ABSTRACT
After WHO European Region determined the 2005 – 2010 Strategic Plan for measles elimination, the number of reported measles cases in Europe fell dramatically. This decrease is related to the vaccination strategy carried out by European countries. This extensive immunization strategy changes the epidemiological patter and could influence the effectiveness and the long-time immunogenicity of the vaccine.
To evaluate the long-time immunogenicity of the measles vaccine in the vaccination era, a pilot study among vaccinated blood donors in Apulia was designed. Of 174 enrolled patients, 93.7% presented an anti-measles IgG titer positive. GMT seems to increase by age (p = 0.001).
The GMT seems to increase by age and this could be related to the exposition to natural boosters, that was more probable before the beginning of universal mass vaccination against measles. Future studies have to focus the correlation between GMT and age.
After the implementation of the actions determined by the WHO European Region 2005 – 2010 Strategic Plan for measles elimination, the number of reported measles cases in Europe fell dramatically.Citation1 This decrease is related to the vaccination strategy carried out by European countries that implemented routine childhood immunization and, in some countries, supplementary immunization activities.Citation2
This extensive immunization strategy changed the epidemiological pattern with a 72% reduction of cases worldwide, from 146 to 40 per million inhabitantsCitation3. Immunization strategies could influence the effectiveness and the long-term immunogenicity of the vaccine, as recent studies have shown for pertussis.Citation4 In fact, long-term immunogenicity of the pertussis vaccine seems to have declined over time and the lack of the natural boosting could partially explain this decline.Citation5 An important reduction in the circulation of the measles virus has been observed and so, it is necessary to carry out a long-term immunogenicity study.
To evaluate the long-term immunogenicity of the measles vaccine in the vaccination era, we designed a pilot study among blood donors at Bari General Hospital located in Apulia. Apulia is a region in the South of Italy (with around 4 millions of inhabitants), where in 2002/2003 a large outbreak of measles (around 20.000 cases) was documented.Citation6 After this outbreak, a National Measles Elimination Plan was issued and provided the adoption of Universal Mass Vaccination. The immunization schedule provides free and active offer of 2 MMR vaccination doses, at the 13th month and at 5–6 y. Two doses of MMR vaccine (with a minimum interval of 4 weeks) are also offered to susceptible adolescents and adults. Immunization coverage in the region has progressively increased reaching 90% and an important decrease in the incidence of measles has been recorded. Despite the (remarkable) increase in immunization coverage, the target coverage has not been achieved and other outbreaks of measles continue to occur.Citation7-10
The study was carried out from May 2011 to June 2012 in the Department of Transfusion Medicine/Blood Bank of Policlinico General Hospital in Bari. Subjects (blood donors) were selected through a convenience sample and written and informed consent was required and obtained from all participants. Participants agreed to carry out additional serological tests (other than routine test for blood donation) and to have their data used in research. Tests were performed free of charge.
The protocol of the study has been approved by the Regional Committee for the Epidemiology (Osservatorio Epidemiologico Regione Puglia). In accordance with Apulian Regional Laws, permission from the Ethics Commitee to carry out this study was not necessary given that both data and sera from patients were collected for routine diagnostic testing. The research was carried out in accordance with the Helsinky declaration.
For each enrolled patient we collected a sample of serum of 5 ml. Anti-Measles IgG in collected sera were analyzed by chemiluminescence (CLIA), using LIAISON® Measles IgG, a semi-quantitative method. We analyzed reactive (positive) samples with a concentration of >16.5 UA/mL which was established by the manufacturer of the test in relation to the sensitivity of the used method. Equivocal test (near the cut off value) were retested; if the second test was also equivocal, it was classified as negative.
For each enrolled patient, we completed a standardized form reporting age, gender and the results of the laboratory test. Forms were anonymized (using initials of name and surname and birth data) and computerized using a database created by FileMaker Pro and data was analyzed by STATA MP12. Chi-square testing was used to compare the proportion of anti-measles IgG positive subjects by number of vaccine doses and multivariate analysis was performed to verify seropositivity rate and demographic data. We calculated the GMT of anti-measles IgG and we performed multivariate analysis to study the correlation between age and GMT. For all tests, a p value of <0.05 was considered as significant.
The study involved 174 blood donors, of which 102 (58.6%) were male, with an average age of 22.3 ± 4.4 (median 22, range 18–40 years).
All blood donors had been previously vaccinated with MMR or Measles monovalent vaccine; only 56 (32.2%) received 2 doses of the vaccine. The time elapsed between the last vaccination and blood sampling was of 6000.8 ± 2777 days.
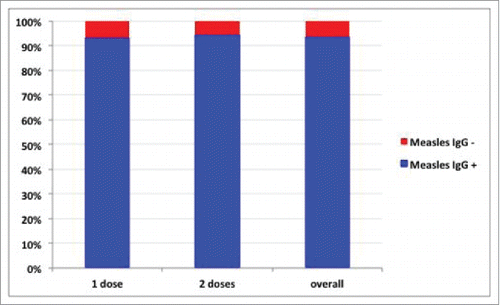
163 (93.7%; 95% CI = 89.0–96.8) enrolled subjects presented an anti-measles IgG titer positive; this proportion did not differ between persons who received one (110/118; 93.2%; 95% CI = 87.1–97) or 2 (53/56; 94.6%; 95% CI = 85.1–98.9) doses (chi-square = 0.12; p = 0.719; ). Multivariate analysis showed that the serological status (anti-Measles IgG+) was not associated with gender, age, number of vaccine doses, the time from the last dose of the vaccine or the serological test (p > 0.05).
