ABSTRACT
Recurrent herpes simplex labialis caused predominantly with herpes simplexvirus 1(HSV-1) is a major problem, for which various treatments have minimal impact. Given the important role of the immune system in controlling virus infection, an activation of virus-specific immune responses, in particular,using dendritic cell (DCs) vaccines, seems to be a promising approach for the treatment of patients with frequent recurrences of herpes labialis. The current paper presents the results of a pilot study of the safety and efficacy of DC vaccines in 14 patients with recurrent HSV-1 infections. DCs were generated in presence of GM-CSF and IFN-alpha and were loaded with HSV-1 recombinant viral glycoprotein D (HSV1gD). DCs cells were injected subcutaneously as 2 courses of vaccination during 9 months. Immunotherapy with DCs did not induce any serious side effects and resulted in more than 2-fold reduction in the recurrence rate and significant enhancement of the inter-recurrent time during the 9 months of treatment and subsequent 6-month follow-up period. An obvious clinical improvement was accompanied with an induction of an antigen-specific response to HCV1gD and a normalization of reduced mitogenic responsiveness of mono-nuclear cells. According to long-term survey data (on average 48 months after the beginning of therapy), 87% of respondents reported the decreased incidence of recurrent infection. At this time, most patients (85.7%) responded to HCV1gD stimulation. The data obtained suggests that dendritic cell vaccines may be a promising new approach for the treatment of recurrent labial herpes.
Introduction
Herpes labialis is a common skin and mucous membrane infection which is predominantlycaused by herpes simplex virus-1 (HSV-1). The WHO estimates worldwide seroprevalence of HSV-1 as 65–90 %. Primary HSV-1 infection has mild clinical symptoms, and is self-limiting. However, viruses are never eliminatedcompletely and establish a life-long latency. Cellular and humoral factors of innate and adaptive immunity are involved in the control of HSV-1 replication.Citation1 Impaired immunity may cause virus reactivation, generalization and recurrent infection.Citation2
Of all patients with labial herpes, about 10% experience frequent recurrences (≥6 per year). Recurrent herpes labialis is a major problem, since episodes of recurrences may be painful, long-lasting, disfiguring for infected patients and negatively affect a patient's quality of life. Accumulating evidence suggests an important role of virus-specific T helper 1 (Th1) in preventing recurrent disease. Produced by Th1 cells interferon-gamma (IFN-γ) activatesnatural killer (NK) cells and enhances the generation of cytotoxic T-lymphocytes (CTL)capable of eliminating the virus.Citation3,4 Actually, patients with recurrent herpes labialis display a suppression of antigen specific T-cell proliferation and Th1 cytokine production that is often associated with increased regulatory T-cell numbers.Citation5,6,7
Current management of recurrent HSV infection is based on antiviral therapy with acyclic nucleosides. Short-course regimens with oral antivirals decrease the duration of lesion episodes and pain by approximately one day; however, this treatment does not abort further recurrences.Suppressive therapy with long-lasting courses of acyclic nucleosides are more effective than episodictherapy in reducing the frequency of recurrences and prolonging the time to first recurrence.Citation8,9 Nevertheless, the data of meta-analysis demonstrate that the clinical benefit of long-term use of oral antiviral agents remains small.Citation10
Given an important role of immune response to HSV-1 latency, an alternative approach to prevent recurrent episodes is an activation of host immunity. Virus envelope glycoproteins (gD and gB) and their constituent peptides are immunodominant antigens, that can be recognized by antigen-specific CD4 and CD8 T-cells and induce virus-specific T-cell responses.Citation11,12 However, therapeutic HSV vaccines for the management of patients with recurrent herpes labialis have not been developed.Recent research shows a pivotal role of dendritic cells (DCs) in presentation of virus antigens to naïve T cells, an activation of an antigen-specific Th1 response, a generation of cytotoxic T-lymphocytes, andmaintaining efficient immune responses.Citation13,14 Interaction of HSV with DCs suppresses DC maturation and affects DC functions, in particular,inhibits the capacity of DCs to produce IL-12 and to stimulate T-cell proliferation, as well as induces DC apoptosis.Citation15,16,17 Therefore, the use of ex vivo generated antigen-loaded DCs is considered to be a new strategy to prevent and to treat a recurrent HSV infection.
