ABSTRACT
The last case of paralytic poliomyelitis caused by wild poliovirus in Brazil occurred in 1989. The interruption of the indigenous poliovirus transmission was obtained through mass immunization campaigns to eligible children and an active epidemiological and laboratorial surveillance of all cases of acute flaccid paralysis (AFP) among children under 15 y of age. This paper describes and evaluates the performance of the AFP surveillance system in different geographic areas of Brazil between 2005 and 2014, using indicators recommended by WHO. AFP surveillance indicators as well as virological investigation of polio and non-polio enteroviruses in stool samples received in the laboratory were assessed from 2005–2014. During the period, 5463 cases of AFP were investigated. Of these, 55% were males and 45% were females. Those under 5 y of age represented 48% of all cases reported and investigated. AFP notification rate was within the acceptable values with mean value of 1.3 (North), 1.4 (Northeast), 1.1 (Southern), 1.0 (Southeast) and 1.4 (Midwest) cases of AFP per 100.000 population aged 15 y as well as the adequacy of fecal specimens received in the laboratory. Sabin- related polioviruses accounted for 1.7% of the isolates while, 6.7% were non-polio enterovirus with the values ranging from 5.0% to 8.9 %. No wild-type polio was found. The AFP epidemiological and laboratorial surveillance activities have been kept at appropriate levels in Brazil. These data are a very strong indication, which supports the status of country free of polio.
Introduction
In 1985, the Pan American Health Organization (PAHO) initiated a regional poliomyelitis eradication program.Citation1 The tremendous success of this program lead the World Health Organization (WHO) to launch, in 1988, the Global Polio Eradication Initiative (GPEI), whose main goal was the eradication of polio by the year 2000.Citation1
Although polio has not yet been eradicated, the number of wild poliovirus cases reported in 2015 (66 cases – data as December 2015) in the 2 remaining endemic countries (Afghanistan and Pakistan) is very small when compared with ∼1000 cases/day in 1980s.Citation2
The infection by a given wild poliovirus ranges in severity from a non-specific illness to severe acute flaccid paralysis (AFP), a complex clinical syndrome characterized by rapid onset of weakness, including less frequently weakness of the respiratory and swallowing muscles and with a broad array a potential etiologies which can cause permanent disability or even death.Citation3 Although polioviruses may primarily strike in any age, paralytic poliomyelitis affects mainly children under 5 y of age in a paralysis rate of one in 200–1000 infections. Few people among those paralyzed die due to involvement of breathing muscles. Since the vast majority of cases occur in asymptomatic way, the virus can spread widely to other regions before cases of paralysis can be observed.Citation4,5
The consistent utilization of Oral Poliovirus Vaccine (OPV) in Brazil through Mass campaigns of immunization began in 1980Citation6 and the last case of paralytic poliomyelitis caused by endemic transmission of wild poliovirus occurred in March 1989 in the Northeast region of the country. After the last wild poliovirus isolation in 1991, in Peru, the entire American Region was certified as polio-free, in 1994.Citation7 However, since wild polioviruses are still in circulation in the world, both AFP and environmental surveillance are necessary to be maintained combined with high coverage of polio vaccine.
Since 2012, Brazil applies a sequential IPV-OPV schedule with 2 doses of IPV (inactivated polio vaccine) at 2 and 4 months of age followed by 2 doses of oral polio vaccine (OPV) at 6 and 15 months of age. Additionally, one annual National Immunization Day (NID) offers OPV to all children under 5 y old regardless their polio vaccination status.Citation8 In general, the coverage with OPV and IPV has been high throughout the country, although in some areas it is < 95% among children under 1 y.Citation8
Recently, a wild PV1 and type 2 VDPV polioviruses were isolated from environmental samples in the State of São Paulo, Brazil. The wild PV1 was closely related (99.6% identity) to the wild PV1 currently circulating in Equatorial Guinea while the VDPV shows to be highly evolved (8,6% VP1 nucleotide differences from its parental Sabin vaccine).Citation9 Also, a rare type of poliovirus recombination in a type3/type 2 Sabin-related poliovirus isolated from a AFP case in Brazil has been reported.Citation10 These episodes reflect the need for maintenance of high immunization coverage rates, combined with virologic surveillance of all cases of acute flaccid paralysis.
