ABSTRACT
Ebola hemorrhagic fever, also known as Ebola virus disease or EVD, is one of the most dangerous viral diseases in humans and animals. In this open-label, dose-escalation clinical trial, we assessed the safety, side effects, and immunogenicity of a novel, heterologous prime-boost vaccine against Ebola, which was administered in 2 doses to 84 healthy adults of both sexes between 18 and 55 years. The vaccine consists of live-attenuated recombinant vesicular stomatitis virus (VSV) and adenovirus serotype-5 (Ad5) expressing Ebola envelope glycoprotein. The most common adverse event was pain at the injection site, although no serious adverse events were reported. The vaccine did not significantly impact blood, urine, and immune indices. Seroconversion rate was 100 %. Antigen-specific IgG geometric mean titer at day 42 was 3,277 (95 % confidence interval 2,401–4,473) in volunteers immunized at full dose. Neutralizing antibodies were detected in 93.1 % of volunteers immunized at full dose, with geometric mean titer 20. Antigen-specific response in peripheral blood mononuclear cells was also detected in 100 % of participants, as well as in CD4+ and CD8+ T cells in 82.8 % and 58.6 % of participants vaccinated at full dose, respectively. The data indicate that the vaccine is safe and induces strong humoral and cellular immune response in up to 100 % of healthy adult volunteers, and provide a rationale for testing efficacy in Phase III trials. Indeed, the strong immune response to the vaccine may elicit long-term protection. This trial was registered with grls.rosminzdrav.ru (No. 495*), and with zakupki.gov.ru (No. 0373100043215000055).
Introduction
Ebola hemorrhagic fever, also known as Ebola virus disease or EVD, is one of the most dangerous viral diseases in humans, great apes, lower primates, and other animals. The disease is characterized by a severe course that progresses from fever, headache, malaise, and myalgia to coagulation disorders and multi-organ failure, with mortality up to 90 %.Citation1-3 The causative agent of this disease is Ebola virus, a filovirus (Filoviridae). Since the pathogen was first reported in 1976, more than 10 outbreaks of EVD have occurred. The largest outbreak to date eventually became an epidemic that claimed the lives of more than 11,000 people between December 2013 and June 2016.Citation4
At the beginning of the last outbreak, there was no licensed vaccine against EVD, although several vaccines have been shown to be protective in preclinical and clinical studies. Most of these vaccines consist of different vectors (Ad3, Ad26, Ad5, and VSV) expressing the Ebola virus glycoprotein, typically that of the 1976 Zaire-Mayinga strain or the 1995 Zaire-Kikwit strain.Citation5 On the other hand, the variant that caused the last outbreak, Ebola virus/H.sapiens-wt/GIN/2014/Makona-C15, belongs to a Zaire strain the envelope glycoprotein of which is 97.2 % and 97.7 % similar to those of the 1976 Zaire-Mayinga and 1995 Zaire-Kikwit strains, respectively. Due to these differences, it may be necessary to develop vaccines against the circulating strain. Accordingly, we designed a novel heterologous vaccine against the circulating Zaire strain, noting that heterologous prime-boost vaccination has been shown to induce stronger immune response than homologous vaccination.Citation6-7
We now report safety and immunogenicity data from a clinical trial of this vaccine, which consists specifically of live-attenuated recombinant vesicular stomatitis virus (VSV) and recombinant adenovirus type-5 (Ad5) expressing the envelope glycoprotein of the Ebola virus/H.sapiens-wt/GIN/2014/Makona-C15 strain. The vaccine, which we call GamEvac Combi, was administered by heterologous prime-boost immunization, and the humoral immune response was compared to homologous VSV-prime immunization.
Results
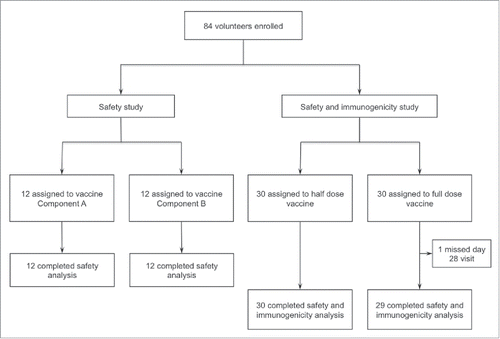
Between September 16 and November 30, 2015, we conducted an open-label, dose-escalation trial to assess safety, side effects, and immunogenicity of GamEvac-Combi in 84 healthy adults of both sexes between 18 and 55 years (). Participants were assigned to receive VSV-glycoprotein (n = 12), Ad5-glycoprotein (n = 12), or sequential injections of VSV-glycoprotein and Ad5-glycoprotein 21 days apart at half (n = 30) or full dose (n = 30, ). Doses were set based on preclinical studies in non-human primates. Baseline characteristics are listed in .