Indeed, preclinical animal studies proved the safety and efficacy of DC-based vaccines for HSV infection. Ghasemi M. et al. demonstrated an induction of a potent protection against acute and latent HSV-1 infection in mice vaccinated with DCs loaded with partially purified viral proteins.Citation18 Clinical studies of DC-based vaccines for the treatment of HSV infection have not been performed. Nevertheless, a recent research shows that volunteers, injected with DCs, pulsed with influenza virus protein, have no side effects and demonstrate a rapid generation of an antigen-specific T-cell response after a single injection of mature DCs.Citation19 Besides, the safety of DCs as antiviral vaccines was proved in chronic hepatitis B and C patients, and in HIV-infected patients.Citation20,21,22
Earlier we reported the preliminary results of a pilot clinical trial on DC- based vaccines for recurrent HSV infection, which demonstrated a tolerance and safety of DCs loaded with HSV antigens in patients with high rate of recurrent episodes.Citation23 As cell platform for antigen delivery, we used monocyte-derived DCs, generated in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interferon-α (IFN-α), termed IFN-DCs. Type I interferons are the potent inducers and activators of DCs. IFN-DCs compared to traditionally obtained DCs (generated upon GM-CSF and interleukin-4) are characterized by a higher migration capacity, more effectively induce the proliferation of cytotoxic T-cells, activate both Th1 and Th2 response and produce IFN-α.Citation24,25,26
The present study aims to investigate the clinical efficacy and safety of DC-based vaccines in an expanded group of patients with recurrent herpes labialis and to investigate both short-term and long-term outcomes of DC vaccination.
Results
The studied group included 14 patients with median age of 38.5 y (ranged from 24 to 34 years) with HSV-1 labial herpes. Clinical baseline characteristics of patients are shown in . All patients enrolled in the study reported a history of recurrent herpes labialis with average disease duration of 15 y (from 10 to 19 years). The median number of recurrences during the past 12 months prior to enrolment into the trial was 10.5 and ranged from 10 to 12 episodes a year. The duration of recurrence-free intervals in this period varied from 30 to 36 d. Immunotherapy with DCs loaded with HSV1gD was well tolerated. None of the patient discontinued the treatment or was hospitalized due to serious adverse events. The reddening, swelling and pain at the injection site as local reactions to vaccination were registered in 57 – 78% of the cases (). Some patients displayed systemic reactions such as weakness (21.4%), chills (57%), sub-febrile fever (50%), headache (21.4%) and malaise (28.6%). These vaccine-related reactions resolved spontaneously during 24–48 hours.
Table 1. Baseline characteristics of patients with Herpes labialis.
Table 2. Vaccine-related reactions.
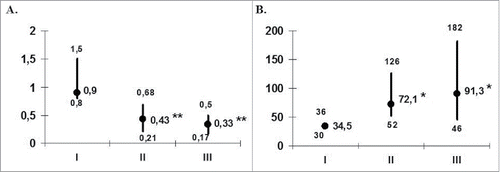
To assess a clinical efficacy of DC-based vaccines we compared the recurrence rate in pretreatment, within treatment and posttreatment periods. Given the difference in time frame periods (12 months before therapy, 9 months during therapy and 6 months after completion of the therapy), we recalculated recurrence incidence per month. The median number of recurrences during therapy was 2-fold lower than during pretreatment period (). The recurrence rate during posttreatment period was 3 times less than before treatment.
Figure 1. Frequency of recurrences (A) and the duration of interrecurrent period (B) in the patients with herpes labialis during DC-vaccination. The data are presented as median and interquartile range. The numbers of recurrences per month (A) and duration of interrecurrent period (B) were analyzed in 14 patients before (I), at the end of vaccination (II) and during the first 6 months of the follow-up period (III). *pU < 0.05 and **pU < 0.01 - the significance of differences with baseline values (U - nonparametric Mann-Whitney test).