The GMT was 2.1 ± 0.5 and multivariate analysis showed a correlation between the GMT and age (coef 0.03; t = 3.39; p = 0.001) but did not show correlations with gender, number of doses of the vaccine and the time from last dose of vaccine or serological test (p > 0.05).
In the literature, there were not several serosurveys of measles among vaccinated subjects. A study carried out in 2012 showed a seropositivity rate of 98.5% 3 y after immunization.Citation11
Another study, carried out in 2011 among 348 vaccinated children, showed a seropositivity rate of 90%.Citation12 In our study, despite the sample is less homogeneous, the time elapsed from vaccine and serological test is much longer than in these studies. The immunogenicity of the measles vaccine observed in our study is consistent with licensure data about MMR and our results seem consistent with the hypothesis that, in this epidemiological pattern, a booster doses of MMR is not needed among vaccinated persons.Citation13
The main limitation of this study is related to the study population composed by blood donors, above all men and in good health, coming from one blood bank only (even if it is the main important blood bank of the Apulia Region), so data could not be representative of the entire regional epidemiological pattern. Thus we are not able to exclude a higher percentage of susceptible people in some settings. e.g. migrants o sub-groups.
The GMT is higher in older persons, who were more likely to be exposed to natural boosters after vaccination, because of wild type measles virus circulation before the implementation of the measles universal vaccination strategy. there was an epidemic of the measles virus. What does this mean? Could GMT progressively decrease in correlation with the reduced circulation of the measles virus? To reach the measles elimination goal, this is a crucial question. Future studies are needed to answer this question and to focus the correlation between GMT and age.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- World Health Organization (WHO) Regional Office for Europe. Guidelines for measles and rubella outbreak investigation and response in the WHO European Region Copenhagen: WHO. Available from: http://www.euro.who.int/__data/assets/pdf_file/0003/217164/OutbreakGuidelines-updated.pdf?ua=1
- Martin R, Wassilak S, Emiroglu N, Uzicanin A, Deshesvoi S, Jankovic D, Goel A, Khetsuriani N. What will it take to achieve measles elimination in the World Health Organization European Region: progress from 2003–2009 and essential accelerated actions. J Infect Dis 2011; 204(Suppl 1):S325-34; PMID:21666181; http://dx.doi.org/10.1093/infdis/jir137
- Muscat M, Ben Mamou M, Shefer A, Jankovic D, Deshevoy S, Butler R. The State of Measles and Rubella in the WHO European region. Rev Esp Salud Publica 2015; 89(4):345-51; PMID:26580789; http://dx.doi.org/10.4321/S1135-57272015000400002
- Warfel JM, Edwards KM. Pertussis vaccines and the challenge of inducing durable immunity. Curr Opin Immunol 2015; 35:48-54; PMID:26091979; http://dx.doi.org/10.1016/j.coi.2015.05.008
- Ausiello CM, Lande R, Urbani F, la Sala A, Stefanelli P, Salmaso S, Mastrantonio P, Cassone A. Cell-mediated immune responses in four-year-old children after primary immunization with acellular pertussis vaccines. Infect Immun 1999; 67(8):4064-71; PMID:10417175
- Lopalco PL, Prato R, Pastore R, Martinelli D, Caputi G, Germinario C. Epidemiological analysis of measles in the Apulian region based on the use of current data sources. J Prev Med Hyg 2005; 46:132-8
- Prato R, Chironna M, Caputi G, Sallustio A, Martinelli D, Falco A, Germinario CA. An outbreak of measles in Apulia, Italy, November 2006 – January 2007. Euro Surveill 2007; 12(14):pii-3168
- Caputi G, Tafuri S, Chironna M, Martinelli D, Sallustio A, Falco A, Germinario CA, Prato R, Quarto M. An outbreak of measles including nosocomial transmission in Apulia, south-east Italy, January-March 2008 - a preliminary report. Euro Surveill 2008; 13(16):pii-18839
- Chironna M, Prato R, Sallustio A, Martinelli D, Germinario C, Lopalco P, Quarto M. Genetic characterization of measles virus strains isolated during an epidemic cluster in Puglia, Italy 2006–2007. Virol J 2007; 4:90; PMID:17888162; http://dx.doi.org/10.1186/1743-422X-4-90
- Cozza V, Chironna M, Leo C, Prato R. Letter to the editor: measles on the cruise ship: links with virus spreading into an emergency department in Southern Italy. Euro Surveill 2014; 19(19):pii-20800; http://dx.doi.org/10.2807/1560-7917.ES2014.19.19.20800
- Knuf M, Zepp F, Helm K, Maurer H, Prieler A, Kieninger-Baum D, Douha M, Willems P. Antibody persistence for 3 years following two doses of tetravalent measles-mumps-rubella-varicella vaccine in healthy children. Eur J Pediatr 2012; 171(3):463-70
- Paulke-Korinek M, Fischmeister G, Grac A, Rendi-Wagner P, Kundi M, Mohsenzadeh-Rabbani A, Moritz K, Fenninger B, Jarisch R, Jasinska J, et al. Persistence of antibodies in 4–8 year old Austrian children after vaccination with hexavalent DTaP-HBV-IPV/Hib and MMR vaccines. Vaccine 2011; 29(32):5130-6; PMID:21624412; http://dx.doi.org/10.1016/j.vaccine.2011.05.046
- Lee CY, Tang RB, Huang FY, Tang H, Huang LM, Bock HL. A new measles mumps rubella (MMR) vaccine: a randomized comparative trial for assessing the reactogenicity and immunogenicity of three consecutive production lots and comparison with a widely used MMR vaccine in measles primed children. Int J Infect Dis 2002; 6(3):202-9; PMID:12718836; http://dx.doi.org/10.1016/S1201-9712(02)90112-8