Table 1. Baseline characteristics.
Safety
Overall, 74 (89 %) participants reported at least one solicited adverse reaction within 42 days of vaccination with VSV-glycoprotein (n = 9), Ad5-glycoprotein (n = 9), half dose of both (n = 29), and full dose of both (n = 27, ). These systemic or local reactions were mild or moderate in severity, generally occurred on the day after vaccination, and resolved within the next 3 days. Reactions were considered moderate if treatment of symptoms was required. There was no significant difference in the nature and severity of reactions to half and full dose vaccines, although the frequency of such events was higher in the former (), noting that one participant who received a full dose withdrew from the study at day 28 for personal reasons. In any case, serious vaccine-associated adverse events were not reported.
Figure 2. Summary of surveilled local and systemic adverse events after vaccine administration. For groups receiving VSV-glycoprotein and Ad5-glycoprotein in one or 2 doses, reactions occurring within 7 days of vaccination are reported. For groups receiving half and full dose of both vectors, reactions occurring within 42 days of vaccination are reported. In groups receiving half and full dose of both vectors reactions occurring within 7 days of vaccination of VSV-glycoprotein are reported as I injection, reactions occurring within 7 days of vaccination of Ad5-glycoprotein are reported as II injection.
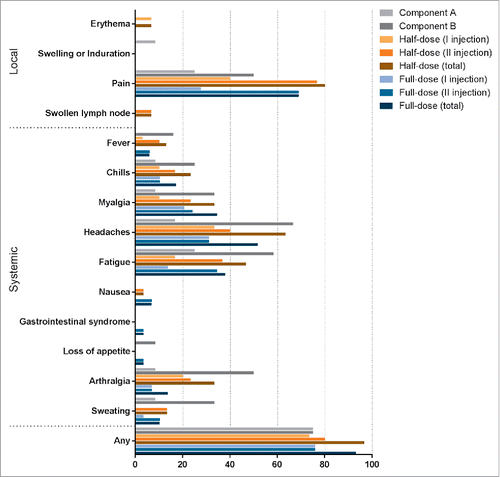
Systemic reactions included elevated axillary temperature (≥ 37°C), which was observed in 16.7 %, 50 %, 36.7 %, and 55.2 % of participants vaccinated with VSV-glycoprotein, Ad5-glycoprotein, half dose of both, and full dose of both. Fever (≥ 38°C axillary temperature) was observed in 0 %, 16.6 %, 13.3 %, and 6.9 % of volunteers, along with symptoms associated with toxicity, including headache (16.7 %, 66.7 %, 63.3 %, and 51.7 %), fatigue (25 %, 58.3 %, 46.7 %, and 37.9%), myalgia (8.3 %, 33.3 %, 33.3 %, and 34.5 %), and arthralgia (8.3 %, 50 %, 33.3 %, and 13.8 %). Isolated cases of loss of appetite, nausea, and gastrointestinal distress were noted. Urticarial and anaphylactic allergic reactions were variable.
Local reactions were mild and did not require additional therapeutic treatment with antihistamines. Local reactions included pain at the injection site (25 %, 50 %, 80 %, and 69 %). In volunteers who received both components in sequence, most pain reactions occurred after the second vaccination. In volunteers vaccinated with both components at half dose, erythema (n = 2, 6.7 %), axillary lymphatic node enlargement (n = 2, 6.7 %), and rash (n = 1, 3.3 %) were observed. Swelling or induration at the site of injection was observed in one volunteer (8.3 %) immunized with VSV-glycoprotein.
Average blood count was comparable before vaccination and during active follow-up after vaccination, and was within normal values. In some volunteers, however, vaccination affected a range of laboratory indicators of liver and kidney function, including alanine aminotransferase, aspartate amino transferase, creatinine, and creatine phosphokinase (). Nevertheless, these indicators returned to baseline within 7 days after vaccination, suggesting that effects were transient. Finally, vaccination did not significantly alter urine indices.