The reduction inrecurrence frequency was accompanied by an increase of relapses-free intervals from 35 d before treatment to 72 d during therapy and up to 91 d in a 6-month posttreatment period (). Individual patient analysis showed that all 14 patients displayed a decreased recurrence rate during the treatment, and 10 of them (71.4%) experienced the reduced frequency of recurrences during the 6-month posttreatment follow-up as compared to pretreatment relapse rate. The proportions of patients who were recurrence free at the end of the treatment period were 3 out of 14 (21.4%) patients. Three patients (including one without relapses during treatment) had no relapses during the 6-month follow-up
Long-term outcomes of DC-based vaccines we evaluated via written surveys of patients whose follow-up period exceeded 24 months since beginning therapy. Nine out of 14 patients matched these criteria, and 8 patients (57%) agreed to take part in the survey. The time interval from the start of the therapy and the time of the interview varied in these patients from 36 to 84 months with a median of 48 months. Seven patients agreed to make additional immunological tests at the time of the interview.
As shown in , the obvious improvement was indicated by most patients. All patients noted the shortening of recurrence duration, and 7 patients (87.5%) reported a decrease in relapses rate and a reduction of affected areas. Six out of 8 respondents noted that general signs and symptoms of recurrent episodes (chills, fever, weakness, headache and muscle pains) when they occur tended to be milder. No patients registered any worsening, in particular, a rise in relapse incidence to initial level. Finally, no respondents expressed any regrets for participating in the clinical trial.
Table 3. Long-term outcomes of DC vaccination estimated by interviewing the patients with herpes labialis followed for over a year.
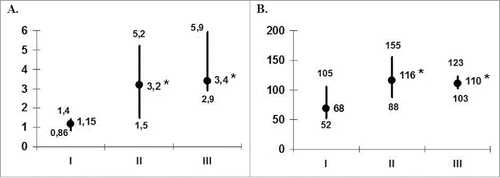
Since cell-mediated immunity is crucialin preventing herpes recurrences,the next stepwe assessed whether vaccination with HSV1gD-pulsed DCs elicited antigen-specific immune response and influenced mitogenic T-cell reactivity. Before therapy,patientMNCsfailed to proliferate upon stimulation with recombinantHSV1gD (). The median value of stimulation index of HSV1gD-induced MNC proliferation was 1.15. Analysis of individual data revealed the obvious antigen-specific MNC responses (SIHSV1gD > 1.5) inonly3 out of 14 (21%) patients. Vaccination with HSV1gD-pulsed DCs resulted in aninduction of an antigen-specific response with a 2-fold increase of median SIHSVgD (up to 3.2) following the treatment. At this time antigen-specific responses were registered in 11 out of 14 (78.6%) patients. In a 6-month follow-up period a median SIHSVgD was maintained at posttreatment level (SIHSVgD = 3.4) and the responsiveness to antigenic stimulation was detected in 12 patients.
Figure 2. Antigen and mitogen reactivity of patients' MNCs (n = 14) during DC-vaccination. The data are presented as stimulation indexes (IS; median and interquartile range) of HSV1gD (A) and ConA (B) on the proliferative response of peripheral blood MNC from 14 patients with herpes labialis assessed before treatment (I), at the end of vaccination (II) and during the first 6 months of the follow-up period (III). *pU < 0.05 - the significance of differences with baseline values (U - nonparametric Mann-Whitney test).
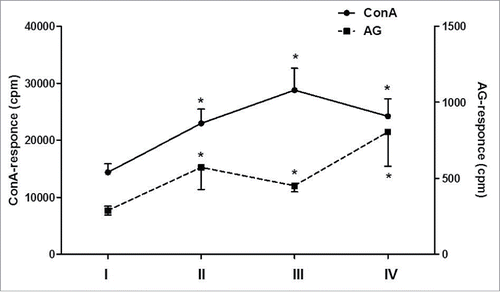
The evaluation of MNC reactivity to T-cell mitogen stimulation showed that before the therapy patient MNCs exhibited decreased proliferative responses to Con A compared with donor MNCs (17150 ± 1470 vs 24280 ± 2120 cpm; pU < 0.05). To the end of the therapy, we noted a significant increase in ConA-induced proliferation of MNCs (on average to 22620 cpm, pU < 0.05), which remained at the same high level after a 6 month follow-up (pU < 0.05). At this time the values of ConA stimulation indexes significantly exceeded those before vaccination (). Of note, there were no correlations between the antigen-specific or mitogenic responses (rS = −0.2; p = 0.31). Seven patients were assessed for antigen- and ConA-induced proliferative responses in a long-term period with a median of48 months (). The increase of proliferative response to viral antigen observed in a 6 mo after the therapy was also maintained in a long-term follow-up period and was registered in 6 out of 7 (85.7%) patients, with median value of SI HSVgD 2.6. Besides, patients in this period maintained a normal level of mitogen-induced MNC proliferation which was restored after DC vaccination.