Due to the risk of importation from regions where the disease has not been eliminated, besides the requirements to retain their polio-free status, the WHO recommends that the countries improve the AFP surveillance. Performance indicators should be used to evaluate the quality of AFP surveillance, such as 1) at least one case of non-polio AFP detected annually per 100 000 children under 15 y of age, 2) adequacy and condition of stools upon arrival at the laboratory, 3) non-polio enteroviruses isolation rate, and 4) timeliness of specimen processing at the laboratory.Citation11 Furthermore, assessing poliovirus surveillance efficacy in all countries (developed and developing ones) is essential to support the global disease eradication efforts.
Thus, this paper discusses the epidemiological and geographic distribution of AFP cases in Brazil between the years of 2005 and 2014, evaluating the performance of the surveillance system for poliovirus, identifying steps that require strengthening and showing that poliovirus surveillance system goes beyond the polio eradication.
Results
Patients gender and age
A total of 5463 cases of AFP were studied from 2005 to 2014 in all geographic regions of the country. The relative contribution by region was North, 11.9%, Northeast, 36.1%, Southern, 12.2%, Southeast, 30.9% and Midwest, 8.9% (). Among the specimens analyzed, 55% were from males with minimum and maximum values between 46.3% and 59.5%, respectively, and 48% were from children under 5 y old ().
Table 1. Acute Flaccid Paralysis (AFP) according to age, gender and region in Brazil, 2005 to 2014.
AFP surveillance sensitivity
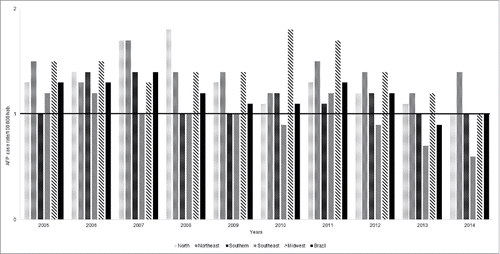
In order to evaluate the sensitivity of the AFP surveillance system, we have been following up the evolution of this indicator over the past 10 y. As shown in , all the regions reached acceptable values (0.9–1.8), with the mean value of 1.3 (North), 1.4 (Northeast), 1.1 (Southern), 1.0 (Southeast) and 1.4 (Midwest). The mean value for the country was 1.2 between 2005 and 2014. However, the southeast region showed a decreasing in the AFP case rate during the last 3 y of study.
Adequacy and quality of clinical samples received
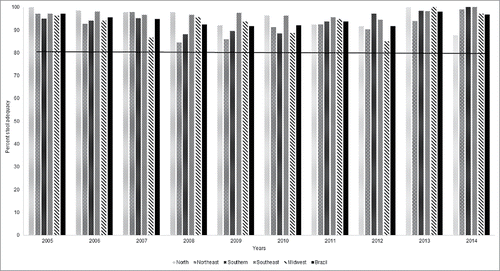
shows the proportion of AFP cases with adequate stool specimens. This indicator was defined as the arrival of the fecal samples in the lab in an adequate container, cold and with no evidence of leakage, although in sufficient quantity (at least 8 g). The minimum target should be 80%. During the evaluated period (2005–2014) the proportion of the stool adequacy by region was 95.4% (87.8–100), 92.5% (84.5–99), 93.9% (88.1–100), 97.1% (94.4–100) and 93.2% (84.9–100) for North, Northeast, Southern, Southeast and Midwest, respectively, while in the Brazil the mean value was 94%. However, some these samples came without documentation or with incomplete information.
Virus isolation and timeliness
During the last 10 years, 94 (1.7%) polioviruses were isolated from the 5463 AFP cases. Based on ITD tests and genome sequencing of the VP1 gene, all polioviruses were classified as Sabin-like based on the number of mutations encountered (PV1 Sabin and PV3 Sabin ≤ 9 nt differences from the reference PV1 and PV3 sequences; PV2 Sabin ≤5 nt differences from the reference Sabin 2 VP1 sequence).
