ABSTRACT
Objective: To evaluate the coverage and timeliness of seasonal influenza vaccine vaccination (SIV) among children aged 6 months to 3 years from the 2010–11 through the 2014–15 seasons. Methods: Children registered in Zhejiang Provincial Immunization Information System (ZJIIS) and needed 2 seasonal influenza vaccine doses in a given season from 2010–11 to 2014–15 were enrolled. Socio-economic information and SIV records of target children were extracted from ZJIIS on 1 January 2016. Any (≥1 dose) and full (2 doses) vaccination coverage by December 1 and March 31 as well as interval between 2 doses were calculated. Trends of coverage over time and determinants on fully vaccination coverage and interval between 2 doses were assessed. Results: Full SIV overage by Mar 31 increased from the 2010–11 to the 2014–15 seasons (2.60% vs 2.92%). Less than 1% of children received 2 doses by December 1. The interval between 2 doses among fully vaccinated children decreased over time (2010–11: 68.32 days; 2014–15: 49.51 days; p < 0.05). Age, socio-economic development level of resident areas were inversely associated with full vaccination coverage and resident children had a significantly higher full vaccination coverage. Younger age, resident children, receiving vaccination from higher service frequency clinics and clinics with morning and afternoon sessions were positive determinants of a shorter interval between 2 doses. Conclusion: Majority of children aged 6 months to 3 years remained at risk of incomplete and delayed SIV. The importance of the 2-dose SIV recommendation needs to be emphasized and effective interventions needs to be implemented to improve the completeness and the timeliness of SIV.
Introduction
Influenza virus is one of the major causes of morbidity and mortality worldwide. It is responsible for over 3 million cases of severe illness, including almost 500000 deaths annually.Citation1 Young children are particular vulnerable to influenza infection, resulting in severe illness, complication, and possibly even death.Citation2 Vaccinations have been shown to be the most cost-effective prevention measure to reduce viral infection, especially for young children. At present, the major strategy for preventing influenza and its complications is seasonal influenza vaccine vaccination (SIV), which is also recommended by the World Health Organization (WHO).Citation3 As circulating influenza virus strains can change from year to year, one or more antigens of seasonal influenza vaccine need to be changed to protect against influenza virus expected to cause disease in the upcoming season every year.Citation3 Hence, unlike other vaccinations, seasonal influenza vaccine needs to be given yearly. Based on these theories, Zhejiang provincial center for disease control and prevention (ZJCDC) recommends annual SIV for young children because of their high risk of serious complications due to influenza resulting in hospitalization or death.
Zhejiang is a developed province with a large population of 80 million located in East China. Only trivalent inactivated seasonal influenza vaccine (TIV), which contains 3 inactivated virus strains: type A(H1N1), type A(H3N2), and type B, is available in Zhejiang province since 2000. TIV is a category II (parent-pay) vaccine in China and the SIV is voluntary. To achieve high levels of vaccine-induced antibodies and reduce the risk of disease caused by virus strains that are antigenically similar to those strains included in TIV, ZJCDC specifically recommends that children aged 6 months to 3 years shall be given 2 doses of TIV separated by at least 28 days in each influenza season. Poor compliance with the 2-dose SIV schedule has been reported in other countries,Citation4-6 however, there are few reports from China and these published data are limited to selected ages and seasons. Therefore, a detailed description of SIV coverage for children aged 6 months to 3 years and spans of multiple seasons is necessary. Furthermore, the timeliness of SIV may be particularly important for effectiveness, ideally before the onset of influenza epidemic, while previous studies have traditionally described coverage by the early spring rather than the autumn.Citation7-9 Additionally, it has been indicated that migrant children are at a high risk of under or delayed vaccination in general,Citation10,11 but few studies have assessed completeness and timeliness of the 2-dose SIV schedule in this vulnerable population.
In this study, we used data from Zhejiang provincial Immunization Information System (ZJIIS) to evaluate the coverage and timeliness of SIV among children aged 6 months to 3 years from the 2010–11 through the 2014–15 seasons. The predictors of SIV from both socio-demographic and vaccination service providing aspects were also explored.
Results
A total of 8551791 children fulfilled 2 eligibility criteria from the 2010–11 to the 2014–15 seasons. The number of children aged 6 to 35 months for each season ranged from 1553978 in the 2010–11 season to 1837555 in the 2014–15 season. The socio-demographic characteristics of enrolled children, the service frequency and the service time of vaccination clinics that these children visited were shown in .