Figure 3. Proliferative response of patients' MNCs (n = 7) to mitogen or antigen stimulation during DC vaccination and 48-month follow-up period. The proliferation of patients' MNC to mitogen (ConA; the left vertical axis) or viral antigen (HSV1gD; the right vertical axis) was analyzed in 7 patients with herpes labialis before DC therapy (I), at the end of vaccination (II), 6 months (III) and 48 months (IV) after vaccination. Data are shown as M ± SE (cpm). *pW < 0.05 - the significance of differences with baseline values (W - non-parametric Wilcoxon test for paired samples).
Discussion
Considering the fact that antiviral treatments are not capable to completely eliminate the herpes virus, and antiviral T-cell responses play a crucial role in preventing herpes recurrences, immunotherapyappears to be a promising approach in management of recurrent herpes labialis. Among various types of immunotherapies,DC-based vaccines are of special interest asDCs can induce a potent specific T-cell response to viral antigens.
In the present study,we firstly assessed the safety and efficacy of DC-based vaccines in patients with frequently recurrent herpes labialis. The data obtained showed a good tolerability of HSV1gD-pulsed DCs. DC vaccination resulted in the reduction of the recurrence rate by 2.3-fold and the enhancement of the inter-recurrent time from 35 to 72 d during 9-month treatment period.
Similar results demonstrated 2-fold decrease in the incidence of recurrent herpes labialis were obtained in the patients who received acyclovir, 400 mg twice daily, for 4-month treatment period and during a 6-month follow-up after antiviral therapy.Citation9 However, in our research the reduction in the number of recurrences was observed in the patients who did not receive acyclic nucleosides. Besides, we registered the decrease in recurrence rate for a longer treatment period that lasted 9 months. Importantly, the therapeutic effect during 6 months after treatment was even more obvious and manifested by 3-fold decrease in median recurrence incidence and an increase in recurrence-free period up to 94 d. According to long-term survey data (on average 48 months after the beginning the therapy), 87% of respondents reported the decreased incidence of clinical recurrences.
Notably, clinical improvement we noted was accompanied with an induction of an antigen-specific response to HSV1gD, which was utilized for both DC loading and ex vivo MNC stimulation. While baseline testing did not reveal any potent proliferative responses of patient MNCs to HSV1gD (SI < 1.5), the stimulation indexes of HSV1gD increased up to 3.2 to the end of the therapy and to 4.0 - in a 6-month follow-up period. To this time most patients (85.7%) responded to HSV1gD stimulation. Studies from both animal models and humans indicate that T-cells specific for HSV-1 contribute to protective immunity against herpes infection.Citation15 HSV1gD is an immunodominant viral coat protein, bearing epitopes recognized by both CD4+ and CD8+ T-cells.Citation11,30 Therefore, the fact that reduction of recurrent rate following DC vaccinations correlated with the induction of antigen-specific response confirms the significance of T-cell immunity in preventing herpes labialis recurrences.
Along with an antigen-specific response, we also measured a mitogen-induced MNC proliferation to ConA. The mitogenic responses of patient MNCs before therapy were decreasedas compared with donors MNCs but considerably increased to the end of the therapy and persisted at a normal level for a long-term period. Since the values of antigen-specific proliferation did not correlated with mitogenic responses, we suggested that the enhancement of ConA-stimulated proliferation was not directly related to DC vaccination but rather reflected the attenuation of virus-induced immunosuppression. Actually, impaired cell-mediated immunity caused by HSV-1 in recurrent herpes infection is a well-known phenomenon mediated by the shift in the Th1/Th2 balance and expansion of regulatory T-cell.Citation5,6,7 Given that impairment of ConA-induced proliferation is also a typical feature of virus-induced immunosuppression,Citation7 the restored res-ponses of MNCs to ConA may be the result of diminished suppressive effects of viruses due to reducing relapse rate.
