ABSTRACT
Background: Children with cystic fibrosis (CF) are at higher risk of severe respiratory syncytial virus (RSV) infection, which can lead to a decline in lung function. A monoclonal antibody, palivizumab (PMB), effectively prevents RSV hospitalizations; however, the high cost of PMB, approximately C$10,000 per patient per RSV season, limits its widespread use. We assess the cost-effectiveness of PMB prophylaxis in CF children less than 2 y of age from the Canadian healthcare payer's perspective. Methods: In 2014, a Markov cohort model of CF disease and infant RSV infections in the Canadian setting was developed based on literature data. Infants were treated with monthly PMB injections over the 5-month RSV season. Lifetime health outcomes, quality-adjusted life years (QALYs) and 2013 $CAD costs, discounted at 5%, were estimated. Findings are summarized as incremental cost-effectiveness ratios (ICERs) and budget impact. Deterministic sensitivity analysis was conducted to assess parameter uncertainty. Results: Implementation of a hypothetical Canadian RSV prophylaxis program resulted in ICERs of C$652,560 (all CF infants) and C$157,332 (high-risk CF infants) per QALY gained and an annual budget impact of C$1,400,000 (all CF infants) and C$285,000 (high-risk CF infants). The analysis was highly sensitive to the probability of severe RSV, the degree of lung deterioration following infection, and the cost of PMB. Conclusions: Our results suggest PMB is not cost-effective in Canada by commonly used thresholds. However, given the rarity of CF and relatively small budget impact, consideration may be given for the selective use of PMB for immunoprophylaxis of RSV in high-risk CF infants on a case-by-case scenario basis.
Introduction
Cystic fibrosis (CF) is the most common fatal genetic disease affecting Canadian children and young adults. In Canada, approximately 1 in 3,600 live births are affected with CF and the prevalence is approximately 12 per 100,000 population.Citation1 These rates are similar to those reported in other developed countries such as Australia.Citation2 Respiratory syncytial virus (RSV) is the leading viral cause of lower respiratory tract infection in infants and young children.Citation3 For children with CF, especially those with deteriorating lung function, RSV poses a particular threat.
Healthy children are at risk of RSV and associated complications, but the severity of RSV is intensified among high-risk groups. The risk of hospitalization from RSV in the first 2 y of life is 1–3% in otherwise healthy infants.Citation4 Children born prematurely with chronic lung disease, or congenital heart disease, have up to 10% risk of hospitalization.Citation5,6 Infants with CF are at higher risk of RSV hospitalization, with a recent meta-analysis reporting a hospitalization rate of 12.6%.Citation7-13
There is evidence that severe viral illnesses in children with CF have sustained pulmonary implications, with studies demonstrating higher acquisition of Pseudomonas aeruginosa,Citation14 and more rapid deterioration in lung function.Citation15
Risk factors that place a child at higher risk of severe RSV include: November-January birth month, >5 family members, small size for gestational age, attending day care and/or having school age siblings.Citation16 Approximately 20% of infants in the general population would be considered ‘high-risk’ for severe RSV disease using a high risk algorithm based on these criteria.Citation16
Palivizumab (PMB) is effective at preventing RSV hospitalization in infants with prematurity, chronic lung disease, and congenital heart disease.Citation5,6 PMB is a humanized murine monoclonal antibody given as intramuscular monthly injections of 15mg/kg over the 5-month RSV season. While the literature is limited and conflicting, the best available data from a study of 75 CF infants suggests a possible benefit from PMB.Citation9 A Cochrane review found no clinically significant benefits of PMB in infants with CF, but found only one randomized controlled trial with a small sample size to review.Citation17 Observational data on RSV hospitalization rates in infants with CF who have received PMB ranges from 1–2%,Citation7,12 which is substantially lower than estimates in those who have not received PMB.Citation7
Despite the limited direct evidence for efficacy of PMB in CF, the American Cystic Fibrosis Foundation recommends that PMB be considered for use in all infants with CF in the first 2 y of life.Citation18 The Canadian Pediatric Society and American Academy of Pediatrics do not routinely recommend PMB for children with CF, but both state that it may be considered if other RSV risk factors are present.Citation3,19 In a North American survey, 75% of responding respirologists said they prescribe PMB to infants with CF, and 40% considered it standard of care.Citation20
PMB has the potential to prevent early hospitalization, as well as potentially ameliorate some of the lung disease progression that leads to earlier morbidity and mortality in CF. However, the high cost of PMB, approximately $10,000 CAD per patient per RSV season, has limited its widespread use. The objective of this study is to assess the cost-effectiveness of PMB prophylaxis in CF children less than 2 y of age from the Canadian healthcare payer's perspective.
