ABSTRACT
Background: Two kinds of regimens (2-1-1 and 1-1-1-1-1) can be selected after Zagreb regimen(2-1-1)of PVRV was officially approved in Beijing in January 2015. Up to now, the subjects for most studies about the comparison between Zagreb and Essen regimen are under 50 y old, rarely at and above. Aging of the immune system may result in decreasing efficacy of vaccination, especially for adults aged above 65–70 y. This study compared the safety and immunogenicity of the Zagreb and Essen regimen in Chinese adults aged 50 and above with the goal to provide a supplemental data for this age group. Methods: A total of 114 cases were divided into 2 groups randomly, received PVRV under the Zagreb and Essen regimens respectively. Serum samples were collected at D0, D7, D14, D42, D180 and D365 to determine the rabies serum neutralizing antibody by rapid fluorescent focus inhibition test (RFFIT). Safety analyses were made by comparing the AEs in day-3, day-7, and day-(7 + 21) in Zagreb or day-(7 + 28) in Essen by gender and age cohorts. Results: 617 blood samples were obtained. Two groups showed similar immunogenicity, the neutralizing antibody titer of all subjects at D14 and D42 showed >0.5 IU/ml. Under the same regimen, Subjects ≥65 y had lower GMC than those who <65 years from D7 to D365 within 2 groups. This difference was significantly shown on D7, D14, D180 in Zagreb group, and on D180 in Essen group (t = 2.38, p = 0.02; t = 3.78, p < 0.001; t = 2.30, p = 0.03; t = 4.42, p < 0.001). Subjects<65 years had higher seroconversion rate compared to ≥65 y on D7, D180 and D365 in both 2 groups, this difference was also significantly shown on D180, D365 in Zagreb group and on D180 in Essen group (χ2 = 20.66, p < 0.001; χ2 = 6.56, p = 0.02; χ2 = 10.96, p = 0.002). Two regimens all showed favorable performances with mildly or common adverse events (AEs). The incidence of local AEs after 3 d in Essen group was higher than Zagreb group (χ2 = 9.69, p = 0.002). The most common local AE was pain, the incidences (8.8%) in Zagreb group was higher than Essen group (8.4%, χ2 = 5.12, p = 0.02). All AEs for Zagreb group and 52.3% of AEs for Essen group occurred during the first 72 hours. During the first 72 hours, subjects aged <65 in Zagreb group (16.26%) had higher incidences of AEs than Essen group (8.57%, χ2 = 4.54, p = 0.03), males in Zagreb group (16.05%) had higher incidence of AEs than Essen group (5.71%, χ2 = 5.34, p = 0.02). The incidences of AEs close in during the first 7 d. Conclusion: The Zagreb and Essen regimens demonstrated the similar safety and efficacy of PVRV in Chinese adults aged 50 and above. People ≥65 y showed reduced immune response to both regimens. More AEs for the Zagreb regimen were observed within the first 72 hours, especially for male and people < 65 y.
Introduction
Rabies is a zoonotic disease effecting more than 100 countries and regions. About 60,000 people die of rabies each year, and most deaths occur in remote areas of developing countries in Asia and Africa.Citation1-2 It is estimated that about 327,000 people die of rabies in Asia and Africa each year if prophylactic vaccination is not given after exposure.Citation3 In 2010, 3 intramuscular PEP regimens were recommended by the WHO: the Essen IM regimen, a 5-dose regimen that involves the administration of one dose of the vaccine on D0, D3, D7, D14, and D28; the 2-1-1 regimen, a 4-dose regimen in which 2 doses of vaccine are administered on D0 (one dose at each of the 2 deltoids or the 2 thighs) followed by one dose of vaccine on D7 and D21; a new 4-dose regimen in which 4 doses of vaccine are administered intramuscularly on D0, D3, D7, and D14Citation4;. In China, 5-dose regimen has been used for many years. Each year, 12–15 million doses are given.Citation1,5 PVRV manufactured by Liaoning Cheng Da Co., Ltd. Shenyang, China, was approved in China in 2004, and approved with 2-1-1 regimen in Beijing in January 2015. Although the intramuscular PEP recommended for about 20 y by WHO, 2 kinds of regimens can be selected until 2015 in Beijing.