Collectively, the data obtained show that DC-based immunotherapy in patients with recurrent herpes labialis reduces the recurrence incidence and increases the duration of recurrence-free periods. Clinical improvement is associated with induction of antigen-specific immune response and restoration of mitogenic T-cell responsiveness. These facts make interferon-α-induced DCs a promising candidate for therapy of recurrent herpes virus infection.
Patients and methods
Study design and patients
Recruiting, assessment and treatment of patients were conducted from May 2008 till December 2014 in a Clinic of Immunopathology affiliated with Institute of Fundamental and Clinical Immunology. Clinical tests were carried out as a prospective pilot study with “before – after” monitoring according to the protocol approved by the Local Ethics Committee. All procedures of cell product collection, manufacturing, and testing took place under the auspices of the Bone Marrow Transplantation Center which currently holds a license for cell technology application.
The study included both male and female patients between 18 and 60 y who signed a written agreement. The criteria for patient selections were recurrent herpes labialis infection with frequent recurrences (more than 6 episodes per year), absence of prolonged suppressive antiviral therapy, patient willingness to abstain from self-administration of antiviral medications during the clinical study, and evidence of herpes remission at the time of enrollment (2 weeks minimum since the last recurrence). The exclusion was based on non-conforming to selection criteria; immunocompromised status due to immunosuppressive therapy or human immunodeficiency virus infection; use of antiviral medications for 4 weeks before the clinical trial or during the trial; participation in another clinical study; pronounced chronic heartand pulmonary disease, respiratory distress, decompensated liver and renal diseases, decompensated diabetes mellitus; malignant diseases; blood diseases; mental disorders; pregnancy.
Primary end points were treatment effects assessed as recurrence rate and mean recurrent-free interval during vaccination as compared to those during 12 months immediately preceding the study. An active monitoring of patients was carried out via telephone and e-mail interviews conducted once a month. As monitoring periods differed and the time frames comprised 12 months before vaccination and 9 months during therapy, we recalculated the number of recurrences per month to compare recurrence rates. The mean inter-recurrence intervals were defined as a ratio of observation period duration (in days) to a number of recurrences.
Secondary end points included: 1) tolerance and safety assessment defined as local and systemic adverse events; 2) recurrence rate/medianinter-recurrence interval during a 6-month follow-up period after immunotherapy as compared to the corresponding values before therapy; 3) analysis of survey data for patients with follow-up period 24 months and more 4) evaluation of immune responses. The questionnaire included 4 questions: 1) Do you feel any improvement after the therapy (reduction of recurrence rate, shortening the time to healing, diminishing of rash affected areas); 2) Do you feel any improvement in general condition after treatment (less severity of fever, weakness, headaches and muscle pains during recurrences); 3)Did you experience any health deterioration (increase in recurrence rate, exacerbation of chronic diseases) after therapy; 4)Do you regret for taking part in the clinical trial. The written survey was conducted in patients who completed therapy more than 24 months ago. On average questioning took place at 48 months after start of treatment. The effect of therapy on the immune response was tested by ex vivo measurement of an antigen-specific and mitogen-induced proliferation of mononuclear cells. Monitoring of immunological parameters was carried out before vaccination, after 2 courses of vaccines(i.e.,after 9 months) and in 6 months following completion of the vaccination (i.e., after 15 months).