Results
Base case results
summarizes the findings in the model discounted at 5%, 3%, and undiscounted. For the ‘All CF’ population, estimated life expectancies (LE) per person, with and without PMB, were similar at 16.12 and 16.09 years, respectively (35.95 and 35.92 y undiscounted). The estimated QALYs per child were 14.99 QALYs with PMB prophylaxis versus 14.96 QALYs without PMB prophylaxis (32.82 and 32.72 QALYs undiscounted). The average cost of PMB per CF infant for 2 y was C$19,587.20. The discounted expected lifetime healthcare costs per CF patient were C$294,702 and C$275,125 in the PMB and no PMB groups respectively (C$801,085 and C$780,364 undiscounted). Therefore the ICER for PMB prophylaxis in All CF infants under 2 y of age was C$652,560 per QALY gained (C$207,207 per QALY gained undiscounted) ().
Table 1. Base case results for palivizumab treated infants compared to no palivizumab treated infants by risk for severe RSV disease.
Table 2. Variables used in the model along with associated ranges used for the sensitivity analyses.
Evaluating only CF infants considered at high risk for severe RSV disease, the estimated discounted LE per child was 16.29 and 16.16 for the PMB and no PMB groups, respectively (35.89 and 35.78 y undiscounted). The estimated QALYs gained per child were 14.92 and 14.81 discounted for the PMB and no PMB groups, respectively (32.62 and 32.27 undiscounted QALYs). The ICERs were more favorable in this population with a discounted ICER of C$157,332 per QALY gained and an undiscounted ICER of C$61,550 per QALY gained ().
In 2011, there were 145 CF infants under the age of 2 across Canada.Citation1 Assuming 20% are at high risk for severe RSV disease,Citation16 the absolute budget impact would be C$1,420,072 per year to administer PMB to All CF infants < 2 y of age, or C$284,014 to target only the HR CF infants.
Sensitivity analysis
We performed 1-way sensitivity analyses over the range of plausible values for all variables in the model (). Within both the HR and All CF groups, under nearly all scenarios, providing PMB resulted in small health benefits at high costs.
None of the sensitivity analyses resulted in an ICER less than $50,000 per QALY. If the cost of PMB decreased by 35%, the expected low for a generic biological medication,Citation34 then the ICER would drop to C$400,660 per QALY gained and C$89,815 per QALY gained for All CF and HR CF groups, respectively. RSV hospitalization rates, or severe infections, would need to approach 30% to be considered cost-effective at a threshold of $50,000 per QALY gained. Utilities, children's weights, and RSV hospitalization costs had little impact. The precise risk of CF-related lung progression following a severe RSV infection is unknown and thus a wide sensitivity analysis was used ranging from 1 (ie; none) to 15 times the risk of progression. The sensitivity analyses are summarized in the Tornado diagrams ().
Figure 1. Tornado diagram of the relative impact on the ICER of each of the variables included in the model for a) All CF infants and b) the high risk CF infants only. The x-axis represents the range of the ICER when the parameters are varied by the ranges shown in brackets. The vertical line indicates the base-case ICER for PMB immunoprophylaxis among a) All CF infants (C$652,560 per QALY gained) and b) the high risk CF infants (C$157,332 per QALY gained).
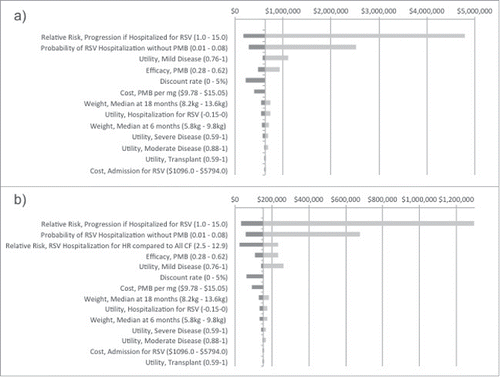
Figure 2. Two-way sensitivity analyses of they key parameters identified in one-way sensitivity analyses for the high-risk CF infants. Analyses were conducted using net benefit with a willingness to pay threshold of $50,000 per QALY. These graphs show the change in the optimal decision strategy over values of 2 variables: A) probability of RSV hospitalization vs. relative risk of progression if hospitalized with RSV, B) probability of RSV hospitalization versus effectiveness of PMB, C) cost of PMB per mg of body weight vs. PMB effectiveness, and D) cost of PMB per mg body weight versus probability of RSV hospitalization. The light gray region represents combinations of the variables for which the “No PMB” strategy is preferred while the dark gray region represents combinations of the variables for which the “PMB” strategy is preferred. The dotted lines represent the base-case values used in the analyses.
