ABSTRACT
A quadrivalent, inactivated, split-virion influenza vaccine containing a strain from both B lineages (IIV4) has been developed, but its safety and immunogenicity in young children has not been described. This was a phase III, randomized, double-blind, active-controlled, multi-center study to examine the immunogenicity and safety of IIV4 in children 3–8 y of age (EudraCT no. 2011-005374-33). Participants were randomized 5:1:1 to receive the 2013/2014 Northern Hemisphere formulation of IIV4, an investigational trivalent comparator (IIV3) containing the B/Victoria lineage strain, or the licensed Northern Hemisphere IIV3 containing the B/Yamagata lineage strain. Participants who had not previously received a full influenza vaccination schedule received 2 doses of vaccine 28 d apart; all others received a single dose. 1242 children were included. For all 4 strains, IIV4 induced geometric mean haemagglutination inhibition titres non-inferior to those induced by the IIV3 comparators. For both B strains, geometric mean antibody titres induced by IIV4 were superior to those induced by the IIV3 with the alternative lineage strain. Similar proportions of participants vaccinated with IIV4 and IIV3 reported solicited injection-site reactions, solicited systemic reactions, and vaccine-related adverse events. A single vaccine-related serious adverse event, thrombocytopenia, was reported 9 d after vaccination with IIV4 and resolved without sequelae. In conclusion, in children aged 3–8 y who received one dose or 2 doses 28 d apart, IIV4 had an acceptable safety profile, was as immunogenic as IIV3 for the shared strains, and had superior immunogenicity for the additional B strain.
Introduction
Current trivalent influenza vaccines contain a single B strain, but since the 1980s, 2 distinct genetic lineages of influenza B virus, Victoria and Yamagata, have been co-circulating worldwide, both of which are responsible for influenza illnesses.Citation1,2 In the US, in half of the Northern Hemisphere influenza seasons between 1999/2000 and 2011/2012, the B lineage included in the trivalent vaccine was not the same as the dominant circulating B lineage.Citation3 Quadrivalent influenza vaccines containing both B lineages are becoming available and should help solve the problem of B strain selection. Influenza B strain viruses disproportionately affect children and adolescents, who may benefit the most from adding a second B strain lineage.
A quadrivalent, inactivated, split-virion influenza vaccine (IIV4) has been developed containing one A/H1N1 strain, one A/H3N2 strain, and one B strain from each lineage. In children/adolescents aged 9 to 17 years, adults aged 18 to 60 years, and elderly adults, IIV4 was as immunogenic as the comparator trivalent inactivated influenza vaccine (IIV3) for each of the 3 shared influenza strains and superior for the additional B strain.Citation4,5 In all age groups, IIV4 has had a safety profile similar to that of the licensed IIV3, with no unexpected safety signals,Citation4,5 but its safety and efficacy in young children has not been described. Here, we describe the results of a phase III clinical trial to assess the immunogenicity and safety of this vaccine in children aged 3 to 8 y of age.
Results
Participants
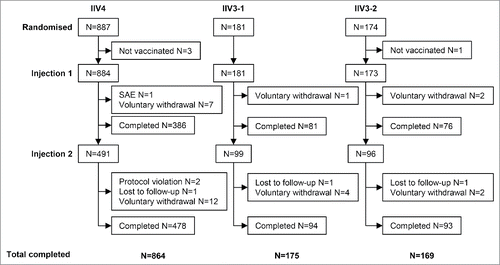
A total of 1242 children were included between September 12 and November 13, 2013, and the study was completed on June 25, 2014. The included children were randomized to IIV4 (n = 887), an IIV3 containing the B/Victoria lineage strain (IIV3-1) (n = 181), or and an IIV3 containing the B/Yamagata lineage strain (IIV3-2) (n = 174) (). All but 4 participants were vaccinated. Of the 1238 vaccinated participants, 1208 completed the study. The main reason for not completing the study was voluntary withdrawal not related to an adverse event (AE). One participant discontinued due to a vaccine-related serious adverse event (SAE) (thrombocytopenia).