Clinical protocol
After inclusion, patients underwent a standard blood effusion and blood samples obtained were used to prepare DC vaccines according to the protocol described below. The treatment consisted of 2 courses of antigen-specific therapy. The first course included 6 subcutaneous injections of DCs (5 × 106 cells per injection), loaded with recombinant HSV-1glicoprotein D (HSV1gD) with a 2-week interval (total duration – 3 months). The second course consisted of 4–6 vaccinations injected with a month interval (total duration – 4– 6 months). As adjuvant, we used human recombinant interleukin-2 (Roncoleukin, Biotech, St. Petersburg)administered subcutaneously in the dose of 250000 units. Vaccinations were delivered in 4 points in the interscapular region;interleukin-2 was administered in 4 points neighboring the vaccination site.
Vaccine preparation
DCs were generated from adherent fraction of peripheral blood mononuclear cells (MNCs). MNCs were isolated by Ficoll-Verographin (Sigma, Cat № F 8016) density-gradient centrifugation.The adhering to plastic MNCs were cultivated in tissue culture flasks (BD BiosciencesFalcon, Cat № 353110) for 3 d in RPMI-1640 medium (Sigma,Cat № R8758) in the presence of 40 ng/mlGM-CSF (Neostim, FDS Pharma LLP) and 1000 U/mlIFN-α (Roferon-A, F. Hoffmann-La Roche Ltd). For antigen loading, DCs were incubated for 1 hour with recombinant HSV1gD (ProSpec, Cat № HSV-221) in the dose of 5 µg/ml. After washing DCs underwent maturation by culturing with Azoximer bromide (Petrovaks; 2 ng/ml) for additional 24 hours. This water-soluble cationic polymer, which hasbeen specifically designed as vaccine adjuvantCitation27 activates anti-inflammatory signaling pathways and enhances antigen presentation and effector cell maturation.Citation28,29 Cells were then washed, harvested, aliquoted at concentration of 5 × 106 DCs/ml, frozen in phosphate buffer solution/10% DMSO (Sigma Aldrich, Cat № 276855) and stored in a freezer (Sanyo Ultra Low) at −80°C until use. The purity of DCs was assessed by flow cytometry analysis (FACSCalibur; Becton Dickinson) using anti-HLA-DR, anti-CD86 and lineage (anti-CD3+, anti-CD20+, anti-CD56+) monoclonal antibodies (Becton Dickinson). The obtained DCs contained not less than 70% of HLA-DR+, Lin- (CD3+, CD20+, CD56+) cells among which CD86+ cells comprised 50% and more. Sterility and viability (evaluated by trypan blue exclusion) was assessed prior each cell injection. The viability of thawed DCs in all cases exceeded 85%.
Cell mediated immunity
To assess antigen-specific T-cell responses, MNCs were cultured for 5 days in the presence and absence of HSV1gD (5 µg/ml). The proliferative response of MNCs to ConcanavalinA (15 µg/ml; Sigma Aldrich, Cat №C5275) was tested in 3-day cultures. The intensity of proliferation was assessed by Citation3Н-thymidine incorporation. Stimulation Index (IS) was calculated as the level of proliferation in cultures with antigen(mitogen)/the level of proliferation in cultures without antigen(mitogen).
Statistical analysis
We evaluated the data by means of Statistica 6.0 for Windows software package. To verify the differences in compared samples we used nonparametric criteria: Mann–Whitney (for non-linked samples) and Wilcoxon (for linked samples). The differences were considered valid if significance value was p<0.05. Correlation analysis was conducted by Spearmen's rank-order correlation (rS).
Abbreviations
| DC | = | dendritic cells |
| HSV-1 | = | herpes simplex virus-1infection |
| MNCs | = | peripheral blood mononuclear cells |
| gpD | = | glycoprotein D |
Disclosure of potential conflicts of interest
The authors have declared that no competing interests exist.