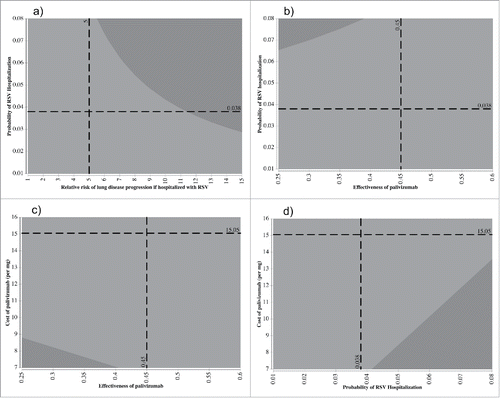
Figure 3. Model schematic for both the high risk and All CF groups. (HS1 = Mild Disease (FEV1 ≥ 70%), HS2 = Moderate Disease (40% ≤ FEV1 <70%), HS3 = Severe Disease 3 (FEV1 < 40%), HS4 = Heart and/or Lung Transplant).
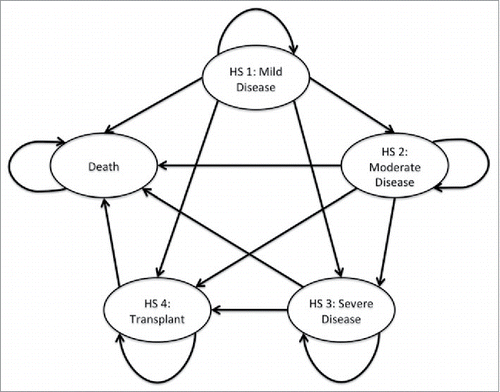
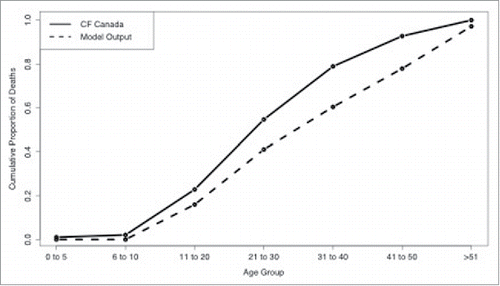
Figure 4. Comparison between model output (dashed line) and CF Canada statistics Citation41 (solid line) on cumulative probability of death for each age group.
The two-way sensitivity analyses do not result in plausible combinations of parameter values where the PMB strategy is cost-effective in “All CF” at a threshold of $50,000 per QALY gained. However, in the HR CF group, varying the relative risk of progression and the probability of RSV hospitalization simultaneously resulted in PMB prophylaxis being cost-effective if the relative risk of progression was 8 or greater and the risk of RSV hospitalization was ≥ 5.4%, both of which were within the range of plausible values (). There were small fluctuations from varying PMB effectiveness between 38% and 72% (); however, combining variables with lower costs of PMB demonstrates significant improvements in cost-effectiveness ().
Discussion
Our analysis indicates that administering PMB to all infants with CF results is not considered cost-effective in Canada under commonly used thresholds. By restricting use to CF infants with other RSV risk factors, PMB was still not considered cost-effective but was much closer to commonly used thresholds. The ICERs calculated were approximately C$650,000 per QALY for All infants with CF and C$160,000 per QALY for HR infants with CF.
The most important variables in the model were the relative risk of lung disease progression following a severe RSV infection, the probability of an infant with CF having severe RSV, and the cost of PMB. A 20–35% decrease in cost is expected with generic production of biologic medications, such as PMB.Citation34 In this scenario the ICERs would significantly decrease to approximately C$400,000/QALY and C$90,000/QALY in All CF and HR CF infants, respectively. In Canada, such a biologic is covered by the provincial health insurance plans. In other settings, where palivizumab may be available at a higher cost to private insurers, the ICER may be higher than calculated here.