Figure 1. Study design and patient flow. Participants were randomized 5:1:1 to receive IIV4, IIV3-1, or IIV3-2. IIV4 contained the 4 Northern Hemisphere 2013/2014 influenza strains recommended by the World Health Organization and the European Union: A/California/7/2009 (H1N1), A/Texas/50/2012 (H3N2), B/Brisbane/60/2008 (B Victoria lineage), and B/Massachusetts/02/20122012 (B Yamagata lineage). IIV3-1 contained both A strains and the B Victoria lineage strain. IIV3-2 contained both A strains and the B Yamagata lineage strain. All participants received one vaccination at day 0. Participants who had not received 2 doses of seasonal influenza vaccine during a previous season (i.e., unprimed participants) received a second dose of vaccine on day 28.
Ages, ethnicities, and geographical distributions were similar in all 3 groups (). Nearly equal numbers of boys and girls were included in the IIV4 and IIV3-2 groups, but the ratio of boys to girls was 1.7 in the IIV3-1 group. Approximately 45% of participants in all groups were primed (i.e. had received a full schedule of seasonal influenza vaccine during a previous influenza season).
Table 1. Participant characteristics.
Immunogenicity
Non-inferiority and superiority
The primary objective of non-inferiority of IIV4 vs. IIV3, analyzed in the per-protocol population, was met for all 4 strains as indicated by a lower limit of the 2-sided 95% confidence interval (CI) of the ratio of haemagglutination inhibition (HAI) geometric mean titres (GMTs) between groups > 1/1.5 (). Results were similar when the analysis was performed in all randomized subjects (Table S1). In addition, HAI antibody responses to both B strains in IIV4 were superior to those induced by IIV3 containing the alternate B strain lineage. Sensitivity analysis based on stratification by previous vaccination status confirmed non-inferiority of IIV4 vs. the IIV3 containing the matched strains and superiority of IIV4 vs. IIV3 containing the alternate B strain lineage (data not shown).
Table 2. Non-inferiority and superiority comparisons.
Immunogenicity of IIV4
Seroprotection rates for IIV4 were relatively high at baseline (76.4% for A(H1N1), 78.1% for A(H3N2), 60.8% for B/Brisbane [Victoria lineage], and 51.7% for B/Massachusetts [Yamagata lineage]), and GMTs were 142 for A(H1N1), 209 for A(H3N2), 61.6 for B/Brisbane, and 46.3 for B/Massachusetts) (). Despite these relatively high HAI antibody responses at baseline, vaccination with IIV4 increased GMTs by at least 6-fold for all vaccine strains (6.86 for A(H1N1), 7.49 for A(H3N2), 17.1 for B/Brisbane, and 25.3 for B/Massachusetts). Also, following vaccination with IIV4, nearly all subjects were seroprotected for all strains (98.7% for A(H1N1), 99.8% for A(H3N2), 98.7% for B/Brisbane, and 99.4% for B/Massachusetts). The associated rates of seroconversion or significant increase in HAI titres were 65.7% for A(H1N1), 64.8% for A(H3N2), 84.8% for B/Brisbane, and 88.5% for B/Massachusetts.
Table 3. HAI antibody responses.
Influence of previous vaccination
Irrespective of priming status, post-vaccination seroprotection rates in the IIV4 group were >97% for all 4 strains. As expected, baseline GMT and seroprotection rates for all 4 vaccine strains were higher for vaccine-primed participants (Table S2). After vaccination, seroconversion/significant increase rates tended to be higher in unprimed participants for both A strains (54–58% for primed vs. 73–76% for unprimed) and B strains (77–88% for primed vs. 90–94% for unprimed). This was linked to higher post-vaccination GMTs in unprimed participants. No obvious differences between IIV4 and the IIV3s were detected in immunogenicity measures or in the effect of priming.
Relative immunogenicity of IIV3-1 vs. IIV3-2
GMTs, seroprotection rates, GMT ratios, and seroconversion/significant increase rates vs. strains A(H1N1) and A(H3N2) were similar in the IIV3-1 and IIV3-2 groups (Table S3).
Safety
Solicited reactions
Solicited injection-site reactions were reported by 62.4% of participants in both the IIV4 and pooled IIV3 groups (). Solicited systemic reactions were reported by 48.9% of participants in the IIV4 group and 45.5% in the pooled IIV3 group. At the site of injection, pain was the most frequently reported solicited reaction (56.5% for IIV4, 55.4% for IIV3) (). The most common solicited systemic reactions were malaise (30.7% for IIV4, 28.2% for IIV3), myalgia (28.5% for IIV4, 26.8% for IIV3), and headache (25.7% for IIV4, 19.2% for IIV3).
