ABSTRACT
Tick-borne encephalitis (TBE) is an acute febrile illness with neurological manifestations that is prevalent in forested areas of moderate climate in Europe and Asia. TBE virus is transmitted by ticks and rarely by unpasteurized milk and dairy products. The disease burden is attributed mainly to resulting long-term disability, especially in individuals over 50 y of age. Currently, there is no causative treatment, but a very effective vaccination is available with a good safety profile. The vaccination requires 3 basic doses to be fully effective and regular boosters afterwards. An accelerated vaccination schedule enables a patient to reach reasonably protective titres within 3 to 4 weeks from the first injection. The risk of travel-related TBE is estimated to be less than the risk of acquiring typhoid fever while visiting highly endemic regions in South Asia, but more than the risk of acquiring Japanese encephalitis, meningococcal invasive disease, or rabies. The pre-travel risk assessment of acquiring TBE should consider known risk factors which include 1) the country and regions to be visited; 2) April to November season; 3) altitude less than 1500 m above the sea level; 4) duration of stay; 5) the extent of tick-exposure associated activities including leisure and professional outdoor activities within the endemic area; and 6) age and comorbidities of the traveler. A major challenge, however, is the very low awareness of the risk of contracting TBE in those who travel to industrialized European countries.
Introduction
Tick-borne encephalitis (TBE) is an arboviral infection prevalent in forested areas of moderate climate in Europe and Asia, which manifests as an acute febrile illness with neurological impairment. TBE is caused by a tick-borne encephalitis virus (TBEV), a member of the genus Flavivirus within the family Flaviviridae. There are 3 subtypes of the virus – European/Western, Siberian, and Far Eastern subtypes.Citation1
Reservoir and vectors
Humans are generally accidental hosts for the TBE virus, and usually become infected after a tick bite or rarely by consumption of unpasteurized milk and dairy products. The vectors and at the same time reservoirs for the virus are hard ticks, namely castor bean/sheep tick (Ixodes ricinus) in Central and Eastern Europe (), and taiga tick (Ixodes persulcatus) in Siberia and the Far East.Citation2 There is an overlap in distribution of both types of ticks in Scandinavia, the Baltic states, and the European part of Russia. The circulation of the virus in tick populations is maintained by co-feeding on small vertebrates. Rodents appear to have persistent viremia for days to weeks without developing any signs of illness and may represent an important reservoir of the virus in nature. The virus can infect all stadia of the ticks and remains with them for life, even through interstadial metamorphosis. Approximately 30% of TBE cases in people who report no history of tick bite may have become infected by hardly visible tick larvae or nymphs.Citation3
Figure 1. Life stages of Ixodes ricinus ticks: larvae- size 0.8 mm (A), nymphs – size 1.2×1.5 mm (B), adult male - size 1.5×2.5 mm (C), unfed female – size 2×4 mm (D), and fully engorged female 7×11 mm (E). (photo by Jan Erhart).
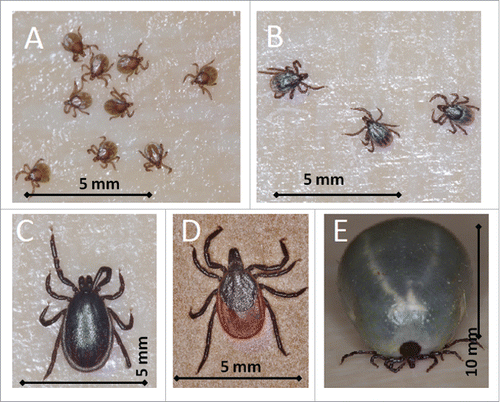
The prevalence of TBEV in ticks in endemic areas range from 0.5% to 5%, exceptionally up to 30%, and there are pockets and hotspots with very variable proportion of ticks carrying the TBEV even within the same geographical area. The highest density of ticks can be found in forested areas and grasslands although infected ticks have been recovered in some urban parks.Citation4 Overall, the risk of TBE infection after a single tick bite is estimated to be less than 1:100. Depending on the climate, there are one or two-peak seasonal patterns of TBE cases; from March to November.Citation1,5
Human epidemiology and endemic regions
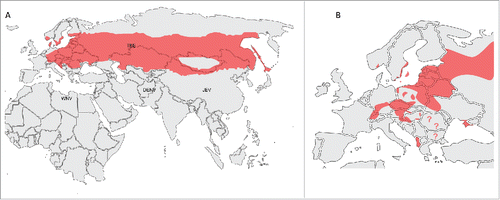
There are about 10,000 – 15,000 cases of TBE reported annually worldwide, Russia alone accounting for nearly half of this number.Citation3 Austria, southern Germany, the Czech Republic and Slovenia, along with Baltic States and southern parts of Scandinavia are the most affected regions in Europe. Endemic regions of TBE appear to have expanded recently, with Switzerland, north-eastern France and northern Italy now established as endemic locations. TBE cases are also reported from the Balkans as far as Greece. Belarus and Ukraine along with European part of Russia have endemic foci from St Petersburg in the north to Crimea in the south. In Asia, the whole of Siberia and Russian far east, northern Japan and 9 provinces in north west and north east of China constantly report cases of TBE (called locally Spring-summer meningoencephalitis in Russia and Forest encephalitis in China).Citation6 Natural TBEV foci are also present in Korea ().Citation7-8
Figure 2. Areas of TBEV distribution in the Eurasian region (A) with possible overlapping regions of other major flavivirues (WNV – West Nile virus, DENV – Dengue virus, JEV – Japanese encephalitis B virus). A map of TBEV distribution in Europe (B).