There are a number of important limitations to consider when interpreting the results of this analysis. Reliable FEV1 data were not available in children under 8 y of age, therefore health states were extrapolated from 8-year-old children to those under 8 y of age. While this extrapolation is meant to be conservative, without reliable FEV1 data in this age group, our model and results are limited by this assumption. Future studies incorporating infant pulmonary function testing may provide much needed objective measures of pulmonary progression in young children with CF.
Similarly, data on severe RSV infection is limited in infants with CF. Hospitalization was used as a proxy of severity; however, it is likely that these values underestimate the true burden of infection, potentially underestimating the benefits of PMB. As emphasis for ambulatory care increases in patients with CF, alternative markers of severity, aside from hospitalization, should be evaluated. In addition, quantifying the risk of progression is challenging. Multiple studies suggest there is a deterioration in pulmonary function testsCitation14,15,25 and increased pseudomonas acquisitionCitation24 following significant lower respiratory tract infections in children with CF. The analysis assumes that preventing these infections would slow this deterioration, though there is no direct evidence supporting this. As CF is a progressive disease characterized by deteriorating lung function, lifetime costs are extremely high. Delaying progression of lung disease using PMB in the first 2 y of life may not contribute substantially to offsetting the overall costs in a patient's lifetime, a result reflected by the high ICERs calculated in our model.
There are no controlled trial data defining the effectiveness of PMB in CF and we assumed similar effectiveness to premature infants with chronic lung disease. Our model and results are limited by this assumption; however, our sensitivity analysis showed limited impact of varying PMB effectiveness within the 95% confidence intervals demonstrated from this trial.Citation5 Clinical trials on RSV infections in children with CF, the associated deterioration in lung function, and the effectiveness of palivizumab would add precision to the estimate of the cost-effectiveness. However, since a previously attempted randomized controlled trial on PMB in CF infants lacked power to demonstrate outcomes, our analysis provides important cost-estimates to policy makers and physicians considering PMB in this population.Citation13,35
The analysis was conducted from the Canadian health-care payer perspective and does not include travel time and lost productivity, which would potentially improve cost-effectiveness.
This model has a number of strengths. To the best of our knowledge, this is the first cost-effectiveness analysis evaluating PMB for use in CF. Given that CF is a relatively rare disease, PMB has been difficult to study in controlled trials. PMB is recommended by most respirologists surveyed Citation20 and the American Cystic Fibrosis Foundation;Citation18 it is therefore important to understand the financial and health care implications of implementing routine use. This model provides cost-effectiveness estimates based on the best available data and we hope it can guide clinicians and policy makers to consider selective use of the medication in order to optimize the health benefits while minimizing costs.
Decision analyses have been published on PMB use in other infant populations: premature infants, late-preterm infants, infants with congenital heart disease and chronic lung disease, as well as in infants living in remote towns.Citation36-38 While the studies found PMB to be cost-effective with ICERs ranging from C$7,000 per QALYCitation38 to C$24,750 per QALYCitation36 in select patient populations, they tended to estimate ICERs out of the cost-effective range for more general patient populations. One study calculated ICERs for low risk infants at around C$180,000 per QALY, and high risk infants at C$7,000 per QALY.Citation38 These models utilized different and more simplistic methodologies making direct comparisons challenging.
Our Markov model incorporates CF-related costs specific to the age and health state of the patient. Further, CF disease progression is explicitly incorporated. Potential benefits related to slower progression, including delaying transplantation, improved QALYs, and reduced costs can thus be estimated. We did not have detailed Canadian data available on a lifetime cohort of CF patients. However, the published Australian cohort used in this study had detailed cost and outcome data on a cross-sectional cohort, which was similar in demographic characteristics to the Canadian CF population.Citation21,22 Finally, our model follows the cohort over their entire lifetime, allowing fluctuations in health states at yearly intervals to provide the most accurate cost-effectiveness estimate.
Commonly used cost-effectiveness thresholds such as the $50,000 per QALY gained have been challenged as arbitrary and not generalizable across different health care settings.Citation39 It is therefore important to consider any implementation of costly therapies within the local context as opposed to adhering to strict thresholds. It is important to note that our analyses are relevant in the Canadian context, and may not be generalizable to other populations.
In summary, our results suggest that PMB is not cost-effective under commonly used thresholds. More research and better knowledge of the impact of RSV infections on CF progression and a potentially lower future price for PMB may change this conclusion. However, given the arbitrary nature of these thresholds, the rarity of CF, and the relatively small absolute budget impact, consideration may be given for selective use of PMB for immunoprophylaxis of RSV in high-risk CF infants.