Table 2. Number of cases (percentage) with increased values of indicators of liver and kidney function in first 7 days after vaccination. In Half-dose and Full-dose groups number of cases after first injection and second injection are divided by slash.
Immunogenicity
Immunogenicity was evaluated based on several indices of humoral and cellular immune response. In particular, humoral response was assessed by enzyme-linked immunosorbent assay (ELISA) against glycoprotein-specific IgG in serum, and by virus neutralization assay to detect neutralizing antibodies. On the other hand, cell-mediated immune response was assessed by ELISA against interferon-γ secreted from glycoprotein-activated peripheral blood mononuclear cells, and by flow cytometry to measure glycoprotein-induced proliferation of CD4+ and CD8+ T cells. Results are summarized in .
Figure 3. Humoral immune response. A) Glycoprotein-specific antibodies at days 21, 28, and 42, as measured by ELISA, in volunteers immunized at half or full dose of VSV-glycoprotein and Ad5-glycoprotein, and at 42 days in volunteers immunized with VSV-glycoprotein only. B) Results plotted as reciprocal end-point titres, with curves showing the distribution of individual antibody titres in each group at days 28 and 42. C) Neutralization antibodies at days 0 and 28 in volunteers immunized at full dose. *, p < 0.001.
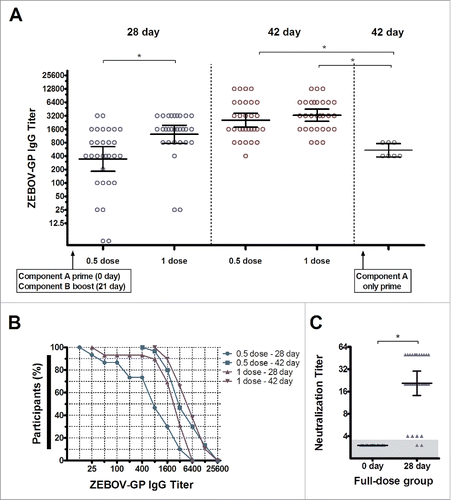
ZEBOV Makona glycoprotein-specific antibodies were detected on day 28 in 93 % and 100 % of volunteers immunized at half and full dose, but in all participants on day 42 (). Indeed, antibody geometric mean end-point titer was 2,540 (95 % confidence interval 1,769–3,647) in volunteers vaccinated at half dose, and 3,277 (95 % confidence interval 2,401–4,473) in volunteers immunized at full dose on day 42. Antibody titres were higher in the latter than in the former (p = 0.0003) on day 28, but were comparable on day 42 (p = 0.26), indicating that the antibody response matured more quickly at full dose. Notably, the geometric mean antibody titer on day 42 was significantly lower at 538.4 (95 % confidence interval 382.2–758.4) in volunteers immunized with VSV-glycoprotein alone (p < 0.0001). On the other hand, neutralizing antibodies to ZEBOV Mayinga were detected in 27 (93.1 %) volunteers immunized at full dose (), with geometric mean antibody titer 20 on day 28 that suggests cross-reactive immunogenicity from Makona immune response to Mayinga.
Remarkably, the glycoprotein-specific antibody response in volunteers immunized at half dose was inversely correlated (correlation coefficient −0.4) on day 28 (p = 0.03) and 42 (p = 0.04) with the concentration of pre-existing neutralizing antibodies against Ad5, which have been reported to lower the efficacy of Ad5-based vaccines. However, these correlations were absent in volunteers immunized at full dose (p > 0.09). We note that pre-existing neutralizing antibodies against Ad5 were detected at > 1:10 in 100 % of participants immunized at half and full dose, of whom 63.3 % and 51.7 %, respectively, had high levels (> 1:200).
The mononuclear cell response was evaluated on days 0, 28, and 42 by interferon-γ secretion, as measured by ELISA and reported as fold increase in secretion upon exposure to Ebola Zaire glycoprotein (). In volunteers immunized at half dose, the median interferon-γ concentration was 1.31 (interquartile range 1.00–1.75) at day 0, 15.83 (interquartile range 5.66–33.91) at day 28, and 10.44 (interquartile range 3.46–22.30) at day 42. In volunteers immunized at full dose, the median interferon-γ concentration was 1.00 (interquartile range 1.00–1.50) at day 0, 22.57 (interquartile range 7.03–38.89) at day 28, and 12.86 (interquartile range 3.87–21.97) at day 42. In general, interferon-γ response was detected on day 28 in 96.7 % and 100 % of volunteers immunized at half and full dose, and in 90 % and 100 % of participants on day 42 ().