Reported incidence rates vary greatly between the countries and within their counties, from 0.7/100,000 in Austria to 55/100,000 in some regions of Latvia and 76/100,000 in Koroska region of Slovenia or similarly in Tomsk region of Russia.Citation3 In general, the notified cases have increased in all endemic countries in the last 20–30 y by an appalling 400%.Citation9 Therefore, one should assume the current risk for a traveler is significantly higher than has been in the recent past.Citation10
The incidence rates in resident populations cannot be extrapolated to assess the risk of infection in the unvaccinated traveler due to difference in exposure and very variable proportion of vaccinated population in various resident groups.
Unless diagnosed overseas or in non-endemic country, imported cases of TBE are usually not published, and therefore only a few cases have been published of TBE imported to the UK, the Netherlands, USA, and Australia.Citation11-14 There are a few dozen cases of TBE imported annually from one endemic country to other endemic country,Citation15 and based on this we could expect that many TBE cases may have gone undiagnosed or misdiagnosed with other infections, including other flaviviruses.Citation16
The uncertainty in quantification of TBE as travel risk has been very well documented in a recent review by SteffenCitation15 and only crude estimates of incidence and prevalence extrapolated from various data sources and populations are available, with both numerators and denominators being far from precise. Such estimates use the principle of “guesstimate” calculations pioneered by Enrico Fermi (1901–1954, Nobel Prize winner in physics in 1938). Fermi's assumptions generally provide rough estimates, within the range of one log, but in situations where no better data are available, they may be used as a benchmark to be validated or modified in future studies. This method has been successfully used in other estimates of disease burden to lay groundwork for future research.Citation17
Based on reported overnight stays, it has been estimated that about 60 international travelers visiting Austria may become infected with TBE every summer, a number equal to the reported TBE cases in all Austria residents, a country with very high vaccination coverage.Citation18-19 The risk for international travelers visiting central European region endemic for TBE is estimated to be similar to acquiring typhoid fever in highly endemic area or developing P. vivax malaria in travelers to India (1:3,000–1:25,000 travelers).Citation19 Another estimate positions the risk of travel-related TBE to be less than the risk of acquiring typhoid fever while visiting highly endemic regions in South Asia, but more than the risk of acquiring Japanese encephalitis, meningococcal invasive disease, or rabies.Citation15
Diagnostics
Diagnosis of TBE is mainly achieved by serological testing, usually by detection of TBEV-specific IgM and IgG antibodies by enzyme-linked immunosorbent assay (ELISA). The antibodies are usually detectable at the beginning of the neurological phase of TBE. Serology using ELISA in non-endemic countries or in patients with a travel history to countries endemic for other flaviviruses may prove a challenge as there is significant cross reactivity with other members of the Flavivirus genus, such as West Nile virus, Japanese encephalitis virus, dengue, etc. In patients previously vaccinated against Japanese encephalitis or yellow fever this may be misinterpreted as post-vaccination titres.Citation20-21 The most sensitive method, which can rule out false positive results from ELISA, is the virus neutralization test (VNT). However, strict biosafety regulations in a number of Western countries restrict the performance of VNT to laboratories equipped with a biosafety level 3 facility (biosafety level 4 in the United States); thus it is rarely performed during routine clinical diagnostics and largely unavailable in the non-endemic countries.Citation20
As a consequence, an alternative to a conventional TBEV VNT was developed based on the virus-like particles expressing a reporter gene. This assay does not require the work with life virus omitting the biosafety restrictions; however, it is not currently available for routine practice.Citation22
Detection of TBEV RNA in serum is limited to the viremic stage of the clinical disease in humans, usually before the onset of neurological symptoms. Thus this method is of limited importance because patients rarely seek medical advice during this phase of the illness. Recently, the presence of TBEV RNA was detected in urine in 2 of the 4 cases investigated during the neurological phase of TBE. This suggests that urine sampling for molecular detection of viral RNA during the neurological phase might represent a new diagnostic opportunity; however, this needs to be verified in a larger patient cohort.Citation23