References
- Stanberry L, Cunningham A, Mindel A, Scott L, Spruance S, Aoki F, Lacey C. Prospects for control of herpes simplex virus disease through immunization. Clin Infect Dis 2000; 30:549-66; PMID:10722443; http://dx.doi.org/10.1086/313687
- Sancho-Shimizu V, Perez De Diego R, Jouanguy E, Zhang S, Casanova J. Inborn errors of anti-viral interferon immunity in humans. Curr Opin Virol 2011; 1:487-96; PMID:22347990
- Van Lint A, Ayers M, Brooks A, Coles R, Heath W, Carbone F. Herpes simplex virus-specific CD8+ T cells can clear established lytic infections from skin and nerves and can partially limit the early spread of virus after cutaneous inoculation. J Immunol 2004; 172:392-97; PMID:14688347; http://dx.doi.org/10.4049/jimmunol.172.1.392
- Zhu J, Peng T, Johnston C, Phasouk K, Kask A, Klock A. Immune surveillance by CD8-α,α+ skin-resident T cells in human herpes virus infection. Nature 2013; 497:494-97; PMID:23657257; http://dx.doi.org/10.1038/nature12110
- McKenna D, Neill W, Norval M. Herpes simplex virus specific immune responses in subjects with frequent and infrequent orofacial recrudescences. Br. J. Dermatol 2001; 144:459-64; PMID:11259999; http://dx.doi.org/10.1046/j.1365-2133.2001.04068.x
- Mysliwska J, Trzonkowski P, Bryl E, Lukaszuk K, Mysliwski A. Lower interleukin-2 and higher serum tumor necrosis factor levels are associated with perimenstrual, recurrent, facial herpes simplex infection in young women. Eur Cytokine Netw 2000; 11:397-406; PMID:11022124
- Zheltova O, Starostina N, Tikhonova M, Leplina O, Chernykh E, Ostanin A. Immunity features of patients with chronic recurrent infections. Immunology 2011; 4:205-9. (Russian).
- Cunningham A, Griffiths P, Leone P, Mindel A, Patel R, Stanberry L, Whitley R. Current management and recommendations for access to antiviral therapy of herpes labialis. J Clin Virol 2012; 53(1):6-11; PMID:21889905
- Rooney J, Straus S, Mannix M, Wohlenberg C, Alling D, Dumois J, Notkins A. Oral acyclovir to suppress frequently recurring herpes labialis. A double-blind, placebo-controlled trial. Ann Intern Med 1993; 18:268-72; PMID:8380540; http://dx.doi.org/10.7326/0003-4819-118-4-199302150-00004
- Chi C, Wang S, Delamere F, Wojnarowska F, Peters M, Kanjirath P. Interventions for prevention of herpes simplex labialis (cold sores on the lips). Cochrane Database Syst Rev 2015; 8:CD010095, doi: 10.1002/14651858; PMID:26252373
- BenMohamed L, Bertrand G, McNamara C, Gras-Masse H, Hammer J, Wechsler S, Nesburn A. Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity. J Virol 2003; 77:9463-73; PMID:12915561; http://dx.doi.org/10.1128/JVI.77.17.9463-9473.2003
- Bettahi I, Zhang X, Afifi R, BenMohamed L. Protective immunity to genital herpes simplex virus type 1 and type 2 provided by self-adjuvanting lipopeptides that drive dendritic cell maturation and elicit a polarized Th1 immune response. Viral Immunol 2006; 19:220-36; PMID:16817765; http://dx.doi.org/10.1089/vim.2006.19.220
- Bedoui S, Greyer M. The role of dendritic cells in immunity against primary herpes simplex virus infections. Front Microbiol 2014; 5:533; PMID:25374562; http://dx.doi.org/10.3389/fmicb.2014.00533
- Lee H, Zamora M, Linehan M, Iijima N, Gonzalez D, Haberman A, Iwasaki A. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J Exp Med 2009; 206:359-70; PMID:19153243; http://dx.doi.org/10.1084/jem.2008-0601
- Pollara G, Speidel K, Samady L, Rajpopat M, McGrath Y, Ledermann J, Coffin R, Katz D, Chain B. Herpes simplex virus infection of dendritic cells: balance among activation, inhibition, and immunity. J Infect Dis. 2003; 187:165-78; PMID:12552441; http://dx.doi.org/10.1086/367675