Figure 4. Cell-mediated immune response to Ebola virus glycoprotein at days 0, 28, and 42 in volunteers immunized at half and full dose of VSV-glycoprotein and Ad5-glycoprotein. A) Fold increase in interferon-γ production by peripheral blood mononuclear cells exposed to glycoprotein. B) Glycoprotein-specific proliferation of CD4+ T-cells. C) Fold increase in interferon-γ production by peripheral blood mononuclear cells exposed to glycoprotein. Curves show the distribution of individual interferon-γ production in each treatment group at days 0, 28, and 42. D) Glycoprotein-specific proliferation of CD8+ T-cells. *, p < 0.0001.
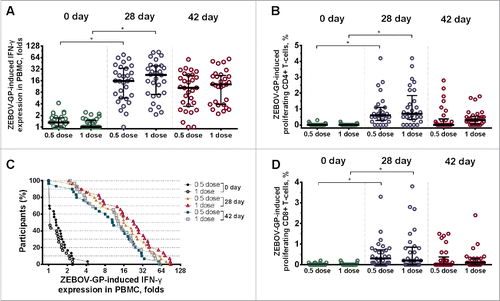
T cell response was measured at days 0, 28, and 42 by flow cytometry, and is reported as frequency of CD4+ and CD8+ cell proliferation upon exposure to Ebola Zaire glycoprotein. Cells from vaccinated participants proliferated significantly in response to Ebola glycoprotein, especially on day 28 (), with no significant differences due to dose. At day 28, 83.3 % of volunteers immunized at half dose and 82.8 % of participants immunized at full dose had developed a glycoprotein-specific CD4+ T cell response, while 73.3 % and 58.6 %, respectively, had developed a glycoprotein-specific CD8+ T cell response. At day 42, CD4+ T cell response was detected in 40 % and 75.9 %, while CD8+ T cell response was detected in 43.3 % and 62.1 %, respectively.
Discussion
The development of vaccines against Ebola virus started soon after it was identified, and early work focused mainly on attempts to inactivate the virus. In 1980, the first vaccine based on inactivated Ebola virus elicited 100 % protection in guinea pigs.Citation8 In subsequent years, a large number of other candidate vaccines was developed, some of which have been tested in clinical trials.Citation5 Today, 8 vaccines, including DNA vaccines, virus-like particles, and recombinant viral vectors, are in different phases of clinical testing.Citation5 Most are recombinant viral vectors expressing the Ebola virus envelope glycoprotein, one of the most protective antigens. These include recombinant vectors derived from human Ad5 and Ad26, simian adenoviruses such as chimpanzee adenovirus serotype 3, modified vaccinia Ankara, VSV, and combinations thereof.Citation5,9-15 At the same time, it was shown in multiple animal models and clinical trials that heterologous prime-boost immunization generally elicits superior immune response in comparison to repeated doses of the same vaccine.Citation7 Thus, on the basis of the accumulated literature on Ebola vaccines, we developed a heterologous vaccine consisting of VSV and Ad5 vectors expressing Ebola virus glycoprotein.
In preclinical studies, the vaccine was found to induce humoral and cellular immune response. In particular, the vaccine elicited glycoprotein-specific humoral response in non-human primates, with IgG titres 32,000–128,000 on day 42, neutralizing antibody titres 250–1,250, and durable 100 % protection for 5 months (Supplementary Fig. 1). Similarly, Stanley et al.Citation6 reported that homologous vaccination with a recombinant chimpanzee adenovirus serotype 3 vector elicited durable protection in only 1 of 3 nonhuman primates (33.3 %), while heterologous vaccination with the same vector as prime and modified vaccinia Ankara as boost elicited durable protection in all 3 immunized nonhuman primates (100 %).