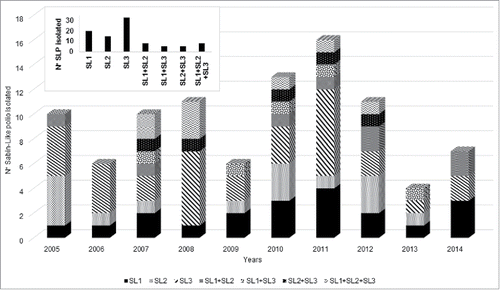
Thus, the 94 poliovirus identified during 2005–2014 were classified as: Sabin like 1 (SL1), 20 isolates (19%); Sabin like 2 (SL2), 15 isolates (14%); Sabin like 3 (SL3), 33 isolates (31%); (SL1) + (SL2), 8 isolates (8%); (SL1) + (SL3), 5 isolates (5%); (SL2) + (SL3), 5 isolates (5%); (SL1) + (SL2) + (SL3), 8 isolates (8%) ().
Figure 3. Proportion of Sabin-like polio isolated from AFP cases per year, Brazil 2005–2014. The inset represents the total number in the last 10 y per serotype.
Regarding the timeliness of specimen processing at the laboratory, >80% of the samples received had results released within the deadline recommended by WHO.
Non-polio isolation rate
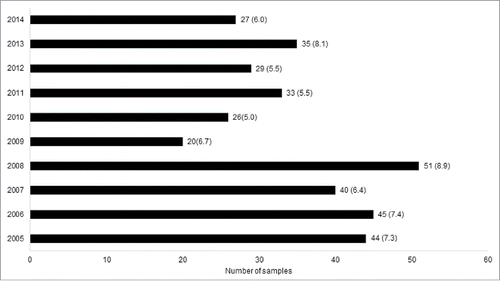
Other important parameter to evaluate the polio surveillance sensitivity is the non-polio enterovirus (NPEV) isolation rate. The WHO suggests that, at least, 10% of all fecal specimens received in to the reference laboratory should be positive regarding non-polio enterovirus isolation. This indicator points out the laboratory ability to perform enterovirus isolation in a routinely way. shows non-polio enterovirus isolation rate from 2005 to 2014 where 350 non-polio enteroviruses were isolated from the 5463 stool specimens investigated. We can observe that, during the last 10 years, the NPEV isolation rate from AFP cases was lower than 10%, with the values ranging from 5.0 % (2010) to 8.9% (2008). During this period (2005–2014) the mean value was 6.7%.
Discussion
The present work represents an effort to better understand the national surveillance system of AFP in Brazil and to report the results of the last 10 y (2005–2014) of evaluation of AFP surveillance indicators. Furthermore, we show that Brazil has a functional AFP surveillance system that operates relatively well in spite of challenges such as a large national geographic territory and economic constraints.
During this study, we assessed the age distribution of AFP cases that represent an important point to evaluate the adverse effect of the vaccine. During the analysis, we found that 48% of AFP cases occurred in children under 5 y old. Similar results were obtained in other regions such as Marches and Lombardy in Italy, which reported 37% and 57%,Citation12,13 respectively and 2 other studies that reported higher values in children <5 years: 74. 4% in Ghana and 67.2% in South Africa.Citation14,15
Regarding the incidence of AFP by gender, we observed similar distribution with 55% and 45% for male and female, respectively. Our results are very similar to studies performed in other areas such as Ghana, 55.8%, South Africa 54.3% for male.Citation14,15
The temperature in which the fecal samples were received at the laboratory was another important indicator analyzed. In recent study, Walker and coworkers demonstrated the effect of transport temperature on the viability of poliovirus in stool specimens suggesting that this point is important to be analyzed in tropical regions.Citation16 In this study, more than 80% of clinical samples arrived at the laboratory in good condition, independently of geographic distribution.
One important criterion in the surveillance system is the investigation of all AFP cases in children <15 y of age. In polio-free regions, the incidence of AFP should be at least 1 case per 100.000 children <15 y of age.Citation11 This indicator remained above the reference value from 2005 until 2012 except for the Southeast region, where it was below the minimum target during 2010 and 2012–2014, although the overall AFP reporting rate in the country was >1/100.000 inhabitants during this period. It is difficult to identify if these results represent normal fluctuations or signs of declining of the surveillance performance.