Up to now, subjects for most studies about the comparison of Zagreb and Essen regimen are under 50 y old, rarely at and above. Aging of the immune system begins at the level of the haematopoietic stem cell (HSC), results in the increased susceptibility to infectious disease and decreased efficacy of vaccination in elders,Citation6-7 especially for the older subjects aged above 65–70 y with reduced in the quality and quantity of immune responses.Citation8-9 In similar researches, which were observed that the antibody seroconversion rate of 2-1-1 regimen is higher than 5-dose regimen in people aged 60 or above,Citation10 Essen regimen is not favorable for older people aged 50 or above due to reduced immune response.Citation6,8,9 And, there was no data about this age group in Beijing, also age and gender factors are not considered at current vaccination programs.
This study compared the safety and immunogenicity of the Zagreb and Essen regimen in Chinese adults aged 50 and above with the goal to provide a supplemental data of this age group.
Results
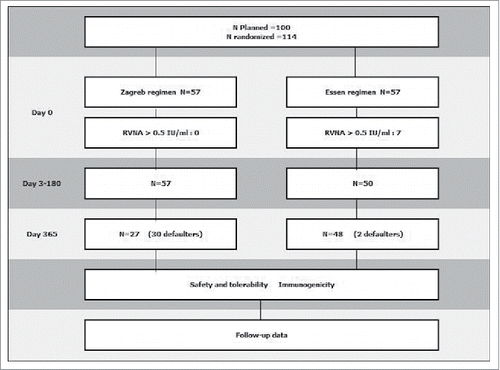
A total of 114 subjects were recruited and assigned to Zagreb and Essen regimens randomly. After first sampling (D0), 7 subjects were excluded from the study as their RVNA> 0.5 IU/ml. 107 subjects completed serum collection at 5 time points (D0-D180). As the serum collection at D365 was in Chinese Spring Festival vacation and the time interval was 180 days, only 75 subjects completed serum collection at the sixth time point ().
The homogeneity of age and gender of subjects were compared between 2 groups. As shown, the distributions of demography characteristics were homogeneous in Zagreb and Essen group ().
Table 1. Socio demographic characteristics of subjects.
Immunogenicity
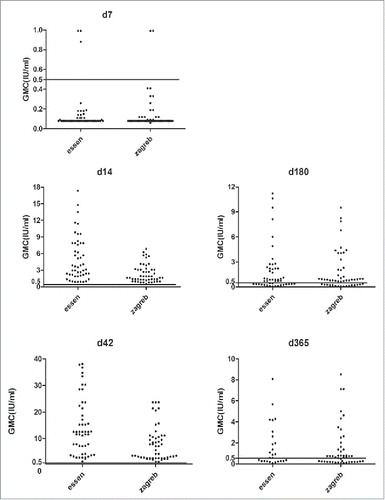
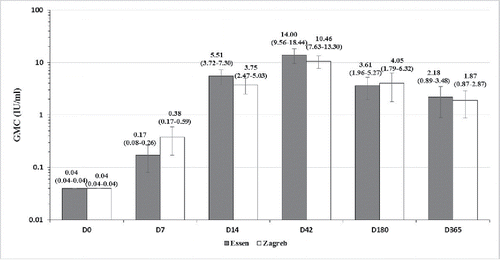
On D0, the geometric mean concentration (GMC) were ≤ 0.04 IU/ml of 107 subjects in 2 groups, indicating that 107 subjects reached the inclusion criterion. On D14, rabies virus neutralizing antibody (RVNA) levels in both group were higher than recommended level from WHO (>0.5 IU/ml),Citation11 the seroconversion rates were 100%. The ratio of GMC between Zagreb and Essen regimens (GMR) was 0.60, 95% confidence interval (95% CI): 0.54–0.66), which met the non-inferiority standard (lower limit of the 95% CI GMR >0.5). The non-inferiority was established. As administration of the dose on D7, antibody level increased significantly. On D42, the antibody level of more than 80% of subjects in both groups was 5 times as much as the standard level recommended by WHO. Subjects ≥ 65 y had lower GMC than <65 years from D7 to D365 in 2 groups, and on D7, D14, D180 in Zagreb group, D180 in Essen group are different significantly (t = 2.38, p = 0.02; t = 3.78, p < 0.001; t = 2.30, p = 0.03; t = 4.42, p < 0.001). Subjects<65 years had much higher seroconversion rate compared to ≥ 65 y on D7, D180 and D365 in 2 groups, D180, D365 in Zagreb group, D180 in Essen group which are different significantly (χ2 = 20.66, p < 0.001; χ2 = 6.56, p = 0.02; χ2 = 10.96, p = 0.002) (, ). The seroconversion rate of subjects ≥70 y on D7, D180 and D365 were 0% in both group, and were 100% on D14 and D42. No more comparison as small sample size for subjects 70 and above.