We now report that in Phase I/II clinical trials in Russia, the vaccine induces strong humoral and cellular immune response in healthy volunteers. In particular, the vaccine elicited antibody response in 100 % of participants, with significantly increased titres 28 days after vaccination, and even higher titres on day 42. At day 42, glycoprotein-specific antibody titres were 2,540 in volunteers immunized at half dose, and 3,277 in volunteers immunized at full dose, in line with previous results from other clinical trials.Citation9-13 Moreover, the data suggest that heterologous VSV-prime Ad5-boost vaccination induced significantly stronger antibody response than immunization with VSV alone. Similarly, glycoprotein-specific response from peripheral blood mononuclear cells and T cells peaked at day 28 before decreasing at day 42, reminiscent of previous Ebola vaccines based on chimpanzee adenovirus serotype 3.Citation14-15
Neutralization assays against the Mayinga strain were performed in order to compare clinical results and preclinical studies in nonhuman primates. In clinical trials, neutralizing antibodies were detected in 93 % of participants immunized at full dose, with titres comparable to those in nonhuman primates. We note that in nonhuman primates, titres of neutralizing antibodies correlate with protection from a lethal dose of Ebola virus Mayinga. While data on cross-reactive immunogenicity were not collected in humans, results in nonhuman primates indicate that the vaccine induces comparable levels of immune response to the Kissidougou 2014 and Mayinga 1976 strains. Notably, clinical trials of VSV-based vaccineCitation16-17 showed that immunization with GP Kikwit led to production not only Kikwit GP-specific antibodies, but also Mayinga GP and Makona/Gueckedou GP (2014). Animal studies of EBOV-GP nanoparticles showed that mice immunized with GP Makona produced NtAb to ZEBOV Mayinga and were protected from lethal dose of ZEBOV Mayinga.Citation18 All these data suggest that there is cross-protection from the immune responses to Makona/Gueckedou, Mayinga, and Kikwit.
One of the main issues limiting the use of vectors based on Ad5 is widespread pre-existing immunity to Ad5. Indeed, pre-existing immunity to Ad5 is negatively correlated with glycoprotein-specific immune response.Citation9,19 Thus, heterologous vaccination with a VSV prime and an Ad5 boost may compensate for the negative effects of pre-existing immunity to Ad5. Accordingly, the glycoprotein-specific humoral and cellular immune response in volunteers immunized at full dose was not correlated with pre-existing neutralizing antibodies to Ad5 (p > 0.09). In any case, it is necessary to directly compare different vaccines based on Ad5, VSV, chimpanzee adenovirus serotype 3, Ad26, and modified vaccinia Ankara, in order to identify optimal vaccine vector(s). At present, this cannot be accomplished, since extensive clinical studies of all vectors under the same clinical and laboratory testing conditions are not available.
Importantly, the data indicate that vaccine based on VSV-glycoprotein and Ad5-glycoprotein are safe in healthy adult volunteers. Indeed, no serious adverse events were reported during the clinical trial, and there were no significant adverse effects on blood, urine, and immune indices. Based on Guidelines for Preparing Core Clinical Safety Information on Drugs,Citation20 some of these adverse events were very common (myalgia, headache, fatigue, arthralgia, and pain at the injection site) or common (erythema, swollen lymph node, nausea, gastrointestinal syndrome, and loss of appetite). However, these adverse events are common to all vaccines based on viral vectors, and are not unexpected.Citation9,11-13,21 The frequency of side effects was also in line with previous viral vector vaccines against Ebola.Citation9,11-13 Thus, we conclude that, except in individual cases of intolerance, our vaccine is well-tolerated, taking into account the nature and severity of adverse events reported, the lack of significant negative impact on quality of life, and the absence significant impact on laboratory parameters, vital systems, and organs. We note, however, that while the frequency of vaccine-associated fever was lower in our trial than in other trials of VSV-based Ebola vaccine,Citation11,16,17 vaccine-associated hyperthermia (> 38°C) may cause confusion in the field during outbreaks, as this symptom could be construed as active Ebola infection. Nevertheless, a VSV-based Ebola vaccine effectively prevented Ebola virus disease when delivered during an outbreak.Citation10
In summary, the data collectively show that the vaccine is highly immunogenic, and induces strong humoral and cellular immune response in up to 100 % of healthy adult volunteers. The data also demonstrate that a vaccine based on VSV-glycoprotein and Ad5-glycoprotein does not cause serious side effects, and has a good safety profile in healthy adult volunteers. Accordingly, the Ministry of Health of the Russian Federation approved the registration of the vaccine on December 28, 2015, with registration number LP-003390.
