district hospital. Pediatrics 2004; 114(6):e713-9; PMID:15574603; http://dx.doi.org/10.1542/peds.2004-0007
- Meningococcal Reference U, Gray SJ, Trotter CL, Ramsay ME, Guiver M, Fox AJ, Borrow R, Mallard RH, Kaczmarski EB. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J Med Microbiol 2006; 55(Pt 7):887-96; PMID:16772416
- Doganci L, Baysallar M, Saracli MA, Hascelik G, Pahsa A. Neisseria meningitidis W135, Turkey. Emerg Infect Dis 2004; 10(5):936-7; PMID:15200836; http://dx.doi.org/10.3201/eid1005.030572
- Kilic A, Urwin R, Li H, Saracli MA, Stratton CW, Tang YW. Clonal spread of serogroup W135 meningococcal disease in Turkey. J Clin Microbiol 2006; 44(1):222-4; PMID:16390974; http://dx.doi.org/10.1128/JCM.44.1.222-224.2006
- Tsolia MN, Theodoridou M, Tzanakaki G, Kalabalikis P, Urani E, Mostrou G, Pangalis A, Zafiropoulou A, Kassiou C, Kafetzis DA, et al. The evolving epidemiology of invasive meningococcal disease: a two-year prospective, population-based study in children in the area of Athens. FEMS Immunol Med Microbiol 2003; 36(1-2):87-94; PMID:12727371; http://dx.doi.org/10.1016/S0928-8244(03)00083-X