Table 1. Characteristics of enrolled children in influenza seasons from 2010–11 to 2014–15, Zhejiang province.
From the 2010–11 to the 2014–15 seasons, both any and full SIV coverage by Mar 31 increased significantly for all age groups (). Full vaccination coverage increased from 8.34% to 10.02% among 6–12 month age group, from1.86% to 2.16% among 13–24 month age group, from 0.09% to 0.15% among 25–36 month age group. After adjusting for age, any SIV coverage by Mar 31 increased significantly from 13.15% to 13.77% from the 2010–11 to the 2014–15 seasons, and full SIV coverage increased significantly from 2.59% to 2.92% in the same period. Most of the children lacked any or full SIV by Dec1, although both also improved over time. Specifically, any SIV coverage by Dec 1 increased significantly from 2.64% to 2.82% from the 2010–11 to the 2014–15 seasons, and full SIV coverage increased significantly from 0.65% to 0.81% in the same period (). For each season, SIV coverage was highest among children aged 6–12 months, followed by 13–24 months and 25–36 months (). Of children ultimately fully vaccinated by Mar 31, the interval between the 2 doses was significantly decreased from 68.32 days (95%CI: 66.14–70.83) in the 2010–11 season to 49.51 days (95%CI: 46.69–51.51) in the 2014–15 season (p < 0.05).
Figure 1. Any (≥1 dose) and full (2 doses) SIV vaccination coverage by Mar 31/ Dec 1 for influenza seasons from 2010–11 to 2014–15, Zhejiang province.
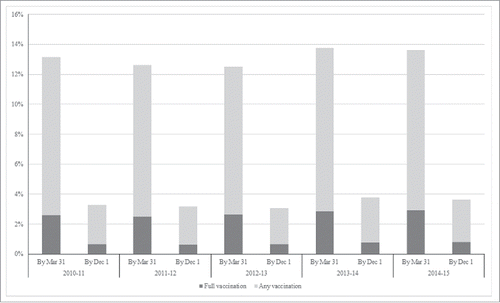
Table 2. Any (≥1 dose) and full (2 doses) SIV vaccination coverage by Mar 31 for influenza seasons from 2010–11 to 2014–15, Zhejiang province.
Age of children [25–36 months of age V.S. 6–12 months of age, AOR = 0.20(95% CI: 0.18–0.61)], socioeconomic development level of resident areas [high V.S. low, AOR = 1.35 (95% CI: 1.16–2.04)] were inversely associated with full SIV coverage, and resident children had a significantly higher full SIV coverage compared with migrant children [AOR = 1.87(95% CI: 1.25–2.72)]. Younger age [25–36 months of age V.S. 6–12 months of age, AOR = 0.70(95% CI: 0.47–0.85)], resident children [AOR = 1.31(95% CI: 1.16–2.07)], receiving vaccination from higher service frequency clinic [≥5 times per week V.S. ≤ 3 times per month, AOR = 1.60(95% CI: 1.29–2.17)] and clinic with morning and afternoon sessions[AOR = 2.05(95% CI: 1.65–2.97)] were positive determinants of a shorter interval between the 2 doses among fully vaccinated children ().
Table 3. Determinants associated with full SIV vaccination by Mar 31 and interval between two doses in the 2014–15 influenza season, Zhejiang province.
Discussion
Our study was the first evaluation of coverage of the 2-dose schedule SIV since the recommendations for universal SIV among children aged 6 months to 3 years were issued, based on data from ZJIIS during several influenza seasons. As we hypothesized but not verified before, the overall coverage of SIV, including any vaccination and full vaccination, among children aged 6 months to 3 years had significantly improved from the 2010–11 to the 2014–15 seasons, and these findings were consistent with trends of coverage of other vaccines whether included in the national immunization program (NIP) or not.Citation12 Our study revealed that less than 3% of eligible children had received full SIV by the end of each season, which was lower than that from other countries including the United States. For example, the coverage of SIV among children aged 6–23 months was 74.2%, 78.2% for the 2011–12 season and the 2012-13 seasons in the United States, respectively.Citation13 Moreover, we found missed opportunity of the second dose of TIV was relatively common as the coverage of partial SIV was over 10%. That meant the second dose was not received by roughly four-fifths of children who received an initial dose by each season.