Methods
We created a decision analytic model to assess the cost-effectiveness of PMB prophylaxis in CF children less than 2 y of age compared to no PMB prophylaxis from the Canadian health-care payer's perspective. Health outcomes included life years and quality-adjusted life years (QALYs). Costs included all publicly-funded healthcare costs: RSV prophylaxis and treatment costs for RSV infection and CF. Primary outcomes were QALYs, costs (2013 Canadian dollars (C$), where C$1 is US$0.95), incremental cost-effectiveness ratios (ICERs), assessed at a willingness to pay threshold of $50,000 per QALY gained, and absolute budget impact. ICERs were calculated as the difference in cost between the intervention and control group divided by the difference in effectiveness (QALYs) in the intervention and control group. QALYs and cost were discounted at 5% but also presented using discount rates of 0% and 3%.
Model design
We developed a Markov cohort model of CF disease and infant RSV infections. The CF disease history was comprised of 5 distinct health states representing the levels of lung function, consistent with another published CF Markov model ().Citation21 Health states included mild disease (forced expiratory volume in 1 second (FEV1) ≥ 70% predicted), moderate disease (40% ≤ FEV1 < 70%), severe disease (FEV1<40%), heart and/or lung transplant, and death. Disease progression was assumed to occur only in a forward direction, where no individuals were able to recover from their current health state.Citation21 We used a cycle length of one year.
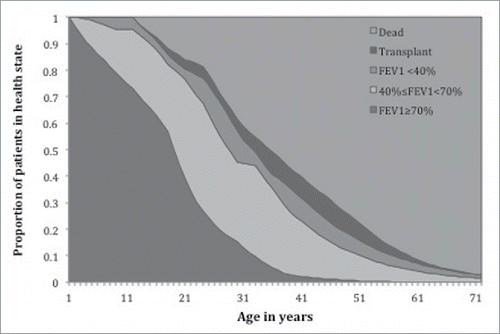
In the absence of Canadian data, health state transition probabilities were adapted from a 2003–2005 Australian registry cohort study.Citation21 These data were thought to be a good proxy for Canada as both countries have similar CF age distributions and universal health care.Citation22 Australian data were available for patients 8–45 y. Therefore we extrapolated from 8 y of age to birth and from 45 y of age to death. We assumed that transition probabilities for children <8 y of age were the same as for the 8 y olds from the Australian cohort.Citation21 We inserted transition probabilities for those > 45 y of age, and compared this to the Canadian age distribution, and median survival, of CF patients.Citation1 Survival and health state proportions in our model were validated against published data from the Canadian CF foundation registry by visually and statistically comparing graphs of health states and survival.Citation1
Two patient populations were considered: All CF patients (the “All CF” group) and high-risk CF patients only (the “HR” group), as defined using the Paes et al. 2009 risk scoring tool moderate and high-risk categories.Citation16
Key parameters
RSV hospitalizations
Only RSV hospitalizations in the first 2 y of life were considered in the model given that RSV prophylaxis is only indicated in this age group, and would not impact RSV hospitalizations in older children. The safety and effectiveness of PMB has not been studied in children over 2 y of age.Citation23 If hospitalized, it was assumed that CF pulmonary disease would progress faster than if the patient had not been hospitalized based on past studies citing a drop in FEV1 for respiratory exacerbations.Citation24,25 The baseline risk of RSV hospitalizations without PMB was estimated as the weighted average of the hazard rates of RSV hospitalizations among children with CF.Citation9,13,26-28
Palivizumab effectiveness was based on a previous randomized controlled trial reporting a 55% reduction in RSV hospitalizations in premature infants.Citation5 The risk of severe RSV was assumed to be higher for the HR group than for the All CF group, as has been demonstrated in premature infants.Citation16 Hospitalization was considered the proxy for severe RSV disease. We estimated a 3.6 times increased risk of hospitalization, assuming 20% of CF infants would have a moderate to high RSV risk score.Citation16
Utilities
We derived utility estimates, or the preference for specific health states, for CF from a study where a standard gamble approach among adolescents with CF aged 12–18 y was used.Citation29 Heart and/or lung transplant utilities were derived from a cost-effectiveness analysis of transplantation which also used the standard gamble approach.Citation30 We derived the disutility of RSV hospitalization from a study in premature infants.Citation31
Costs
The cost for RSV prophylaxis, provided by the pharmacy at the Hospital for Sick Children, Toronto, Ontario, was C$1,505/100mg vial (PMB from MedImmune). Because infants with CF visit the clinic frequently, no additional physician visits were assumed to receive PMB. RSV associated hospitalization costs were estimated from the Ontario Case Costing Initiative (OCCI) which collects case cost data for acute inpatient, day surgery and ambulatory care cases, as well as complex continuing care, rehabilitation, mental health and community care access centers cases.Citation32 An additional 5% was added to OCCI to account for physician costs based on previous studies analyzing health administrative data.Citation33 Due to the lack of Canadian specific data, we obtained costs relating to the different health states for each age from a published Australian cohort.Citation21 The yearly costs included CF-related hospitalizations, prescription medications, dietary supplements, clinical visits, oxygen therapy, home-IV therapy, laboratory testing, and costs associated with organ transplantation.Citation21 All costs were converted into 2013 Canadian Dollars using conversion rates as of November 15, 2013. All cost estimates, parameter values, and ranges for the sensitivity analysis are shown in .