Materials and methods
Vaccine
The vaccine GamEvac-Combi consists of a live-attenuated recombinant vesicular stomatitis virus expressing Ebola virus glycoprotein (VSV-glycoprotein), and a recombinant replication-defective adenovirus type-5 expressing the same glycoprotein (Ad5-glycoprotein). Both components were developed, manufactured, and stored by Gamaleya Federal Research Center of Epidemiology and Microbiology (Moscow, Russia) according to good manufacturing practices. A full dose of the vaccine was 2.5 × 107 plaque forming units per dose for VSV-glycoprotein, and 2.5 × 1011 viral particles per dose for Ad5-glycoprotein.
Study design and volunteers
An open-label, dose-escalation trial was performed to assess safety, side effects, and immunogenicity after 2 doses in 84 healthy adults of both sexes between 18 and 55 years. The study protocol did not require uniform distribution of men and women among treatment groups, although volunteers were nevertheless distributed among groups so that each group had about the same ratio of men and women. All participants provided written informed consent, and were immunized at half or full dose. The study was reviewed and approved by the appropriate national and local competent authorities, including the Ethics Committee of the Ministry of Health of the Russian Federation. This trial is registered with grls.rosminzdrav.ru (No. 495*), and with zakupki.gov.ru (No. 0373100043215000055).
Trial design
In the first module of the study, the tolerability of VSV-glycoprotein and Ad5-glycoprotein were separately assessed in 12 healthy volunteers each, who received a single half dose of the intended therapeutic dose (0.25 mL). Injection-site reactions, systemic reactogenicity, and medication use to alleviate such symptoms were monitored for 5 days after injection and at day 7.
In the second module, safety and immunogenicity were assessed in 60 volunteers, who received VSV-glycoprotein as the first dose, and Ad5-glycoprotein as the second dose 21 days thereafter. Thirty volunteers were immunized at half dose, while the other 30 received the full dose (0.5 mL). Injection-site reactions, systemic reactogenicity, and medication use to alleviate such symptoms were monitored for 5 days after the first dose, for 3 days after the second dose, and at follow-up on days 7, 28, 35, and 42. Volunteers underwent clinical and laboratory evaluation at each study visit. Laboratory analyses included complete blood and urine counts, creatinine, urea, glucose, total protein, alanine aminotransferase, aspartate aminotransferase, total bilirubin, and creatine phosphokinase.
In all cases, injections were administered intramuscularly into the deltoid muscle (musculus deltoideus).
Immunogenicity
Humoral immune response
Serum samples were collected on 0, 28, and 42 days after the first injection. Glycoprotein-specific antibodies were measured by ELISA using as probe the glycoprotein of 2014 Zaire Ebola virus (subtype Zaire, strain H.sapiens-wt/GIN/2014/Kissidougou-C15) (Sino Biological, SB40442). Briefly, 96-well immunoplates (SPL, SP32296) were coated at 4°C overnight with 100 μL 1 μg/mL glycoprotein in coating buffer (137 mM NaCl, 8.1 mM Na2HPO4, 2.7 mM KCl, and 1.5 mM KH2PO4). Plates were then washed 3 times with PBS containing 0.05 % Tween20 (TPBS), and blocked for an hour at 37°C in TPBS supplemented with 5 % non-fat milk (Applichem, A0830). Subsequently, wells were incubated for 1 hour at 37°C with serum samples (100 μL) serially diluted 2-fold in TPBS from 1:12.5 to 1:102,400. Plates were then washed 3 times with TPBS, labeled for 1 hour at 37°C with 100 μL horseradish peroxidase-conjugated secondary antibody (GE, NA933V) diluted 1:10,000 in TPBS with 5 % milk, and washed 3 times with TPBS. Plates were reacted for 20 minutes at 25°C with 100 μL of the peroxidase substrate TMB (SIGMA, T0440). Reactions were terminated with 4 M H2SO4, and optical density at 450 nm was measured on a Multiscan FC (Thermo Fisher, USA). Relative amounts of glycoprotein-specific antibodies are reported as geometric mean end-point titres with 95 % confidence intervals.
Neutralizing antibodies on day 28 were assayed in serum samples of volunteers immunized at full dose, using infectious Ebola virus Mayinga 1976 as described previously.Citation19 These assays were performed in a BSL-4 laboratory at 48 Central Research Institute of the Ministry of Defense of the Russian Federation.