Another challenge in diagnostics is the risk of dual infection, most frequently Borrelia burgdorferi and Anaplasma phagocytophilum, but also co-infection with Ehrlichia sp., Francisella tularensis, Coxiella burnetii, and rickettsiae have been reported.Citation24-25
Clinical presentation
There are 3 main clinical forms: meningitis, meningoencephalitis, and menigoencephalomyelitis (50%, 40%, and 10% of cases, respectively), and the fourth, rare but most devastating encephalomyeloradiculitis. Mortality ranges from 1% in central Europe to 5–20% in the Far East. Long term morbidity is significant, especially in the encephalitic and encephalomyelitic (and myeloradiculitic) forms, and may last from months to years, ranging from postencephalitic syndrome (cognitive impairment, poor concentration, headaches, fatigue, ataxia, neuropsychiatric disorders and mood disorders) to permanent palsies and seizures.Citation26-27
The disease burden estimate found an average of 3.1 disease-adjusted life-years (DALY) lost per every case, where majority (94%) of the disease burden were due to long-term or permanent neurological sequelae and only small portion due to mortality (5.5%) and a minimal fraction was due to acute illness (0.5%).Citation28
There are several features typical for TBE:Citation2,26,29
About a third of patients will not notice a tick bite. Mere exposure to outdoors in the endemic area within the incubation period of 7–28 d is sufficient to consider TBE;
About two thirds of cases have bi-phasic illness, with initial non-specific viremic illness that may show transaminitis, leukopenia and thrombocytopenia, followed by few days up to 2 weeks of complete resolution of symptoms before neurological impairment with high fevers develop;
At the onset of the second phase, leukocytes and CRP may be elevated, and serology may lag behind the clinical manifestation. Serology shows cross reactions with other flaviviruses in the IgG class;
Initial CSF studies may also reveal predominance of neutrophils and borderline lactate. Repeated lumbar puncture then shows shift to lymphocytic picture;
Brain regions mostly affected by the virus include basal ganglia, nucleus caudatus, brainstem, cerebellum and thalamus and, hence, ataxia including fine tremor with impaired balance and reduced level of consciousness are the main symptoms of encephalitis. Anterior horns of the spinal cord may be affected in the myelitic form resulting in polio-like disease with predominance of weakness in the proximal parts of the upper limbs. Imaging is usually unremarkable; however, T2 and turbo Flair sequences may show non-specific lesions in these regions;
Diagnosis is based on presence of clinical illness, proteo-cytological association in the cerebrospinal fluid and serological confirmation by either positive IgM or significant raise in IgG titres of TBE specific antibodies.
Management is only supportive, based on empirical experience and expert opinion, as there is only one case control study and no randomized trials at all. All other literature of TBE in humans includes case reports, case series and seroprevalence surveys.Citation19
Tick-bite prevention
Given the ecology of ticks and their habitats the highest risk behaviors in an endemic area include leisure outdoor activities including: camping, hiking, canoeing, biking, professional sports and activities of Scouts and Guides. Among professional exposure, the highest risk is encountered in woodcutting, forestry, farming, and military activities.
A universal vaccine against tick bites to cover all tick-borne diseases is still far away.Citation30 Therefore, traditional individual protective measures need to be taken, including avoidance of exposure, wearing long sleeves and long trousers, preferably impregnated with permethrin, and treatment of exposed skin with dimethylmetatoluamide (DEET)-containing repellent every 1–2 hours, along with frequent tick checks and rapid removal of ticks with tweezers, and avoidance of unpasteurized milk and dairy products.Citation31 However, all of these measures have limited effectiveness.Citation32
Vaccination
The only proven and reliable form of prevention is vaccination.Citation33 In regions with high vaccination coverage, there is 80–90% reduction of cases reported.Citation34
There are 4 types of vaccine available, only one of them approved in the UK and until recently in Canada, but not in the United States (TicoVac and TicoVac Junior, a version of FSME Immun and FSME Immun Junior) (). All four vaccines are cell cultured TBEV on chick fibroblasts, inactivated by formaldehyde, adjuvanted with aluminum hydroxide, administered by intramuscular injection. Excipients include gentamicin and neomycin. Allergic reaction to eggs or any of the excipients are also the only reported contraindications of the vaccine.Citation35
Table 1. Vaccination schedule.