Analysis
Outcomes analyzed include life expectancy, QALYs, costs, ICERs, and absolute budget impact. Separate analyses were conducted for the ALL CF group and the HR group. Similar to other Canadian cost-effectiveness studies, a willingness-to-pay threshold of $50,000 per QALY was chosen as a lower boundary of cost-effectiveness. However, understanding the arbitrary nature of this threshold, resulting ICERs should be considered in the local context of cost-effectiveness.Citation39
Deterministic sensitivity analyses were performed to explore parameter uncertainty and to determine threshold values at which the optimal strategy changes. Where data for a parameter was limited, wide ranges of input values were assessed in the sensitivity analysis (). One-way sensitivity analyses varied each parameter separately over plausible ranges. Two-way sensitivity analysis was conducted for clinically meaningful parameters that were found to have the greatest impact in one-way sensitivity analysis. Ranges for the sensitivity analysis were derived from the literature and can be found in .
Validation of the all CF population
The model was found to be a good representation of the true population dynamics of CF in Canada (Fisher's Exact Test, p-value = 0.8832). The model predicted cumulative probability of death for different age groups with the CF Canada data shows a good fit in the Canadian context (). Similarly, the health state distributions by age () suggest that this model reflects the underlying population dynamics of CF in Canada.Citation1 Relatively lower proportions of adults in our model were in health state 1 compared to CF Canada data and more transplants were performed in the Australian cohort than in Canada.
Abbreviations
| RSV | = | respiratory syncytial virus |
| PMB | = | palivizumab |
| CF | = | cystic fibrosis |
| QALY | = | quality-adjusted life year |
| ICER | = | incremental cost effectiveness ratio |
| FEV1 | = | forced expiratory volume in 1 second |
| LE | = | life expectancy |
Disclosure of potential conflicts of interest
The authors have no conflicts of interest to disclose.
Supplementary files
Download MS Word (23.4 KB)References
- The Canadian Cystic Fibrosis Registry: 2011 Annual Report. Cystic Fibrosis Canada. 2011.
- Cystic Fibrosis in Australia 2012: 15th Annual Report from the Australian Cystic Fibrosis Data Registry. The Thoracic Society of Australia and New Zealand.
- Robinson J. Preventing respiratory syncytial virus infections. Paediatr Child Health 2011; 16(8):488-90.
- Eriksson M, Bennet R, Rotzen-Ostlund M, von Sydow M, Wirgart BZ. Population-based rates of severe respiratory syncytial virus infection in children with and without risk factors, and outcome in a tertiary care setting. Acta Paediatr 2002; 91(5):593-8; PMID:12113331; http://dx.doi.org/10.1111/j.1651-2227.2002.tb03282.x
- Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics 1998; 102(3 Pt 1):531-7.
- Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH Jr, Connor EM, Sondheimer HM; Cardiac Synagis Study Group. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003; 143(4):532-40; PMID:14571236; http://dx.doi.org/10.1067/S0022-3476(03)00454-2
- Sanchez-Solis M, Gartner S, Bosch-Gimenez V, Garcia-Marcos L. Is palivizumab effective as a prophylaxis of respiratory syncytial virus infections in cystic fibrosis patients? A meta-analysis. Allergol Immunopath 2015; 43(3):298-303; http://dx.doi.org/10.1016/j.aller.2013.09.003
- Speer ME, Fernandes CJ, Boron M, Groothuis JR. Use of palivizumab for prevention of hospitalization as a result of respiratory syncytial virus in infants with cystic fibrosis. Pediatr Infect Dis J. 2008; 27(6):559-61; PMID:18434935; http://dx.doi.org/10.1097/INF.0b013e3181673c15
- Giebels K, Marcotte J-E, Podoba J, Rousseau C, Denis MH, Fauvel V, Laberge S. Prophylaxis against respiratory syncytial virus in young children with cystic fibrosis. Pediatr Pulmonol 2008; 43(2):169-74; PMID:18085710; http://dx.doi.org/10.1002/ppul.20751
- McCormick J, Southern KW. A survey of palivizumab for infants with cystic fibrosis in the UK. Arch Dis Chil 2007; 92(1):87-8; PMID:17185451; http://dx.doi.org/10.1136/adc.2006.0105338
- Sorrentino M, Powers T, Grp POS. Effectiveness of palivizumab: evaluation of outcomes from the 1998 to 1999 respiratory syncytial virus season. Pediatr Infect Dis J. 2000; 19(11):1068-71; PMID:11099087; http://dx.doi.org/10.1097/00006454-200011000-00007
- Paes B, Mitchell I, Li A, Lanctot KL. Respiratory hospitalizations and respiratory syncytial virus prophylaxis in special populations. Eur J Pediatr 2012; 171(5):833-41; PMID:22203430; http://dx.doi.org/10.1007/s00431-011-1654-8
- Cohen A, Boron M. C. D. A phase IV study of the safety of Synagis (Palivizumab) for prophylaxis of respiratory syncytial virus disease in children with cystic fibrosis. American Thoracis Society International Conference; 2005; San Diego, CA.
- Armstrong D, Grimwood K, Carlin JB, Carzino R, Hull J, Olinsky A, Phelan PD. Severe viral respiratory infections in infants with cystic fibrosis. Pediatr Pulmonol 1998; 26(6):371-9; PMID:9888211; http://dx.doi.org/10.1002/(SICI)1099-0496(199812)26:6%3c371::AID-PPUL1%3e3.0.CO;2-N
- Wang EE, Prober CG, Manson B, Corey M, Levison H. Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. N Engl J Med 1984; 311(26):1653-8; PMID:6504106; http://dx.doi.org/10.1056/NEJM198412273112602
- Paes B, Steele S, Janes M, Pinelli J. Risk-Scoring Tool for respiratory syncytial virus prophylaxis in premature infants born at 33–35 completed weeks' gestational age in Canada. Curr Med Res Opin 2009; 25(7):1585-91; PMID:19469698; http://dx.doi.org/10.1185/03007990902929112
- Robinson KA, Odelola OA, Saldanha IJ. Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis. Cochrane Database Syst Rev 2014; 5:CD007743; PMID:24851825
- Borowitz D, Robinson KA, Rosenfeld M, Rosenfeld M, Davis SD, Sabadosa KA, Spear SL, Michel SH, Parad RB, White TB, et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr 2009; 155(6 Suppl):S73-93; PMID:19914445; http://dx.doi.org/10.1016/j.jpeds.2009.09.001
- Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014; 134(2):415-20; PMID:25070315; http://dx.doi.org/10.1542/peds.2014-1665
- Giusti R. North American synagis prophylaxis survey. Pediatr Pulmonol 2009; 44(1):96-98; PMID:19085922; http://dx.doi.org/10.1002/ppul.20922
- van Gool K, Norman R, Delatycki MB, Hall J, Massie J. Understanding the costs of care for cystic fibrosis: an analysis by age and health state. Value Health 2013; 16(2):345-55; PMID:23538187; http://dx.doi.org/10.1016/j.jval.2012.12.003
- Cystic Fibrosis Data Network: National and International Cystic Fibrosis Data Reports. 2013; http://www.cysticfibrosisdata.org/Reports.htm. Accessed June 12, 2015.
- Synagis prescribing information. 2014; http://www.medimmune.com/docs/default-source/pdfs/prescribing-information-for-synagis.pdf. Accessed June 12 2015.