Based on previous studies and our analysis on potential predictors, there were probably several reasons for under or incomplete SIV observed here. First, the complexity of SIV schedule may make it difficult for vaccination providers to identity which children need to receive 2 doses in each season. One investigation found that 40% vaccination nurses did not know that children should be administrated 2 doses of TIV.Citation14 Second, the knowledge on the importance and necessity of SIV is limited among parents. ShulerCitation15 noted that parents might not know the importance and appropriate time for the second dose of TIV. Another evidence suggested that parents from lower socio-economic development areas would be less likely to know the recommendations of SIV while be more likely to question the effectiveness and concern the safety of vaccine.Citation16 Our previous study in Zhejiang province found that children from a relative lower socio-economic development area tended to experience more drop outs for diphtheria-tetanus-pertussis combined vaccine.Citation12 These evidence might well explain the reason for a lower coverage of full SIV found in children from low socio-economic development areas. Third, parents may not be able or willing to overcome barriers, like missing work or school, for their children's SIV. According to our previous study,Citation17 we assumed this phenomenon would be more significant among migrant children, whose parents had not enough time for primary healthcare and were less aware of the information on vaccination. Furthermore, the poorer education level of migrant parents would also influence their awareness and capacity to seek and take advantage of vaccination service and other public health service.Citation18 Fourth, TIV is still a non-NIP vaccine and needs an out of pocket expense. Lower family income, especially for migrant family or family from low economic development areas, may restrict the expense on parent-pay vaccines including TIV. BlankCitation19 had verified the importance of free of charge policy when setting for goals of high vaccination coverage. We assume that a high SIV coverage will be expected if TIV is included in NIP. Fifth, system based factors like well-child visit and schedule of NIP vaccines may have an impact on SIV coverage. These factors disproportionately affect elder children as they had fewer health facility visits than the youngers. The elder children sometimes need a separate, special visit for SIV, while the younger children can get SIV at the same time of a well-child visit or receiving other vaccines. We assumed this was one of the possible reasons for a higher coverage of SIV observed in younger children. Lastly, the frequency of sessions and the service time of vaccination clinic also play important roles in SIV. Our previous studyCitation18 had demonstrated that frequent sessions and extending service time were the most beneficial interventions to working mothers and could improve the mothers' compliance with the vaccination schedule and allow for better coverage rates.
In this study, we found that the second dose was usually delayed far beyond the recommended interval of 28 days among fully vaccinated children. Furthermore, we found few children finished the 2-dose schedule before 1 Dec in each season, which was the shortest time to get adequate immunity against influenza. We also found age of children, frequency of session and service time of vaccination clinic were determinants for long interval of 2 doses of TIV. Plausible explanations for these findings might be similar to the reasons for fully SIV mentioned above. Compared with elder children, younger children had more opportunities to visit health facilities for public health service including vaccination. Besides, frequent session schedule and extending service time also made parents more convenient to bring their children for administering vaccines.
Our findings were particular concerning because high vaccination coverage and complete SIV were pivotal for establishing an adequate herd immunity and reducing incidence of influenza in community, especially among children who were at greater risk of infection and transmission compared with other subsets of population. Moreover, it was indicated that a high SIV coverage among children could prevent infections among household contacts and even reduce incidence rates among older individuals.Citation20 Continuously monitoring SIV coverage, in addition to full vaccination coverage, is an efficient measure to evaluate the success of SIV program and to provide evidence for policy making or taking interventions. As such, we suggest that provincial and local health departments, vaccination clinics take actions as followed. First, increasing the vaccination provider's awareness on the importance and necessity of administering 2 doses of TIV to adequately protect children from influenza is needed. It is also important to improve the provider's capacity of identifying eligible children for 2-dose schedule of TIV through training. Second, improving provider-parent communication on efficacy, safety, and 2-dose schedule of TIV may be valuable. Since people believe that vaccination providers are an authoritative source of information and their explanation is a good opportunity to correct misinformation and misunderstanding, eliminating suspicion about the quality and effectiveness of the vaccines, providers can make more informed decisions and more precise recommendations on a case-by-case basis for parents when necessary.Citation21 Third, effective interventions such as appointment at a more appropriate time, walk-in vaccination clinics, standing orders, extending service hours need to be implemented to improve timely completion of SIV. Using reminder-recall system supported by ZJIIS can promote timely receipt of TIV.Citation22 Reminder-recall system can remind providers about the number and timing of recommended doses based on the vaccination history, and generate reminder text messages for children who are due or overdue for vaccination. Fourth, as a recent study indicated that school vaccination clinics resulted in 88% of eligible children received 2 doses of TIV in the 2011–12 season,Citation23 an optimal SIV coverage might be anticipated in alternative vaccination settings.