Table 2. Immunogenicity.
Table 3. Comparison of AEs incidences between 2 regimens.
Safety
Both regimens appeared safe and were tolerated by the subjects. No immediate adverse event developed within 30 minutes after each vaccination in 2 regimens. Both local and systemic AEs were common and mild in intensity, no severe adverse reaction. No AE caused any subjects to drop out.
The incidence of local AEs during 72 hours to the 7 d after the last vaccination in the Essen group was higher than in the Zagreb group (χ2 = 9.69, p = 0.002). The most common local AE was pain, the incidences (8.8%) in Zagreb group was higher than Essen group (8.4%) significantly (χ2 = 5.12, p = 0.02).
The incidences of systemic AEs in 72 hours were 4.67% of Zagreb group and 2.40% of Essen group (χ2 = 0.03, p = 0.86). The most common systemic AE was fatigue, with incidences of 3.51% in the Zagreb group and 3.60% in the Essen group (χ2 = 0.00, p = 0.96) (). All AEs for Zagreb group and 52.3% of AEs for Essen group occurred during the first 72 hours.
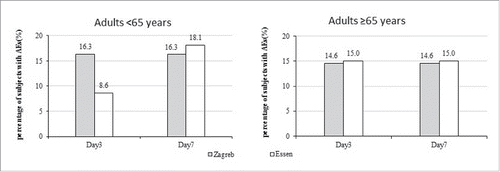
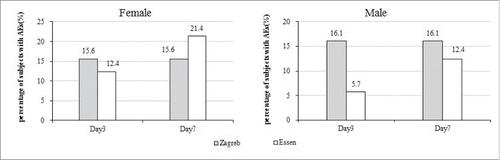
Comparing by age cohort, subjects aged <65 in Zagreb group (16.26%) had higher incidences of AEs than Essen group (8.57%) during 3 d after first vaccination (χ2 = 4.54, p = 0.03), the incidences of AEs close in during 7 d. No significant difference in adults ≥65 y (). Comparing by gender cohort, males in Zagreb group (16.05%) had higher incidence of AEs than Essen group (5.71%) during 3 d after first vaccination (χ2 = 5.34, p = 0.02), the incidences of AEs close in during 7 d ().
Discussion
Not only could the Zagreb regimen reduce medical expenses, but it also could increase patient compliance. Although Essen regimen could bring reliable PEP, many patients especially in some developing countries still could not afford it. To reduce cost and offer a simpler regimen than Essen, Zagreb regimen was recommended by WHO in 1992.Citation12 Rabies vaccine is classified as Class II vaccine in China, all paid by those being vaccinated. Essen and Zagreb regimen charge¥275 ($45) and ¥220 ($37) for one complete treatment, respectively. In 2015, with the same purpose Zagreb regimen of PVRV was approved by the regulatory authority in Beijing. Immunization cost directly affects compliance of subjects and drop-out rate. Low economic status was one of the 3 leading factors of delayed rabies immunization in India.Citation13 In Zhejiang, the compliance of Zagreb regimen was 92.38% higher than that of Essen regimen (84.39%) obviously, and in Beijing the compliance of I,II,III exposures to the Essen regimen were 33.3%, 77.1% and 78.0% respectively, the 4th and 5th dose were most commonly missed (day 14 and 28).Citation14-15 Increase with the number of dog raised in city and the rabies incidence of surrounding provinces, not only local rabies cases have occurred, the number of injuries by animals also presented a rising trend in Beijing since 2005. Although the rabies status of the offending animals with laboratory confirmation is <2.2%Citation16, dog rabies in Beijing following the strict implementation of dog ownership and population control laws has become rare indeed. There were 16 districts in Beijing. In 2015, the numbers of II, III exposures reported were 26326 and 10318 respectively in Chaoyang district, and 19.64% (7198) were victims whose age >50 y.