Table 4. Safety summary: unsolicited AEs, solicited reactions, and SAEs after any injection.
Table 5. Solicited reactions with 7 d after any injection.
Overall, there were no major differences in the rates, types, or severity of solicited reactions between the IIV4 and pooled IIV3 groups (). In almost all cases, solicited reactions started within 3 d after injection lasted 3 d or less, and were grade 1 (Figure S1 and data not shown). Less than 6% of participants in the IIV4 and pooled IIV3 groups had grade 3 (severe) injection-site reactions (5.7% for IIV4, 4.2% for IIV3) and less than 5% had grade 3 systemic reactions (4.2% for IIV4, 3.4% for IIV3) (). Rates of solicited reactions were comparable in participants who received a single dose of vaccine and after the second dose in participants who received 2 doses of vaccine (Figure S1).
Unsolicited AEs
Unsolicited AEs were reported by 41.5% of participants in the IIV4 group and 35.6% in the pooled IIV3 group (). The most frequently reported unsolicited AEs were nasopharyngitis (7.1% for IIV4, 6.2% for IIV3) and upper respiratory tract infection (3.8% for IIV4, 4.2% for IIV3). Grade 3 non-serious AEs were reported by 2.0% in the IIV4 group and 2.5% in the pooled IIV3 group.
One 3-year-old participant in the IIV4 group experienced severe thrombocytopenia, an adverse event of special interest (AESI), 9 days after a first vaccination. The event resolved after 38 d but led to study discontinuation. On long-term follow-up, the platelet level increased and remained stable within normal ranges, indicating that it was a transient event. However, based on the temporal relationship with the vaccination, the event was considered by the investigator to be related to the vaccination. No other unexpected unsolicited AEs were reported.
Four participants had grade 3 unsolicited AEs considered by the investigator to be treatment-related. In the IIV4 group, one participant experienced severe vomiting and one experienced severe fatigue, and in the pooled IIV3 group, one participant experienced severe oropharyngeal pain and one experienced severe fever.
A participant in the IIV4 group experienced several immediate unsolicited AEs (mild cold sweat, dizziness, and pallor) following vaccination. These events, which were considered as related to the vaccination, resolved on the same day and did not recur after the participant received a second vaccination 28 d later.
Discussion
This randomized, double-blind, active-controlled, multi-center study showed that in children aged 3 to 8 years, antibody responses induced by IIV4 were non-inferior to those induced by the licensed IIV3 for all matched strains. In addition, IIV4 provided superior immunogenicity against influenza B of both lineages when compared to an IIV3 containing the alternate B strain lineage.
Despite the relatively high seroprotection rates at baseline, vaccination with IIV4 increased GMTs by at least 6-fold for all 4 vaccine strains. Seroconversion/significant increase rates were 80–90% for B strains and 60–70% for A strains. Although post-vaccination HAI antibody responses were somewhat lower in primed than unprimed participants, post-vaccination seroprotection rates were always >98% for both IIV3 and IIV4. Also, within the primed and unprimed groups, immunogenicity measures for each strain were similar between IIV4 and the IIV3 containing the strain. Thus, non-inferiority was maintained regardless of priming status.
The vaccine groups were balanced for all baseline characteristics except that the ratio of boys to girls was higher in the group vaccinated with IIV3-1 than in the other groups. Several studies suggest that females develop stronger antibody responses than males.Citation6 However, between subjects vaccinated with IIV3-1 and IIV3-2, we did not find any differences in HAI antibody responses against the shared A strains, indicating that this imbalance should not have influenced our findings.
The high baseline titres observed in this study are not unusual. Even higher baseline titres were found in a phase III study of children and adolescents 9–17 y of age examining the immunogenicity and safety of the same (2013/2014) formulation of IIV4. In that study, seroconversion/significant increase rates 21 d after vaccination were only 24.0% for A(H1N1), 20.0% for A(H3N2), 39.0% for B Yamagata lineage (B/Massachusetts), and 48.0% for B Victoria lineage (B/Brisbane) (Lu et al., unpublished observations). The seroconversion rates in the current study were closer to those in another phase III trial, which reported rates of 62–85% in children and adolescents 9–17 y of age.Citation5 The moderately high baseline titres in the current study were probably mostly due to natural exposure to the same or similar strains in the 2013/2014 formulation of IIV4.