The vaccines are in general well tolerated, with only local reaction at the site of injection and short-lasting raised temperature. No serious or life threatening side effects have been reported in clinical trials and in post-marketing monitoring.Citation36 The Western-subtype-derived vaccines induce protection for the other two, Siberian and Far Eastern subtypes.Citation37 Both vaccines based on the European strains are interchangeable during a course of vaccination.Citation38
Rapid or accelerated schedules for travelers are available depending on the choice and availability of TBE vaccine; the manufacturers recommend either an accelerated schedule of immunization on Day 0, Day 14 and Month 5–7, or rapid schedule of immunization on Day 0, Day 7 and Day 21. The effectiveness of the Western vaccines reaches over 90% in rapid/accelerated schedule after 2 doses and nearly 100% after 3 doses with rapid decline in titres requiring finishing full vaccination regimen. Therefore, there is a need to finish the full schedule – both in children and adults.Citation35,39-40
Risk stratification
Based on the available data, only those who are at risk of significant tick exposure should be vaccinated before traveling to the endemic area.Citation15 The pre-travel risk assessment should additively consider the following risk factors:
Factors of the environment
Region, country and region to be visited (see map of endemic areas, );
Season (high risk from April to November); and,
Lower altitude (less likely in altitude over 1500 m above the sea level).
Factors of the individual
Outdoor activity and its extent (both leisure and professional);
Duration of stay (the longer stay, the higher risk of an infected tick bite, as it is a stochastic event);
Higher age/comorbidities (as in people of higher age and/or with chronic conditions, TBE has higher mortality and more severe long-term consequences).Citation41
Apart from difficulty in estimating the real risk of acquiring TBE, there is another challenge; and it is the perception of safety when traveling to a European industrialized country. The majority of travelers may not seek travel advice prior to travel, or at least not early enough to be able to finish the course of vaccination.
Conclusion
The principal information a traveler should be advised of regarding TBE is that it is a febrile illness affecting the brain and spinal cord. It is transmitted by hard ticks in many countries of moderate climate in Europe and Asia. Unpasteurized milk and dairy products may also be a source of infection. The illness is rare in travelers, which may be partially due to misdiagnosis and underreporting. TBE has low mortality; however, there is a very high burden of long-term morbidity, especially neurological deficits. Tick avoidance is a particularly important factor when involved in outdoor activities, but the only proven prevention is vaccination. Vaccination may be administered in an accelerated schedule relatively shortly (several weeks) before travel. TBE vaccines are in general very effective and well tolerated.
There are various on-line resources of information concerning tick-borne diseases including TBE:
Abbreviations
| TBE | = | tick-borne encephalitis |
| TBEV | = | tick-borne encephalitis virus |
| ELISA | = | enzyme-linked immunosorbent assay |
| VNT | = | virus neutralization test |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The study has been supported by the Czech Science Foundation projects 14-29256S and 16-20054S, Ministry of Health of the Czech Republic, grant No. 16-34238A, and by project LO1218 with financial support from the Ministry of Education, Youth and Sports of the Czech Republic under the NPU I program.