- Collinson J, Nicholson KG, Cancio E, Ashman J, Ireland DC, Hammersley V, Kent J, O'Callaghan C. Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax 1996; 51(11):1115-22; PMID:8958895; http://dx.doi.org/10.1136/thx.51.11.1115
- Smyth AR, Smyth RL, Tong CY, Hart CA, Heaf DP. Effect of respiratory virus infections including rhinovirus on clinical status in cystic fibrosis. Arch Dis Child 1995; 73(2):117-20; PMID:7574853; http://dx.doi.org/10.1136/adc.73.2.117
- Abman SH, Ogle JW, Butler-Simon N, Rumack CM, Accurso FJ. Role of respiratory syncytial virus in early hospitalizations for respiratory distress of young infants with cystic fibrosis. J Pediatr 1988; 113(5):826-30; PMID:3183835; http://dx.doi.org/10.1016/S0022-3476(88)80008-8
- Hiatt PW, Grace SC, Kozinetz CA, Raboudi SH, Treece DG, Taber LH, Piedra PA. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics 1999; 103(3):619-26; PMID:10049966; http://dx.doi.org/10.1542/peds.103.3.619
- Garcia DF, Hiatt PW, Jewell A, Schoonover SL, Cron SG, Riggs M, Grace S, Oermann CM, Piedra PA. Human metapneumovirus and respiratory syncytial virus infections in older children with cystic fibrosis. Pediatr Pulmonol 2007; 42(1):66-74; PMID:17123316; http://dx.doi.org/10.1002/ppul.20546
- Yi MS, Britto MT, Wilmott RW, Kotagal UR, Eckman MH, Nielson DW, Kociela VL, Tsevat J. Health values of adolescents with cystic fibrosis. J Pediatr 2003; 142(2):133-40; PMID:12584533; http://dx.doi.org/10.1067/mpd.2003.51
- Ramsey SD, Patrick DL, Albert RK, Larson EB, Wood DE, Raghu G. The cost-effectiveness of lung transplantation. A pilot study. University of Washington Medical Center Lung Transplant Study Group. Chest 1995; 108(6):1594-601; PMID:7497767; http://dx.doi.org/10.1378/chest.108.6.1594
- Chirico G, Ravasio R, Sbarigia U. Cost-utility analysis of palivizumab in Italy: results from a simulation model in the prophylaxis of respiratory syncytial virus infection (RSV) among high-risk preterm infants. Ital J Pediatr 2009; 35(1):4; PMID:19490659; http://dx.doi.org/10.1186/1824-7288-35-4
- The Ontario Case Costing Initiative. http://www.occp.com/mainPage.htm. Accessed Nov 15, 2013.
- Sander B, Kwong JC, Bauch CT, Maetzel A, McGeer A, Raboud JM, Krahn M. Economic appraisal of ontario's universal influenza immunization program: A cost-utility analysis. PLoS Med 2010; 7(4):e1000256; PMID:20386727; http://dx.doi.org/10.1371/journal.pmed.1000256
- Calo-Fernandez B, Martinez-Hurtado JL. Biosimilars: company strategies to capture value from the biologics market. Pharmaceuticals (Basel). 2012; 5(12):1393-408; PMID:24281342
- Robinson KA, Odelola OA, Saldanha I, McKoy N. Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis. Cochrane Database Syst Rev 2010; (2):CD007743; PMID:20166098
- Tam DY, Banerji A, Paes BA, Hui C, Tarride JE, Lanctot KL. The cost effectiveness of palivizumab in term Inuit infants in the Eastern Canadian Arctic. J Med Econ 2009; 12(4):361-70; PMID:19900071; http://dx.doi.org/10.3111/13696990903442155
- Lanctot KL, Masoud ST, Paes BA, Tarride JE, Chiu A, Hui C, Francis PL, Oh PI. The cost-effectiveness of palivizumab for respiratory syncytial virus prophylaxis in premature infants with a gestational age of 32–35 weeks: a Canadian-based analysis. Curr Med Res Opin 2008; 24(11):3223-37; PMID:18928643; http://dx.doi.org/10.1185/03007990802484234
- Bentley A, Filipovic I, Gooch K, Busch K. A cost-effectiveness analysis of respiratory syncytial virus (RSV) prophylaxis in infants in the United Kingdom. Health Econ Rev 2013; 3(1):18; PMID:23919494; http://dx.doi.org/10.1186/2191-1991-3-18
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness - The curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014; 371(9):796-7; PMID:25162885; http://dx.doi.org/10.1056/NEJMp1405158
- Dieticians of Canada. WHO Growth Charts for Canada. Birth to 24 Months: Boys. 2010.
- Cystic Fibrosis Canada. Canadian Cystic Fibrosis Patient Data Registry Report. Toronto, ON: Cystic Fibrosis Canada, 2010.