Cell-mediated immune response
Whole-blood samples were collected on days 0, 28, and 42 after the first injection. Peripheral blood mononuclear cells were isolated from 6–7 mL samples using ACCUSPIN™ System-Histopaque®−1077 (SIGMA, A7054). These cells were seeded at 200,000 per well in 96-well plates (Corning, CLS3595) containing complete RPMI-1640 medium (PAA Laboratories, G0029.3050) supplemented with 10 % of the volunteer's plasma. Cells were then incubated for 2 hours at 37°C in 5 % CO2, and stimulated with 5 μg/mL 2014 Ebola virus glycoprotein from subtype Zaire, strain H.sapiens-wt/GIN/2014/Kissidougou-C15 (Sino Biological, SB40442). Media were collected 48 hours after stimulation, and analyzed for interferon-γ by ELISA (Human IFN-gamma Platinum ELISA, BMS228CE, eBioscience). Results are reported as fold increase in interferon-γ concentration upon exposure to glycoprotein.
Antigen-specific T cell response was measured by CFSE T Cell Proliferation assay. Briefly, peripheral blood mononuclear cells were stained with Carboxyfluorescein succinimidyl ester (CFSE) Tracer Kit (Invitrogen, USA) as described previously.Citation22-23 Cells were then stimulated with 5 μg/mL 2014 Ebola virus glycoprotein from subtype Zaire, strain H.sapiens-wt/GIN/2014/Kissidougou-C15 (Sino Biological, SB40442). Cells were analyzed by flow cytometry 72 hours later on a FACSAriaIII (BD Biosciences, USA) flow cytometer with BD FACSDiva Software (BD Biosciences, USA). Proliferating CD4+ or CD8+ T lymphocytes were identified by forward and side light scatter, expression of CD3, CD4, CD8, and low fluorescence from CFSE. Proliferation in unstimulated cells were subtracted from that of stimulated cells, and negative differences were set to zero. Results are reported as percent proliferating cells.
Statistical analysis
The sample size of 84 was expected to produce reliable data on adverse events. Fisher's exact test was used to analyze binary data. Paired numerical data were analyzed by Wilcoxon signed-rank test, while unpaired numerical data were analyzed by Mann–Whitney U. Data were analyzed in GraphPad Prism version 6, EXCEL 2010, and STATISTICA 7.0. Antibody response is reported as geometric mean titres with 95 % confidence intervals, while cell-mediated response is reported as median with interquartile range.
Abbreviations
| Ad26 | = | recombinant replication-defective adenovirus type-26 |
| Ad5 | = | recombinant replication-defective adenovirus type-5 |
| CFSE | = | carboxyfluorescein succinimidyl ester |
| VSV | = | live-attenuated recombinant vesicular stomatitis virus |
Disclosure of potential conflicts of interest
The authors report no potential conflicts of interest.
Supplemental Files
Download Zip (681.2 KB)Funding
The study was funded by Ministry of Health of Russian Federation. Funding agencies had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data, and had final responsibility in the decision to publish.
References
- Sanchez A, Geisbert TW, Feldmann H. Filoviridae: Marburg and Ebola viruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields virology fifth edition volume 1. Philadelphia (PA): Lippincott Williams & Wilkins; 2007, p. 1409-48.
- Taylor DJ, Leach RW, Bruenn J. Filoviruses are ancient and integrated into mammalian genomes. BMC Evol Biol 2010; 10:193; PMID:20569424; http://dx.doi.org/10.1186/1471-2148-10-193
- Geisbert TW. Marburg and Ebola hemorrhagic fever (Filoviruses). In: Bennet JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases eighth edition. Philadelphia (PA): Elsevier; 2015, p 1995-9.