References
- Růžek D, Dobler G, Donoso Mantke O. Tick-borne encephalitis: pathogenesis and clinical implications. Travel Med Infect Dis 2010; 8:223-32; http://dx.doi.org/10.1016/j.tmaid.2010.06.004
- Lindquist L, Vapalahti O. Tick-borne encephalitis. Lancet 2008; 371:1861-71; PMID:18514730; http://dx.doi.org/10.1016/S0140-6736(08)60800-4
- Amicizia D, Domnich A, Panatto D, Lai PL, Cristina ML, Avio U, Gasparini R. Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Hum Vaccin Immunother 2013; 9:1163-71; PMID:23377671; http://dx.doi.org/10.4161/hv.23802
- Rendi-Wagner P, Jeschko E, Kollaritsch H. Oesterreichische Expertengruppe für Reisemedizin. Travel vaccination recommendations for Central and Eastern European countries based on countryspecific risk profiles. Wien Klin Wochenschr 2005; 117(Suppl 4):11-9; PMID:16416380; http://dx.doi.org/10.1007/s00508-005-0442-8
- Kollaritsch H, Krasilnikov V, Holzmann H, Karganova G, Barrett A, Süss J, Pervikov Y, Bjorvatn B, Duclos P, Hombach J. Background document on vaccines and vaccination against tick-borne encephalitis (TBE) WHO Position Paper. Geneva: WHO Strategic Advisory Group of experts on immunization. Available at: http://www.who.int/immunization/sage/6_TBE_backgr_18_Mar_net_apr_2011.pdf, Last accessed June 2016
- Wu XB, Na RH, Wei SS, Zhu JS, Peng HJ. Distribution of tick-borne diseases in China. Parasit Vectors 2013; 6:119; PMID:23617899; http://dx.doi.org/10.1186/1756-3305-6-119
- Kim SY, Yun SM, Han MG, Lee IY, Lee NY, Jeong YE, Lee BC, Ju YR. Isolation of tick-borne encephalitis viruses from wild rodents, South Korea. Vector Borne Zoonotic Dis 2008; 8:7-13; PMID:18240970; http://dx.doi.org/10.1089/vbz.2006.0634
- Ko S, Kang JG, Kim SY, Kim HC, Klein TA, Chong ST, Sames WJ, Yun SM, Ju YR, Chae JS. Prevalence of tick-borne encephalitis virus in ticks from southern Korea. J Vet Sci 2010; 11:197-203; PMID:20706026; http://dx.doi.org/10.4142/jvs.2010.11.3.197
- European Centers for Disease Prevention and Control. Annual epidemiological report 2014 — emerging and vector borne diseases. Stockholm: ECDC, 2014:20-3.
- Kunze U. Is there a need for a travel vaccination against tick-borne encephalitis? Travel Med Infect Dis 2008; 6:380-3; PMID:18984485; http://dx.doi.org/10.1016/j.tmaid.2008.06.004
- Reusken C, Reimerink J, Verduin C, Sabbe L, Cleton N, Koopmans M. Case report: Tick-borne encephalitis in two Dutch travellers returning from Austria, Netherlands, July and August 2011. Euro Surveill 2011; 16(44):20003; PMID:22085619
- Centers for Disease Control and Prevention (CDC). Tick-borne encephalitis among US travelers to Europe and Asia - 2000–2009. MMWR Morb Mortal Wkly Rep 2010; 59:335-8; PMID:20339345
- Chaudhuri A, Růžek D. First documented case of imported tick-borne encephalitis in Australia. Intern Med J 2013; 43:93-6; PMID:23324091; http://dx.doi.org/10.1111/imj.12017
- Public Health England. http://webarchive.nationalarchives.gov.uk/20140714084352/ http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/TickborneEncephalitis/ Last accessed June 2016
- Steffen R. Epidemiology of tick-borne encephalitis (TBE) in international travellers to Western/Central Europe and conclusions on vaccination recommendations. J Travel Med 2016; 23(4):taw018; PMID:27087558
- Haditsch M, Kunze U. Tick-borne encephalitis: a disease neglected by travel medicine. Travel Med Infect Dis 2013; 11:295-300; PMID:23916617
- Chrdle A, Mallátová N, Vašáková M, Haber J, Denning DW. Burden of serious fungal infections in the Czech Republic. Mycoses 2015; 58 Suppl 5:6-14; PMID:26449501
- Kunze U. the ISW-TBE. Tick-borne encephalitis (TBE): Report of the 14th Annual Meeting of the International Scientific Working Group on Tick-Borne Encephalitis (ISWTBE). Ticks Tick Borne Dis 2012; 3:197-201; PMID:22765977
- European Centre for Disease Prevention and Control. Epidemiological situation of tick-borne encephalitis in the European Union and European Free Trade Association countries. Stockholm: ECDC; 2012.