In fact, the number of AFP notification seems to decline in 2013 and 2014, mainly in the Southeast and Midwest regions, reflecting the decrease in the AFP notification rate in the country. On the other hand, the decline in the AFP case reported might be due to better medical conditions and greater accuracy in medical examinations, which excluded the possibility of poliovirus infection. Additionally, some doctors may judge that the motor deficit was due to other factors not requiring notification. This is a significant problem for the AFP case report system, which calls for a better awareness of the importance of this notification. Furthermore, in the early stages, polio may be difficult to differentiate from other forms of acute flaccid paralysis, which are independent of polio, such as Guillain-Barré Syndrome. However, any child under 15 y of age with AFP or any person of any age with paralytic illness must be investigated.Citation11
The non-polio enterovirus isolation rate is an important parameter used to evaluate the integrity and viability of stool specimen sent to the laboratory. The WHO suggests that, at least, 10% of stool specimens dispatched to the laboratory should yield NPEV. The results obtained in our work shows that the national annual average enteroviruses isolation is 6.7%, with variations from 5.0% to 8.9%. This result is close to the findings reported in another study conducted in Pakistan, Nigeria, Malaysia and Tunisia, where NPEV were isolated from 8.5%, 7.6%, 6.5% and 5.3% of AFP cases, respectively.Citation17,18,19,20 However, the frequency of NPEV isolation in our study was much lower than reported in other countries such as Egypt (17.6%) and Ghana (20%).Citation21,14 The rates under 10% can reflect a low circulation of NPEV in the country. Moreover, other factors could explain a decrease in the NPEV isolation rate: The loss of sensitivity of the isolation methods or inadequate conditions of sample collection and storage before the transportation. WHO reference laboratories frequently carry out the cell sensibility assay on cells lines (RD and L-20) used in the laboratory routine. In addition to use cell lines (RD and L20) suggested by WHO, we also utilize HEp-2c cell line, which is susceptible to NPEV isolation. Although the conditions of the stool samples upon arrival at the laboratory are satisfactory, we have no control over all steps involved from the collection until shipment of the samples to the laboratory. This may have had some impact on the NPEV isolation rate seen in the last 10 y. However, previous studies suggested that there are populations from which the NPEV isolation rate may be expected to be less than 10% due to climatic factors.Citation22
Overall, the present study shows the improvement in AFP surveillance in Brazil, revealing that surveillance activity is satisfactory in all geographic regions. However, the surveillance program needs to address problems regarding the improvement of specific clinical data and sample documentation. Furthermore, clinicians, surveillance officers and laboratory staff should be trained and motivated to conduct active search for AFP cases.
Material and methods
Experimental ethics
The Enterovirus laboratory at FIOCRUZ is an official Brazilian Ministry of Health Reference Laboratory. The virus isolates utilized in the present study are part of the virus collection of the laboratory. Patients are attended by the local public health system ambulatories where the clinical samples (feces) are collected. This activity was considered as a public health response to the poliomyelitis surveillance and thus did not require review by the review board.
Study background
Brazil is the largest country in South America and the fifth largest in the world, with an area of 8.515.767 km2 divided into 5 different regions (North, Northeast, Midwest, Southeast and South) with an estimated population of around 204 million people (according to 2015 estimates). The number of children under 15 y of age is estimated to be around 47 million. A retrospective study was developed using AFP surveillance data collected by the national surveillance system for notifiable diseases and the WHO Regional Reference laboratory database from 2005 to 2014. All cases reported to the surveillance system and all laboratorial data obtained from the analysis of 5463 AFP cases during this period were included in the study. These cases were obtained from all 5 different geographic regions of the country.