In this study, the non-inferiority was established, Zagreb and Essen regimen showed similar immunogenicity. Both regimens achieved 100% seroconversion rate on D14 and D42, and achieved RVNA concentrations ≥0.5 IU/mL, in excess of adequate titers as defined by the WHO. Compared with PCECV, PVRV achieved higher seroconversion rate on D14 and better protective effect in people aged ≥50 who received intramuscular administration,Citation6 similar results were also observed in people at other ages.Citation17-19 Reduced immune response was shown with the same regimen, the seroconversion and GMC of subjects ≥65 y were lower compared to 50–65 y from D7 to D365, and the GMC was under 0.5 IU/mL at D180 and D365. The antibody levels waned over time, and this decrease is greater among older individuals. For safety study, subjects showed well tolerance to the 2 regimens, with the similar incidence of AEs in people at the same age range.Citation20 There were more AEs for the Zagreb group within the first 72 hours, and pain was the most common local AE, because there were 2 doses on day-0. Males and people aged <65 showed more obviously. Further studies are needed to optimize the Zagreb and Essen regime in China.
The limitations of this study includes a small sample size, simulated exposure by volunteers and unfavorable bias caused by subjects with poor compliance during follow up period, due to the time conflict in Chinese Spring Festival vacation, which was within the one year's time required 6 required blood collection.
In conclusion, this study demonstrated a well performance in immunogenicity and safety in intramuscular vaccination to adults aged 50 and above with PVRV under both Essen and Zagreb regimen. Reduced immune response was shown in adults aged ≥ 65. More AEs for the Zagreb regimen within the first 72 hours, especially for male and people < 65 y. Further studies are needed to validate the characteristics of adults aged 50 and above under Zagreb and Essen regime in China.
Materials and methods
Study design
This study was conducted by Chaoyang Center for Disease Prevention and Control from January 2014 to January 2015. Antibody evaluation was performed by the National Institutes for Food and Drug Control (NIFDC). Recruitment, assignment, vaccination and follow-up were conducted in the rabies vaccination outpatient of Chaoyang District. This study was approved by the Ethics Committee of the Center for Disease Prevention and Control of Chaoyang District, Beijing. The informed consent form was approved and signed by all studied subjects. Our study was conducted in strict compliance with the Declaration of Helsinki, Good Clinical Practice (GCP) and local regulations.
According to associated studiesCitation4,20-21 and considering the feasibility of safety follow-ups and samplings for the people aged ≥50 years, the sample size was set at ≥100 and was approved by the Ethics Review board. A total of 114 subjects were enrolled and randomized to Zagreb and Essen regimen group, from January to March 2014.
Subjects
The subjects included in the study were those who were healthy, able to receive follow-ups after vaccination as schedule in the study protocol and signed the informed consent. Subjects were excluded from the study if any of the following criteria were met: history of animal bites; previous rabies vaccination; significant acute or chronic infectious disease; on steroids or immunosuppressive drugs; known or possible immunodeficiency or allergy to any composition of the vaccine; participating in other clinical study at the same time.
PEP vaccination regimens
The vaccines in this study were all commercially used, and have been approved by China Food and Drug Administration (CFDA) in 2004, and through 2 re-registration valid until 2017-12-31. The potency of the vaccine lots was determined by standard NIH potency tests, performed at NIFDC. The PVRV (Speeda®, lot200509001, Liaoning Cheng Da Co., Ltd., Shenyang, China) was produced by culturing Vero cells with rabies virus of the L. Pasteur vaccine strain PV2061, followed by inactivation by β-propiolactone, freeze-drying and re-dissolution in normal saline. The potency of the vaccines was 4.5 IU/dose, which fully fulfill the WHO recommendation for potency. The vaccines were administered intramuscularly to the deltoid muscle based on the regimen assigned after randomization: 2 doses on Day 1, and one dose on each of Days 7 and 21 for subjects in Zagreb regimen, and one dose on each of Days 1, 3, 7, 14, and 28 for subjects in Essen regimen. Zagreb regimen was initiated in Beijing in January 2015. Up to now, only purified chick-embryo cell vaccines (PCECV) and PVRV have been approved for the Zagreb 4-dose regimen in Beijing.