This study had several limitations. First, our results were based on 10 cities and could not be generalized to the entire population of Zhejiang province. Second, since children not registered in ZJIIS were not enrolled in this study and they might have a lower coverage, we could have overestimated the coverage of SIV. Third, we did not evaluate the potential influence from the vaccine supply as these data were unavailable. Also, we could not explore other predictors for incomplete or delayed SIV mentioned in the previous studies as the limited demographic information extracted from ZJIIS.
Conclusion
This study illustrated incomplete and delayed SIV coverage for the majority of children aged 6 months to 3 years, leaving them susceptible to influenza infection. Steps should be taken to emphasize the importance of the 2-dose SIV recommendation to both providers and parents. Effective interventions such as extending service time, reminder-recall strategy, should also be implemented to maximize all opportunities to fully and timely vaccinate children against influenza.
Methods
Data source
Appropriately anonymized individual records of children, including SIV records were extracted from ZJIIS on 1 Jan 2016. ZJIIS is a computerized, population-based vaccination registration system, containing demographic information and vaccination records for children aged < 7 years living in Zhejiang province.Citation24,25 All vaccination clinics in Zhejiang province (except for Huzhou city) are included in ZJIIS. ZJIIS consists of client software deployed at each vaccination clinic and database deployed in ZJCDC. ZJIIS consolidates data from different vaccination clinics through the Internet and provides a tool for monitoring and evaluating the immunization program. Once any vaccination clinic in Zhejiang is visited for the first time, child will be registered in ZJIIS with a unique identification number. Demographic information (such as name, date of birth, gender, family address, phone number, immigration status) and vaccination records (including historical immunization records for migrant children) are collected by ZJIIS and these information will be updated in real time if there is any change.
Study areas
This study was performed in 10 cities (total of 11cities in Zhejiang Province) and the other city (Huzhou) was not included in this study as vaccination clinics in Huzhou did not participate in ZJIIS. These 10 cities were divided into 3 socio-economic strata (high, middle, and low) by Gross Domestic Product (GDP) per capita according to 2015 statistics from Zhejiang provincial bureau of statistics. Hangzhou (HZ), Ningbo (NB), Zhoushan (ZS) were classified as high level for GDP per capita >15000 USD; Shaoxing (SX), Jinhua (JH), Jiaxing (JX) were classified as middle level for GDP per capita between 10000 and 15000 USD; Taizhou (TZ), Quzhou (QZ), Lishui (LS), Wenzhou (WZ) were classified as low level for GDP per capita <10000 USD.
Target population
Influenza season was defined from Oct 1 through Mar 31 of the next year. For each season assessed (2010–11 through 2014–15), children were eligible for inclusion if they 1) were 6–35 months of age as of Oct 1 to ensure every child had opportunity to finish the 2-dose SIV schedule (for example, children born from 1 Nov 2007 to 1 Apr 2010 were eligible for inclusion of the 2010–11 season); and 2) registered in ZJIIS and not designated as ‘permanently inactive’ (i.e., deceased) or ‘moved or gone elsewhere’. Children could be included repeatedly in all the study seasons if they met these 2 criteria.
Definitions and outcomes
The coverage was defined as the proportion of vaccinated children among the target population. Doses of TIV were considered invalid if they 1) were administered earlier than 4 days prior to 6 months of age, or 2) were administered <28 days from the previous SIV. Invalid doses were excluded from the analysis.
Primary outcomes were any (≥1 dose) and full (2 doses) SIV coverage by Mar 31 for each study season. Additional outcomes included any and full SIV coverage by Dec 1 (given the minimum time period of receiving 2 doses of TIV), and the interval between 2 doses for fully vaccinated children by Mar 31.