IIV4 and the IIV3s had similar safety profiles. The overall frequencies of solicited reactions and of unsolicited AEs were similar between the vaccine groups, and few unsolicited AEs were considered vaccine-related. A single SAE of transient severe thrombocytopenia occurred 9 d after the first vaccination with IIV4 in a 3-year-old participant. Due to the temporal relationship with the vaccination, the event was considered to be vaccine-related, although etiology was not established. Only a few other cases of symptomatic thrombocytopenia following influenza vaccination have been reported, most of which have been in adults.Citation7 The most frequent explanation for thrombocytopenia in children is infection.Citation8 Other possible causes include myelodysplastic syndrome and autoimmune disease.
Data collected in Finland between 1999 and 2012 showed that an estimated 41.7% of all influenza B infections were caused by B viruses of the lineage not included in the trivalent vaccine.Citation9 In Hong Kong between 2000 and 2010, influenza B accounted for the highest rate of infections (41.9%) in children and young adolescents.Citation10 Using a conservative estimate that 15% of all influenza illnesses in children were caused by lineage-mismatched B viruses, coupled with 70% vaccine efficacy against all circulating strains and 30% vaccine-induced cross-protection between B strains, the authors of the Hong Kong study estimated that for every 1000 cases of influenza, 60 could be prevented by replacing a trivalent influenza vaccine with a quadrivalent vaccine containing B strains from both lineages.Citation10 Thus, young children should benefit from the addition of a second B strain lineage to the inactivated, split-virion influenza vaccine. As shown in this study, IIV4 may provide this added coverage without affecting vaccine tolerability and without diminishing the immunogenicity of the 3 other strains.
Patients and methods
Study design and objectives
This was a phase III, randomized, double-blind, active-controlled, multi-center study performed at 4 centers in Poland, 11 in Finland, 4 in Mexico, and 3 in Taiwan during the 2013/2014 Northern Hemisphere influenza season (EudraCT no. 2011-005374-33). The primary objective of the study was to demonstrate, for each strain, non-inferiority of specific antibody responses induced by IIV4 compared with IIV3-1 and IIV3-2. Secondary objectives were to assess the safety profile of each vaccine during the 28 d following each vaccination, to collect SAEs, including AESIs throughout the study; to assess humoral immune response induced by IIV4; and, for the B lineage strains, to demonstrate superiority of antibody responses induced by IIV4 compared to the antibody responses induced by the IIV3 containing the alternate B strain lineage.
Ethics
The study protocol was approved by the relevant ethics committee or institutional review board of each center and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines for Good Clinical Practice. Parents or legal guardians of all participants provided written informed consent at enrolment.
Participants
Healthy children 3 to 8 y of age were considered for enrolment. They were excluded from the study if they had received any vaccine during the 4 weeks preceding the first trial vaccination or planned to receive any vaccination up to 4 weeks after the last trial vaccination; were vaccinated with the current influenza seasonal vaccine formulation in the previous 6 months; had received immune globulins, blood, or blood-derived products in the past 3 months; had known or suspected congenital or acquired immunodeficiency; had received immunosuppressive therapy within the preceding 6 months; had received long-term systemic corticosteroid therapy (prednisone or equivalent for more than 2 consecutive weeks within the past 3 months); had a known systemic hypersensitivity to any of the vaccine components or history of a life-threatening reaction to the vaccines used in the trial or to a vaccine containing any of the same substances; had known or suspected thrombocytopenia, bleeding disorder, or receipt of anticoagulants in the 3 weeks preceding inclusion that contraindicated intramuscular vaccination; had chronic illness that could have interfered with trial conduct or completion; or had moderate or severe acute illness or febrile illness (temperature ≥ 38.0°C) on the day of vaccination.