Every clinical case characterized as acute onset of focal weakness or paralysis in children under 15 y old has to be reported to the health authorities and clinical specimens referred to the National Reference Polio laboratory for diagnostic confirmation. The clinical samples to be investigated consist of fecal specimens (one specimen per case) collected within 14 d from the onset of motor deficit. After the report, follow up is required to verify whether there is residual paralysis at 60 d after the onset. Four parameters of minimum performance standards were used in this work to evaluate the quality of AFP surveillance: proportion of AFP cases with adequate stool specimens, AFP case rate and, non-polio enterovirus isolation rate and timeliness of laboratory results.Citation11
Virus isolation and identification
After received the samples at the laboratory, 200 microliters of each clarified stool sample were used to inoculate RD and L20B cell lines. Cells were incubated at 37°C and examined daily for cytopathic effect (CPE). Culture supernatants showing CPE were collected and frozen at −20°C. All laboratorial procedures were performed according to the standard protocols recommended by WHO.Citation23
Intratypic differentiation tests were carried out by conventional Reverse transcriptase polymerase chain reaction (RT-PCR) or qPCR followed by VP1 sequence of all poliovirus isolated. The rRT-PCR screening kits were made available by the Center for Disease Control and Prevention.Citation24
For sequencing of the major viral capsid surface protein (VP1), viral RNA was extracted from 140 μl of the culture supernatants, that showed CPE, using QIAmp (Qiagen) and used as a template for cDNA construction with random primers (Promega, Madison) and SuperScript III Reverse Transcriptase (Invitrogen) according to the manufacturer's instructions. For single poliovirus serotypes isolates PCR was performed using a primer pair (Q8 and Y7) that amplifies a fragment of approximately 1100 bp within the VP1 gene.Citation25 For poliovirus-containing mixtures PCR was performed using 3 sets of Sabin specific primers targeting the 3′- end of the VP3 gene in combination with a previously described reverse primer that targets the 5′ - portion of the 2A gene, named Q8.Citation26,27 After 32 cycles (94°C for 30 s, 42°C for 30 s, and 60°C for 40 s) in a model 9700 thermocycler (Applied Biosystems), PCR products were analyzed by electrophoresis in 1% agarose gels containing 0.5 mg/mL ethidium bromide and compared to a 100 bp DNA Ladder (Invitrogen) by visualization with a UV transilluminator. Specific products were gel purified with the QIAquick Gel Extraction Kit (QIAGEN) and quantified by comparison with the Low DNA Mass Ladder (Invitrogen) in a 1% agarose gel. Sequencing reactions were performed by using the ABI PRISM BigDye Terminator v3.1 Cycle Sequencing Ready Reaction Kit (Applied Biosystems) in a GeneAmp PCR System 9700 thermocycler (Applied Biosystems) with 25 cycles of 96°C for 15s, 42°C for 30s, and 60°C for 3min. Products were purified by isopropyl alcohol precipitation, and samples were analyzed by using the ABI PRISM 310 Genetic Analyzer (Applied Biosystems). VP1 sequences of the poliovirus isolates were compared to the prototype strains according GenBank number: GQ984141.1 (Polio 1), and AY184220.1 (Polio 2) AY184221.1 (Polio 3).
Abbreviations
| AFP | = | Acute Flaccid Paralysis |
| CDC | = | Centers for Disease Control and Prevention |
| CPE | = | Cytopathic Effect |
| FIOCRUZ | = | Fundação Oswaldo Cruz |
| IPV | = | Inactivated Polio Vaccine |
| ITD | = | Intratypic Differentiation |
| GPEI | = | Global Polio Eradication Initiative |
| NPEV | = | Non-Polio Enterovirus |
| OPV | = | Oral Poliovirus Vaccine |
| PAHO | = | Pan American Health Organization |
| PV | = | Poliovirus |
| RD | = | Rhabdomyosarcoma |
| RT-PCR | = | Reverse Transcriptase Polymerase Chain Reaction |
| SL | = | Sabin Like |
| VDPV | = | Vaccine-Derived Poliovirus |
| VP | = | Viral Protein |
| WHO | = | World Health Organization |
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests.
Funding
This work was funded by a grant from the Brazilian Ministry of Health, Fundação Oswaldo Cruz and CNPq.
References
- Centers for Disease Control and Prevention (CDC). Progress toward global eradication of Poliomyelitis, 1988–1991. MMWR 1993; 42(25):486-487.