Statistical analysis
The coverage rates of any and full SIV by Dec 1 and Mar 31 of the next year, along with 95% confidence interval (CI), were calculated for the 2010–11 through the 2014–15 seasons. Chi-square test was used to examine whether there was any difference in coverage during different seasons. For children fully vaccinated by Mar 31 of the next year, the means and 95% CI were calculated for interval between the 2 doses. Logistic regression model was used to explore the predictors of full SIV coverage by Mar 31, as well as its interval between the 2 doses, from both socio-demographic and vaccination service providing aspects in the 2014–15 season. Adjusted odds ratio (AOR) with 95% CI for each variable was also calculated. We performed all analysis with SAS (SAS Institute, Inc., Cary, NC, version 9.3) and at a significance level of 0.05.
Ethical considerations
This study was exempt from ethical review since it involved examination of de-identified data.
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest.
Author contributions
Yu Hu conceived and designed the experiments; Yaping Chen performed the experiments; Yu Hu analyzed the data; Bing Zhang contributed reagents/materials/analysis tools; Yu Hu wrote the paper.
Acknowledgments
The authors would like to thank Rui Wu from Suzhou Shensu Automatic Technology Co., Ltd. for providing assistance with the ZJIIS data.
References
- Collin N, de Radigues X, World Health Organization HNVTF. Vaccine production capacity for seasonal and pandemic (H1N1) 2009 influenza. Vaccine 2009; 27:5184-6; PMID:19563891; http://dx.doi.org/10.1016/j.vaccine.2009.06.034
- Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine 2002; 20:1831-6; PMID:11906772; http://dx.doi.org/10.1016/S0264-410X(02)00041-5
- Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec 2012; 87:461-76; PMID:23210147
- Jackson LA, Neuzil KM, Baggs J, Davis RL, Black S, Yamasaki KM, Belongia E, Zangwill KM, Mullooly J, Nordin J, et al. Compliance with the recommendations for 2 doses of trivalent inactivated influenza vaccine in children less than 9 years of age receiving influenza vaccine for the first time: a Vaccine Safety Datalink study. Pediatrics 2006; 118:2032-7; PMID:17079576; http://dx.doi.org/10.1542/peds.2006-1422
- Bhatt P, Block SL, Toback SL, Ambrose CS. A prospective observational study of US in-office pediatric influenza vaccination during the 2007 to 2009 influenza seasons: use and factors associated with increased vaccination rates. Clin Pediatr 2010; 49:954-63; PMID:20522609; http://dx.doi.org/10.1177/0009922810370868
- Pabst LJ, Fiore AE, Cullen KA. Completion of the 2-dose influenza vaccine series among children aged 6 to 59 months: immunization information system sentinel sites, 2007-2008 influenza season. Clin Pediatr 2011; 50:1068-70; PMID:21098532; http://dx.doi.org/10.1177/0009922810385928
- Jimenez-Garcia R, Esteban-Vasallo MD, Rodriguez-Rieiro C, Hernandez-Barrera V, Dominguez-Berjon MA, Carrasco Garrido P, et al. Coverage and predictors of vaccination against 2012/13 seasonal influenza in Madrid, Spain: analysis of population-based computerized immunization registries and clinical records. Hum Vaccin Immunother 2014; 10:449-55; PMID:24280728; http://dx.doi.org/10.4161/hv.27152
- Tuppin P, Samson S, Weill A, Ricordeau P, Allemand H. Seasonal influenza vaccination coverage in France during two influenza seasons (2007 and 2008) and during a context of pandemic influenza A(H1N1) in 2009. Vaccine 2011; 29:4632-7; PMID:21550376; http://dx.doi.org/10.1016/j.vaccine.2011.04.064
- Centers for Disease C, Prevention. Seasonal influenza vaccination coverage among children aged 6 months-18 years — eight immunization information system sentinel sites, United States, 2009-10 influenza season. MMWR Morb Mortal Wkly Rep 2010; 59:1266-9; PMID:20930704