Vaccination
IIV4 contained the 4 Northern Hemisphere 2013/2014 influenza strains recommended by the World Health Organization and the European Union: A/California/7/2009 (H1N1), A/Texas/50/2012 (H3N2), B/Brisbane/60/2008 (B Victoria lineage), and B/Massachusetts/02/2012 (B Yamagata lineage). IIV3-1 was the 2013/2014 formulation of the licensed IIV3 (Vaxigrip®, Sanofi Pasteur, Lyon, France) and contained both A strains and the B Victoria lineage strain. IIV3-2 was an investigational formulation of IIV3 containing both A strains and the B Yamagata lineage strain. All study vaccines were presented in 0.5-ml prefilled syringes. Each 0.5-ml dose contained 15 µg of hemagglutinin per strain.
Participants were randomized 5:1:1 to receive IIV4, IIV3-1, or IIV3-2. Randomization was performed with the permuted block method with stratification by site and influenza vaccination status at enrolment (primed or unprimed). Vaccine assignment was via an interactive web or voice response system so that investigators and participants were blinded to the vaccine administered. All participants received one vaccination at day 0. Participants who had not received 2 doses of seasonal influenza vaccine during a previous season (unprimed participants) received a second dose of vaccine on day 28. Participants were kept under observation for 30 min after each vaccination.
Immunogenicity
HAI titres were measured at baseline (day 0) and 28 d after the last vaccination as described previously.Citation4 The lower limit of quantitation was set at the reciprocal of the lowest dilution used in the assay (10) and the upper limit of quantitation as the highest dilution used in the assay (10,240). Seroprotection was defined as a HAI titer ≥ 40. Seroconversion was defined as a HAI titer < 10 on day 0 and a HAI titer ≥ 40 measured 28 d after the last vaccination, and a significant increase was defined as a HAI titer ≥ 10 on day 0 and a ≥ 4-fold increase from baseline in HAI titer 28 d after the last vaccination.
Safety
Parents or legal guardians of each child recorded solicited injection-site (pain, erythema, swelling, induration, and ecchymosis) and systemic reactions (fever, headache, malaise, myalgia, and shivering) up to 7 d after each vaccination in a diary. Severity of solicited reactions were graded 1 for mild, 2 for moderate, and 3 for severe (Table S4). Investigators recorded safety according to the ICH E2A Guideline for Clinical Safety Data Management: Definitions and Standards for Expedited Reporting. Parents or guardians of children participating in the study received a final telephone call 6 months after the last vaccination for the collection of SAEs, including AESIs (anaphylaxis, Guillain-Barré syndrome, encephalitis/myelitis, neuritis, febrile and non-febrile convulsions, thrombocytopenia, and vasculitis).
Sample size
A total of 1225 subjects were planned (875 for the IIV4 group and 175 for each IIV3 group). With a one-sided α level of 2.5%, a non-inferiority margin of 1.5, a standard deviation of log10 transformed titres of 0.6 for A strains and 0.5 for B strains, and 80% participants evaluable in each group, the power for the primary analysis (non-inferiority) was estimated to be 98.6% for each A strain and 96.7% for each B strain. The overall IIV4 group provided a probability of approximately 95% of observing any AE with a true incidence of 0.34%.
Statistical analysis
Statistical analysis was performed using SAS® version 9.2 (SAS Institute, Cary, NC, USA). The primary analysis (non-inferiority) was performed in the per-protocol analysis set, defined as all randomized participants who completed the vaccination schedule, had a post-last vaccination blood sample drawn, and completed the study according to protocol. For each strain, non-inferiority was demonstrated if the 2-sided 95% CI of the ratio of the GMT between IIV4 and that of the comparator IIV3, calculated using a normal approximation of log-transformed titres, was > 2/3. Secondary immunogenicity analysis (superiority) was performed in the full analysis set, defined as all randomized participants who received at least one dose of the study vaccine and had a post-last vaccination blood sample drawn. For each B strain, superiority was demonstrated if the 2-sided 95% CI of the ratio of the GMT between IIV4 and that of the comparator IIV3, calculated using a normal approximation of log-transformed titres, was > 1. For sensitivity analysis of non-inferiority and superiority, the stratified 2-sided 95% CI of the post-vaccination GMTs ratio between the IIV4 and IIV3 group(s) was calculated using an analysis of variance model (type II) of log10-transformed titres, with the vaccination status (primed or unprimed) as the stratifying factor. Descriptive analysis of immunogenicity was performed on the other immunogenicity analysis set, defined as all randomized participants who received the study vaccine and who had available HAI titres at both day 0 and 28 d after the last vaccination, with analysis according to the vaccine actually received. Safety endpoints were assessed in the safety analysis set, defined as all participants who received IIV4 or IIV3 (pooled), with analysis according to the treatment received.