Immunogenicity assessment
Blood samples (about 3–5 ml each time) were collected at 6 time points (Day 0, 7, 14, 42, 180, 356, respectively), and prior to the vaccination on Day 0, 7, 14. The indicators for the assessment of immunogenicity included serums neutralizing antibody level and seroconversion. RFFIT was used to test the neutralizing antibody done by the NIFDC. The level of antibody of < 0.5 IU/ml before immunization was considered negative and the level of antibody of ≥0.5 IU/ml after immunization as positive. According to requirement of data cleaning, data would be excluded in the following cases: if the level of antibody before immunization was higher than that after immunization at any point of time; if the protective antibody before immunization was positive; if rabies immunoglobulin was used; if a combination of hormone drugs was used.
Safety assessment
During the study, any AEs were recorded for 30 minutes after each vaccination by investigators. Subjects were followed up for AEs up to 7 d after the last vaccination, safety followed-up data were recorded in individual case report forms, which collected local and systemic AEs. The local AEs include pain, induration, erythema, edema, tenderness, pruritus. Pain was defined as a severe reaction was disabling or limited motion. Erythema, hematoma or induration with a diameter > 5 cm, or continual pruritus at the injection site. Systemic AEs include fever (T≥ 38°C), myalgia, malaise, nausea, fatigue, loss of appetite and headache. Severe systemic reactions were defined as either fever ≥ 40°C or a severely disabling event resulting in the patient being confined to bed. The relativity of local and systemic AEs to the investigational vaccine was judged by the investigator.
Statistical analysis
The demographic data were used for descriptive statistical analysis, and the homogeneity was compared between groups using analysis of variance and Chi-square test. The serum antibody levels were compared between 2 groups after immunization using the t-test. GMC were calculated by exponentiating the least square means on D0, D7, D14, D42, D180 and D365. The seroconversion rate refers to the percentage of subjects whose antibody reached protective level after immunization (≥0.5 IU/ml). GMR and 95% CI were calculated on 6 time points. If the lower limit of GMR on D14 was greater than 0.5, the non-inferiority was established. The indicators for safety assessments included frequency distribution, composition ratio and percentage for descriptive analysis. The incidences were compared between groups, age and gender cohort. The statistical analysis was conducted by software SPSS (16.0).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was conducted by ChaoYang Center for Disease Prevention and Control. The rabies vaccines used in the study were provided by Chengda, company, Liaoning.
References
- World Health Organization. WHO Expert Consultation on Rabies, Second report. World Health Organ Tech Rep Ser 2013; (982):1-139, PMID:24069724.
- Wilde H, Lumlertdacha B, Meslin FX, Ghai S, Hemachudha T. Worldwide rabies deaths prevention–A focus on the current inadequacies in postexposure prophylaxis of animal bite victims. Vaccine 2016; 34(2):187-9; PMID:26626211; http://dx.doi.org/10.1016/j.vaccine.2015.11.036
- Knobel DL, Cleaveland S, Coleman PG, Fèvre EM, Meltzer MI, Miranda ME, Shaw A, Zinsstag J, Meslin FX. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ 2005; 83(5):360-8; PMID:15976877; http://dx.doi.org/S0042-96862005000500012
- Huang G, Liu H, Tang Q, Yu P, Shen X, Zhang Y, Liu X, Cao Q, Fu C, Liu B, et al. Making rabies prophylaxis more economical: immunogenicity and safety results from a preliminary study using a 2–1 intramuscular regimen in healthy volunteers. Hum Vaccin Immunother 2014; 10(1):114-9; PMID:24008819; http://dx.doi.org/10.4161/hv.26264
- Hu R, Tang Q, Tang J, Fooks AR. Rabies in China: an update. Vector Borne Zoonotic Dis 2009 Feb; 9(1):1-12. Epub 2008 Sep 21.Review; PMID:18803503; http://dx.doi.org/10.1089/vbz.2008.0046
- Dorrington MG, Bowdish DM. Immunosenescence and novel vaccination strategies for the elderly. Front Immunol 2013; 4:171. Print 2013; PMID:23825474.