- World Health Organization (WHO). Polio Global Eradication Initiative. December 2015. Available from:http://www.polioeradication.org/mediaroom/newsletterpolionews.aspx. (Accessed 15 Mar. 2016).
- Marx A, Glass JD, Sutter RW. Differential diagnosis of acute flaccid paralysis and its role in poliomyelitis surveillance. Epidemiologic Rev 2000; 22(2):298-316; PMID:11218380; http://dx.doi.org/10.1093/oxfordjournals.epirev.a018041
- Apostol LN, Suzuki S, Bautista A, Galang H, Paladin FJ, Fuji N, Lupisan S, Olveda R, Oshitani H. Detection of non-Polio enteroviruses from 17 years of virological surveillance of acute flaccid paralysis in the Philippines. J Medical Virol 2012; 84:624-631; PMID:22337302; http://dx.doi.org/10.1002/jmv.23242
- Watkins RE, Martin PAJ, Kelly H, Madin B, Watson C. An evaluation of the sensitivity of acute flaccid paralysis surveillance for poliovirus infection in Australia. BMC Infect Dis 2009; 9:162; PMID:19788763; http://dx.doi.org/10.1186/1471-2334-9-162
- Risi JB, Jr. The control of poliomyelitis in Brazil. Rev Infect Dis 1984; 6: Suppl 2:400-3; http://dx.doi.org/10.1093/clinids/6.Supplement_2.S400
- Pan American Health Organization (PAHO). Third meeting of the international commission for the certification of Poliomyelitis Eradication. Washington, DC: PAHO, 1994; Expanded Program on Immunization.
- Domingues CMAS, Pereira SF, Marreiros ACC, Menezes N, Flannery B. Introduction of sequential inactivated Polio Vaccine-Oral polio vaccine schedule for routine infant immunization in Brazil's National Immunization Program. J Infect Dis 2014; 210(S1):S143-51; PMID:25316829; http://dx.doi.org/10.1093/infdis/jit588
- Cassemiro KMSdM, Burlandy FM, Barbosa MRF, Chen Q, Jorba J, Hachich EM, Sato MIZ, Burns CC, Silva EE. Molecular and phenotypic characterization of a highly evolved Type 2 Vaccine-Derived Poliovirus isolated from seawater in Brazil, 2014. Plos One 2016; 11(3):e0152251; PMID:27019095; http://dx.doi.org/10.1371/journal.pone.0152251
- Cassemiro KMSdM, Burlandy FM, Silva EE. Rare natural type 3/type 2 intertypic capsid recombinant vaccine-related poliovirus isolated from a case of acute flaccid paralysis in Brazil, 2015. J Gen Virol 2016; 97:1545-50; PMID:27082658; http://dx.doi.org/10.1099/jgv.0.000484
- World Health Organization (WHO): recommended surveillance standard of poliomyelitis. Available from: http://www.polioeradication.org/AboutUs/Strategy/Surveillance.aspx (Accessed 16 Aug 2016)
- D'Errico MM, Barbadoro P, Bacelli S, Esposto E, Moroni V, Scaccia F, Tantucci L, Prospero E. Surveillance of acute flaccid paralysis in the Marches region (Italy): 1997–2007. BMC Infect Dis 2008; 8:135; PMID:18844987; http://dx.doi.org/10.1186/1471-2334-8-135
- Pellegrinelli L, Primache V, Fiore L, Amato C, Fiore S, Bubba L, Pariani E, Amendola A, Barbi M, Binda S. Surveillance of acute flaccid paralysis (AFP) in Lombardy, Northern Italy, from 1997 to 2011 in the context of the national AFP surveillance system. Hum Vaccines Immunotherapeutics 2015; 11(1):277-81; PMID:25483546; http://dx.doi.org/10.4161/hv.36164
- Odoom JK, Ntim NAA, Sarkodie B, Addo JN, Minta-Asare K, Obodai E, Eshun E, Ahove VV, Diamenu S, Adjabeng M, et al. Evaluation of AFP surveillance indicators in polio-free Ghana, 2009–2013. BMC Public Health 2014; 14:687; PMID:24996415; http://dx.doi.org/10.1186/1471-2458-14-687
- Khuzwayo LS, Kuonza LR, Ngcobo NJ. Evaluating the acute flaccid paralysis surveillance system in South Africa, 2005–2009 - an analysis of secondary data. Pan African Medical J 2013; 14:86; PMID:23646222; http://dx.doi.org/10.11604/pamj.2013.14.86.2032
- Walker AT, Williams AJ, Gary HE, Jr, Pallansch MA, Wassilak SGF, Oberste MS. Effect of time at temperature on wild poliovirus titers in stool specimens. Virology 2015; 482:28-31; PMID:25817402; http://dx.doi.org/10.1016/j.virol.2015.03.005
- Ahmad A., Rehman A. One year surveillance data of acute flaccid paralysis at Bahwal Victoria Hospital Bahawalpur. Pak J Med Sci 2007; 23(3):308-12.