Abbreviations
| AE | = | adverse event |
| CI | = | confidence interval |
| GMT | = | geometric mean titer |
| HAI | = | hemagglutination inhibition |
| IIV3 | = | trivalent inactivated, split-virion influenza vaccine |
| IIV4 | = | quadrivalent, inactivated, split-virion influenza vaccine |
| SAE | = | serious adverse event |
Disclosure of potential conflicts of interest
S. Pepin, E. Rivas, Y. Hutagalung, J. Menezes, and C. Monfredo are employees of Sanofi Pasteur. H. Szymanski declares serving as the principal investigator for studies funded by Ablynx, Astra Zeneca, GlaxoSmithKline, Novartis, Wyeth, Pfizer, and Sanofi Pasteur. All other authors declare no conflicts of interest for the work presented in this article.
Author contributions
S.P. participated in study design, data collection, data analysis/interpretation, and writing the article. C.M. participated in study design, data collection, and writing the article. All other authors participated in data collection and writing the article.
KHVI_A_1212143_Supplement.zip
Download Zip (107.8 KB)Acknowledgments
The authors thank Dr Jonna Simila (Oulu Vaccine Research Clinic, Finland) for participating as a study investigator. Medical writing was provided by Dr Phillip Leventhal (4Clinics, Paris, France).
Funding
The study and medical writing were funded by Sanofi Pasteur.
References
- McCullers JA, Saito T, Iverson AR. Multiple genotypes of influenza B virus circulated between 1979 and 2003. J Virol 2004; 78:12817-28; PMID:15542634; http://dx.doi.org/10.1128/JVI.78.23.12817-12828.2004
- Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 1990; 175:59-68; PMID:2309452; http://dx.doi.org/10.1016/0042-6822(90)90186-U
- Centers for Disease Control. Past Weekly Surveillance Reports, 2014. Available from: http://www.cdc.gov/flu/weekly/pastreports.htm
- Pepin S, Donazzolo Y, Jambrecina A, Salamand C, Saville M. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine in adults. Vaccine 2013; 31:5572-8; PMID:24016810; http://dx.doi.org/10.1016/j.vaccine.2013.08.069
- Cadorna-Carlos JB, Nolan T, Borja-Tabora CF, Santos J, Montalban MC, de Looze FJ, Eizenberg P, Hall S, Dupuy M, Hutagalung Y, et al. Safety, immunogenicity, and lot-to-lot consistency of a quadrivalent inactivated influenza vaccine in children, adolescents, and adults: A randomized, controlled, phase III trial. Vaccine 2015; 33:2485-92; PMID:25843270; http://dx.doi.org/10.1016/j.vaccine.2015.03.065
- Klein SL, Pekosz A. Sex-based biology and the rational design of influenza vaccination strategies. J Infect Dis 2014; 209 Suppl 3:S114-9; PMID:24966191; http://dx.doi.org/10.1093/infdis/jiu066
- Cecinati V, Principi N, Brescia L, Giordano P, Esposito S. Vaccine administration and the development of immune thrombocytopenic purpura in children. Hum Vaccin Immunother 2013; 9:1158-62; PMID:23324619; http://dx.doi.org/10.4161/hv.23601
- Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am J Hematol 2010; 85:174-80; PMID:20131303; http://dx.doi.org/10.1002/ajh.21833
- Heikkinen T, Ikonen N, Ziegler T. Impact of influenza B lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999-2012. Clin Infect Dis 2014; 59:1519-24; PMID:25139969; http://dx.doi.org/10.1093/cid/ciu664
- Chan PK, Chan MC, Cheung JL, Lee N, Leung TF, Yeung AC, Wong MC, Ngai KL, Nelson EA, Hui DS. Influenza B lineage circulation and hospitalization rates in a subtropical city, Hong Kong, 2000-2010. Clin Infect Dis 2013; 56:677-84; PMID:23074315; http://dx.doi.org/10.1093/cid/cis885