- Kobelt D, Lechmann M, Steinkasserer A. The interaction between dendritic cells and herpes simplex virus-1. Curr Top MicrobiolImmunol 2003; 276:145-61; PMID:12797447
- Bedoui S, Gebhardt T. Interaction between dendritic cells and T cells during peripheral virus infections: a role for antigen presentation beyond lymphoid organs. Curr Opin Immunol 2011; 23:124-30; PMID:21112755; http://dx.doi.org/10.1016/j.coi.2010.11.001
- Ghasemi M, Erturk M, Buruk K, Sonmez M. Induction of potent protection against acute and latent herpes simplex virus infection in mice vaccinated with dendritic cells. Cytotherapy 2013; 15:352-61; PMID:23579060; http://dx.doi.org/10.1016/j.jcyt.2012.11.012
- Dhodapkar M, Steinman R, Sapp M, Desai H, Fossella C, Krasovsky J, Donahoe S, Dunbar P, Cerundolo V, Nixon D, et al. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. JClinInvest 1999; 104:173-80; PMID:1041-1546
- Luo J, Li J, Chen R, Nie L, Huang J, Liu Z, Luo L, Yan X. Autologus dendritic cell vaccine for chronic hepatitis B carriers: A pilot, open label, clinical trial in human volunteers. Vaccine 2010; 28:2497-504; PMID:20117267; http://dx.doi.org/10.1016/j.vaccine.2010.01.038
- Zabaleta A, D'Avola D, Echeverria I, Llopiz D, Silva L, Villanueva L, Riezu-Boj J, Larrea E, Pereboev A, Lasarte JJ, et al. Clinical testing of a dendritic cell targeted therapeutic vaccine in patients with chronic hepatitis C virus infection. Mol Ther Methods Clin Dev 2015; 2:15006; PMID:26029717; http://dx.doi.org/10.1038/mtm.2015.6
- García F, Plana M, Climent N, Leon A, Gatell J, Gallart T. Dendritic cell based vaccines for HIV infection: the way ahead. Hum Vaccin Immunother 2013; 9:2445-52.
- Leplina O, Zheltova O, Borisova A, Starostina N, Ostanin A, Chernykh E. Dendritic cells vaccines in the treatment of herpetic infection. Vestnikural'skoimeditsinskoiakademicheskoinauki 2011; 35:38-39. (Russian).
- Della Bella S, Nicola S, Riva A, Biasin M, Clerici M, Villa M. Functional repertoire of dendritic cells generated in granulocyte macrophage-colony stimulating factor and interferon–α. J LeukocBiol 2004; 75:106-16; PMID:14525963; http://dx.doi.org/10.1189/jlb.0403154
- Korthals M, Safaian N, Kronenwett R, Maihofer D, Schott M, Papewalis C, Diaz Blanco E, Winter M, Czibere A, Haas R, et al. Monocyte derived dendritic cells generated by IFN-alpha acquire mature dendritic and natural killer cell properties as shown by gene expression analysis. J Transl Med. 2007; 5:46-57; PMID:17894866; http://dx.doi.org/10.1186/1479-5876-5-46
- Santini S, Pucchini T, Lapenta C, Parlato S, Logozzi M, Belardelli F. A new type 1 IFN-mediated pathway for the rapid differentiation of monocytes into highly active dendritic cells. Stem cells 2003; 21:357-62; PMID:12743330; http://dx.doi.org/10.1634/stemcells.21-3-357
- Kabanov V. From synthetic polyelectrolytes to polymer-subunit vaccines. Pure Appl Chem 2004; 76:1659-77; http://dx.doi.org/10.1351/pac200476091659
- Dyakonova V, Dambaeva S, Pinegin B, Khaitov R. Study of interaction between the polyoxidoniumimmu nomodulator and the human immune system cells. IntImmunopharmacol 2004; 4:1615-23; PMID:15454114; http://dx.doi.org/10.1016/j.intimp.2004.07.015
- Powell B, Andrianov A, Fusco P. Polyionic vaccine adjuvants: another look at aluminum salts and polyelectrolytes. ClinExp Vaccine Res. 2015; 4:23-45; PMID:25648619; http://dx.doi.org/10.7774/cevr.2015.4.1.23
- Chentoufi A, Zhang X, Lamberth K, Dasgupta G, Bettahi I, Nguyen A, Wu M, Zhu X, Mohebbi A, Buus S, et al. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J Immunol. 2008; 180(1):426-37; PMID:18097044; http://dx.doi.org/10.4049/jimmunol.180.1.426