- Chan TC, Hung IF, Cheng VC, Luk JK, Chu LW, Chan FH. Inadequate knowledge of pneumococcal vaccine of nursing home healthcare workers affects the pneumococcal vaccination uptake of older adults in nursing homes. J Am Geriatrics Soc 2013; 61:1429-30; PMID:23937501; http://dx.doi.org/10.1111/jgs.12384
- Han K, Zheng H, Huang Z, Qiu Q, Zeng H, Chen B, Xu J. Vaccination coverage and its determinants among migrant children in Guangdong, China. BMC Public Health 2014; 14:203; PMID:24568184; http://dx.doi.org/10.1186/1471-2458-14-203
- Hu Y, Chen Y, Guo J, Tang X, Shen L. Completeness and timeliness of vaccination and determinants for low and late uptake among young children in eastern China. Hum Vaccin Immunother 2014; 10:1408-15; PMID:24584000; http://dx.doi.org/10.4161/hv.28054
- Srivastav A, Zhai Y, Santibanez TA, Kahn KE, Smith PJ, Singleton JA. Influenza vaccination coverage of Vaccine for Children (VFC)-entitled versus privately insured children, United States, 2011-2013. Vaccine 2015; 33:3114-21; PMID:25979804; http://dx.doi.org/10.1016/j.vaccine.2015.04.098
- Dominguez SR, Daum RS. Physician knowledge and perspectives regarding influenza and influenza vaccination. Hum Vaccin 2005; 1:74-9; PMID:17038822; http://dx.doi.org/10.4161/hv.1.2.1604
- Shuler CM, Iwamoto M, Bridges CB, Marin M, Neeman R, Gargiullo P, Yoder TA, Keyserling HL, Terebuh PD. Vaccine effectiveness against medically attended, laboratory-confirmed influenza among children aged 6 to 59 months, 2003-2004. Pediatrics 2007; 119:e587-95; PMID:17332179; http://dx.doi.org/10.1542/peds.2006-1878
- Cohen B, Ferng YH, Wong-McLoughlin J, Jia H, Morse SS, Larson EL. Predictors of flu vaccination among urban Hispanic children and adults. J Epidemiol Commun Health 2012; 66:204-9; PMID:20881023; http://dx.doi.org/10.1136/jech.2009.099879
- Zhang J, Gao W, Yang X, Kang J, Zhang Y, Guo Q, Hu Y, Xia G, Kang Y. Tolerogenic vaccination reduced effector memory CD4 T cells and induced effector memory Treg cells for type I diabetes treatment. PloS one 2013; 8:e70056; PMID:23894591; http://dx.doi.org/10.1371/journal.pone.0070056
- Hu Y, Luo S, Tang X, Lou L, Chen Y, Guo J, Zhang B. Does introducing an immunization package of services for migrant children improve the coverage, service quality and understanding? An evidence from an intervention study among 1548 migrant children in eastern China. BMC Public Health 2015; 15:664; PMID:26173803; http://dx.doi.org/10.1186/s12889-015-1998-5
- Blank PR, Schwenkglenks M, Szucs TD. Vaccination coverage rates in eleven European countries during two consecutive influenza seasons. J Infect 2009; 58:446-58; PMID:19446340; http://dx.doi.org/10.1016/j.jinf.2009.04.001
- Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med 2001; 344:889-96; PMID:11259722; http://dx.doi.org/10.1056/NEJM200103223441204
- Lv M, Fang R, Wu J, Pang X, Deng Y, Lei T, Xie Z. The free vaccination policy of influenza in Beijing, China: The vaccine coverage and its associated factors. Vaccine 2016; 34:2135-40; PMID:26917011; http://dx.doi.org/10.1016/j.vaccine.2016.02.032
- Dombkowski KJ, Harrington LB, Dong S, Clark SJ. Seasonal influenza vaccination reminders for children with high-risk conditions: a registry-based randomized trial. Am J Preventive Med 2012; 42:71-5; PMID:22176850; http://dx.doi.org/10.1016/j.amepre.2011.09.028
- Cummings GE, Ruff E, Guthrie SH, Hoffmaster MA, Leitch LL, King JC, Jr. Successful use of volunteers to conduct school-located mass influenza vaccination clinics. Pediatrics 2012; 129 Suppl 2:S88-95; PMID:22383487; http://dx.doi.org/10.1542/peds.2011-0737H
- Li Q, Hu Y, Zhong Y, Chen Y, Tang X, Guo J, Shen L. Using the Immunization Information System to determine vaccination coverage rates among children aged 1-7 years: a report from Zhejiang Province, China. Int J Environ Res Public Health 2014; 11:2713-28; PMID:24603495; http://dx.doi.org/10.3390/ijerph110302713
- Hu Y, Chen Y, Zhang B, Li Q. An Evaluation of Voluntary Varicella Vaccination Coverage in Zhejiang Province, East China. Int J Environ Res Public Health 2016; 13:560.