- Ergunay K, Tkachev S, Kozlova I, Růžek D. A Review of Methods for Detecting Tick-Borne Encephalitis Virus Infection in Tick, Animal, and Human Specimens. Vector Borne Zoonotic Dis 2016; 16:4-12; PMID:26771446
- Mansfield KL, Horton DL, Johnson N, Li L, Barrett AD, Smith DJ, Galbraith SE, Solomon T, Fooks AR. Flavivirus-induced antibody cross-reactivity. J Gen Virol 2011; 92(Pt 12):2821-9; PMID:21900425
- Yoshii K, Ikawa A, Chiba Y, Omori Y, Maeda J, Murata R, Kariwa H, Takashima I. Establishment of a neutralization test involving reporter gene-expressing virus-like particles of tick-borne encephalitis virus. J Virol Methods 2009; 161:173-6; PMID:19481114
- Veje M, Studahl M, Norberg P, Roth A, Möbius U, Brink M, Bergström T. Detection of tick-borne encephalitis virus RNA in urine. J Clin Microbiol 2014; 52:4111-2; PMID:25165082
- Broeker M. Following a tick bite: double infections by tick-borne encephalitis virus and the spirochete Borrelia and other potential multiple infections. Zoonoses Public Health 2012; 59:176-80; PMID:21848518
- Lotric-Furlan S, Petrovec M, Avsic-Zupanc T, Strle F. Concomitant tickborne encephalitis and human granulocytic ehrlichiosis. Emerg Infect Dis 2005; 11:485-8; PMID:15757574
- Kaiser R. Tick-borne encephalitis: Clinical findings and prognosis in adults. Wien Med Wochenschr 2012; 162:239-43; PMID:22695809
- Rostasy K. Tick-borne encephalitis in children. Wien Med Wochenschr 2012; 162:244-7; PMID:22688618
- Šmit R, Postma MJ. The burden of tick-borne encephalitis in disability-adjusted life years (DALYs) for Slovenia. PLoS One 2015; 10:e0144988.
- Chmelik V, Bouzkova M, Houserova L, Zdvorak J, Sirkova I, Slamova I, Jerhotova Z, Trnovcova R, Chrdle A, Cihlova V, et al. Clinical picture of TBE; a retrospective study of 493 cases. Zentralbl Bakteriol 1999; 289:583-4.
- Sprong H, Trentelman J, Seemann I, Grubhoffer L, Rego RO, Hajdušek O, Kopáček P, Šíma R, Nijhof AM, Anguita J, et al. ANTIDotE: anti-tick vaccines to prevent tick-borne diseases in Europe. Parasit Vectors 2014; 7:77; PMID:24559082
- Jensenius M, Parola P, Raoult D. Threats to international travellers posed by tick-borne diseases. Travel Med Infect Dis 2006; 4:4-13; PMID:16887719
- Banzhoff A, Broeker M, Zent O. Protection against tick-borne encephalitis (TBE) for people living in and travelling to TBE-endemic areas. Travel Med Infect Dis 2008; 6:331-41; PMID:18984477
- Bogovic P, Strle F. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J Clin Cases 2015; 3:430-41; PMID:25984517
- Heinz FX. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine 2007; 25:7559-67; PMID:17869389
- TicoVac SP. Available at: https://www.medicines.org.uk/emc/medicine/30324, Last accessed June 2016
- Hombach J, Barrett AD, Kollaritsch H. Vaccines against tick-borne encephalitis. In Plotkin S, Orenstein W, Offit P (eds). Vaccine, 7th edn. USA: Elsevier INC, 2016 ( in press)
- Domnich A, Panatto D, Arbuzova EK, Signori A, Avio U, Gasparini R, Amicizia D. Immunogenicity against Far Eastern and Siberian subtypes of tick-borne encephalitis (TBE) virus elicited by the currently available vaccines based on the European subtype: systematic review and meta-analysis. Hum Vaccin Immunother 2014; 10:2819-33; PMID:25483679
- Prymula R, Pöllabauer EM, Pavlova BG, Löw-Baselli A, Fritsch S, Angermayr R, Geisberger A, Barrett PN, Ehrlich HJ. Antibody persistence after two vaccinations with either FSME-IMMUNR Junior or ENCEPURR Children followed by third vaccination with FSME-IMMUNR Junior. Hum Vaccin Immunother 2012; 8:736-42; PMID:22699436
- Schoendorf I, Ternak G, Oroszlan G, Nicolay U, Banzhoff A, Zent O. Tick-born encephalitis (TBE) vaccination in children: advantage of the rapid immunization schedule (i.e., days 0, 7, 21). Hum Vaccin 2007; 3:42-7; PMID:17297298
- Schondorf I, Beran J, Cizkova D, Lesna V, Banzhoff A, Zent O. Tick-borne encephalitis (TBE) vaccination: applying the most suitable vaccination schedule. Vaccine 2007; 25:1470-5; PMID:17196713
- Süss J, Kahl O, Aspöck H, Hartelt K, Vaheri A, Oehme R, Hasle G, Dautel H, Kunz C, Kupreviciene N, et al. Tick-borne encephalitis in the age of general mobility. Wien Med Wochenschr 2010; 160:94-100; PMID:20300927