- Bassey BE, Gasasira A, Mitula P, Frankson UU, Adeniji JA. Surveillance of acute flaccid paralysis in Akwa Ibom State, Nigeria 2004–2009. Pan African Medical J 2011; 9:32; PMID:22145065; http://dx.doi.org/10.4314/pamj.v9i1.71208
- Saraswathy TS, Nor Zahrin H, Apandi MY, Kurup D, Rohani J, Zainah S, Khairullah NS. Acute flaccid paralysis surveillance: looking beyond the global poliomyelitis eradication initiative. Southeast Asian J Trop Med Public Health 2008; 39(6):1033-39.
- Bahri O, Rezig D, Ben Nejma-Oueslati B, Ben Yahia A, Ben Sassi J, Hogga N, Sadraoui A, Triki H. Enteroviruses in Tunisia: virological surveillance over 12 years (1992–2003). J Medical Microbiol 2005; 54:63-69; PMID:15591257; http://dx.doi.org/10.1099/jmm.0.45695-0
- Afifi SS, Zaki SA, Mohamed AF, Hosseiny HE. Isolation and identification of non-polio enteroviruses from children in different egyptian governorates. Australian J Basic Applied Sci 2009; 3(4):3230-8.
- Sanders R, Maher C, Aylward RB, Bilous J, Schnur A, Sato Y, Omi S, Tangermann RH. Development and coordination of the polio laboratory network in the Western Pacific Region of the World Health Organization. J Infect Dis 1997; 175(8uppl 1):8117-21.
- World Health Organization: Polio Laboratory Manual. 2004; 4th edition, Document World Health Organization/IVB/04.10. Geneva, Switzerland.
- Kilpatrick DR, Yang CF, Ching K, Vincent A, Iber J, Campagnoli R, Mandelbaum M, De L, Yang SJ, Nix A, et al. Rapid group-, serotype-, and vaccine strain-specific identification of poliovirus isolates by real-time reverse transcription-PCR using degenerate primers and probes containing deoxyinosine residues. J Clin Microbiol 2009; 47(6):1939-41; PMID:19386844; http://dx.doi.org/10.1128/JCM.00702-09
- Oberste MS, Nix WA, Maher K, Pallansch MA. Improved molecular identification of enteroviruses by RT-PCR and amplicon sequencing. J Clin Virol 2003; 26(3):375-7; PMID:12637088; http://dx.doi.org/10.1016/S1386-6532(03)00004-0
- Toyoda H, Kohara M, Kataoka Y, Suganuma T, Omata T, Imura N, Nomoto A. Complete nucleotide sequences of all three poliovirus serotype genomes. Implication for genetic relationship, gene function and antigenic determinants. J Mol Biol 1984; 174(4):561-85; PMID:6202874; http://dx.doi.org/10.1016/0022-2836(84)90084-6
- Costa EV, Campos RM, Tavares FN, Grégio CR, Burlandy FM, Silva EE. A RT-PCR method for selective amplification and phenotypic characterization of all three serotypes of Sabin-related polioviruses from viral mixtures. Mem Inst Oswaldo Cruz 2012; 107(5):698-701; PMID:22850966; http://dx.doi.org/10.1590/S0074-02762012000500022