- Dewan SK, Zheng SB, Xia SJ, Bill K. Senescent remodeling of the immune system and its contribution to the predisposition of the elderly to infections. Chin Med J (Engl) 2012; 125(18):3325-31; PMID:22964331.
- Leder K, Weller PF, Wilson ME. Travel vaccines and elderly persons: review of vaccines available in the United States. Clin Infect Dis 2001; 33(9):1553-66; PMID:11588700; http://dx.doi.org/10.1086/322968
- Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis 2008; 46(7):1078-84; PMID:18444828; http://dx.doi.org/10.1086/529197
- He YJ, Liu ZM, Zuo XW. Analysis and comparison of rabies vaccination effects by 5 dose immunization regimen and 2-1-1 immunization regimen. Experimental and Laboratory Medicine, 2014, 22(3):353-4.
- WHO Publication. Rabies vaccines: WHO position paper recommendations. Vaccine 2010; 28:7140-2; PMID:20831913; http://dx.doi.org/10.1016/j.vaccine.2010.08.082
- WHO Expert Committee on Rabies. Guide for post-exposure treatment. Eighth report. WHO technical report 824. Geneva Switzerland: World Health Organization 1992.
- Joseph J, N S, Khan AM, Rajoura OP. Determinants of delay in initiating post-exposure prophylaxis for rabies prevention among animal bite cases: hospital based study. Vaccine 2013; 32(1):74-7; PMID:24188758; http://dx.doi.org/10.1016/j.vaccine.2013.10.067
- He XF, Jiang Ll. Compliance comparison of vaccination procedures of Essen and Zagreb. Zhejiang J Prevent Med 2014, 26(5):490-1.
- Wang Cl, Zhang XW, Yu YX. Study on the compliance and economic cost of rabies vaccination. Chinese J Vaccines Immu 2010, 16(3):254-7.
- Lv YQ. A Survey on the Presence of Rabies Virus and Antibody Production in Response to Inactivated Vaccines in Dogs for Changping district, Beijing. Beijing: Chinese Academy of Agricultural Sciences, 2009: 1-33.
- Liu H, Huang G, Tang Q, Li J, Cao S, Fu C, Cao Q, Liu B, Pan H, Wang M. The immunogenicity and safety of vaccination with purified Vero cell rabies vaccine (PVRV) in China under a 2-1-1 regimen. Hum Vaccin 2011; 7(2):220-4; PMID:21311216
- Hu Q, Liu MQ, Zhu ZG, Zhu ZR, Lu S. Comparison of safety and immunogenicity of purified chick embryo cell vaccine using Zagreb and Essen regimens in patients with category II exposure in China. Hum Vaccine Immunotherapy 2014; 10:1645-9; PMID:24632727; http://dx.doi.org/10.4161/hv.28420
- Ma J, Wang H, Li J, Chang L, Xie Y, Liu Z, Malerczyk C. A randomized open-labeled study to demonstrate the non-inferiority of purified chick-embryo cell rabies vaccine administered in the Zagreb regimen (2-1-1) compared with the Essen regimen in Chinese adults. Hum Vaccine Immunotherapy 2014; 10(10):2805-12. Hum Vaccine Immunotherapy. 2015; 11(5):1295; PMID:25483635; http://dx.doi.org/10.4161/21645515.2014.972773
- Li R, Li Y, Wen S, Wen H, Nong Y, Mo Z, Xie F, Pellegrini M. Immunogenicity and safety of purified chick-embryo cell rabies vaccine under Zagreb 2-1-1 or 5-dose Essen regimen in Chinese children 6 to 17 years old and adults over 50 years: a randomized open-label study. Hum Vaccine Immunotherapy 2015; 11(2):435-42; PMID:25692350; http://dx.doi.org/10.4161/21645515.2014.994460
- Li R, Huang L, Li J, Mo Z, He B, Wang Y, Wu X, Minutello M, Guinet-Morlot F, Pichon S. A next-generation, serum-free, highly purified Vero cell rabies vaccine is safe and as immunogenic as the reference vaccine Verorab® when administered according to a post-exposure regimen in healthy children and adults in China. Vaccine 2013; 31(50):5940-7; PMID:24148575; http://dx.doi.org/10.1016/j.vaccine.2013.10.043