- World Health Organization. Ebola situation report-21 October 2015. Geneva (Switzerland): World Health Organization; 2015 Oct 21. http://apps.who.int/ebola/current-situation/ebola-situation-report-21-october-2015#main-content
- Sridhar S. Clinical development of Ebola vaccines. Ther Adv Vaccines 2015; 3(5–6): 125-38; PMID:26668751; http://dx.doi.org/10.1177/2051013615611017
- Stanley DA, Honko AN, Asiedu C, Trefry JC, Lau-Kilby AW, Johnson JC, Hensley L, Ammendola V, Abbate A, Grazioli F, et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 2014; 20(10):1126-9; PMID:25194571
- Moorthy V, Fast P, Greenwood B. Heterologous prime-boost immunisation in Ebola vaccine development, testing and licensure report of a WHO Consultation held on 21 November 2014. Geneva, Switzerland; 2014. http://www.who.int/immunization/research/meetings_workshops/ebola_primeboost_21nov14/en/
- Lupton HW, Lambert RD, Bumgardner DL, Moe JB, Eddy GA. Inactivated vaccine for Ebola virus efficacious in guineapig model. Lancet 1980; 2(8207):1294-5; PMID:6108462; http://dx.doi.org/10.1016/S0140-6736(80)92352-1
- Zhu FC, Hou LH, Li JX, Wu SP, Liu P, Zhang GR, Hu YM, Meng FY, Xu JJ, Tang R, et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet 2015; 385(9984):2272-9; PMID:25817373; http://dx.doi.org/10.1016/S0140-6736(15)60553-0
- Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, Carroll MW, Doumbia M, Draguez B, Duraffour S, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015; 386(9996):857-66; PMID:26248676; http://dx.doi.org/10.1016/S0140-6736(15)61117-5
- Huttner A, Dayer JA, Yerly S, Combescure C, Auderset F, Desmeules J, Eickmann M, Finckh A, Goncalves AR, Hooper JW, et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis 2015; 15(10):1156-66; PMID:26248510; http://dx.doi.org/10.1016/S1473-3099(15)00154-1
- Tapia MD, Sow SO, Lyke KE, Haidara FC, Diallo F, Doumbia M, Traore A, Coulibaly F, Kodio M, Onwuchekwa U, et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2016; 16(1):31-42; PMID:26546548; http://dx.doi.org/10.1016/S1473-3099(15)00362-X
- De Santis O, Audran R, Pothin E, Warpelin-Decrausaz L, Vallotton L, Wuerzner G, Cochet C, Estoppey D, Steiner-Monard V, Lonchampt S, et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis 2016; 16(3):311-20; PMID:26725450; http://dx.doi.org/10.1016/S1473-3099(15)00486-7
- Ledgerwood JE, DeZure AD, Stanley DA, Novik L, Enama ME, Berkowitz NM, Hu Z, Joshi G, Ploquin A, Sitar S, et al. Chimpanzee adenovirus vector Ebola vaccine - preliminary report. N Engl J Med 2014; PMID:25426834; http://dx.doi.org/10.1056/NEJMoa1410863
- Ledgerwood JE, Sullivan NJ, Graham BS. Chimpanzee adenovirus vector Ebola vaccine–preliminary report. N Engl J Med 2015; 373(8):776; PMID:26287857
- Regules JA, Beigel JH, Paolino KM, Voell J, Castellano AR, Munoz P, et al. A Recombinant Vesicular Stomatitis Virus Ebola Vaccine—Preliminary Report. N Engl J Med 2015; PMID:25830322; http://dx.doi.org/10.1056/NEJMoa1414216
- Agnandji ST, Huttner A, Zinser ME, Njuguna P, Dahlke C, Fernandes JF, Yerly S, Dayer JA, Kraehling V, Kasonta R, et al. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med 2016; 374(17):1647-60; PMID:25830326; http://dx.doi.org/10.1056/NEJMoa1502924
- Bengtsson KL, Song H, Stertman L, Liu Y, Flyer DC, Massare MJ, Xu RH, Zhou B, Lu H, Kwilas SA, Hahn TJ, Kpamegan E, Hooper J, Carrion R Jr, Glenn G, Smith G. Matrix-M adjuvant enhances antibody, cellular and protective immune responses of a Zaire Ebola/Makona virus glycoprotein (GP) nanoparticle vaccine in mice. Vaccine 2016 Apr 7; 34(16):1927-35; http://dx.doi.org/10.1016/j.vaccine.2016.02.033
- Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, Mulangu S, Hu Z, Andrews CA, Sheets RA, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine 2010; 29(2):304-13; PMID:21034824; http://dx.doi.org/10.1016/j.vaccine.2010.10.037
- Good Safety Information Practices. In: Council for International Organizations of Medical Sciences, author. Guidelines for preparing core clinical-safety information on drugs: report of CIOMS working group III. Geneva (Switzerland): World Health Organization; 1995.
- Centers for Disease Control and Prevention. Possible side-effects from vaccines. Atlanta (GA): Centers for Disease Control and Prevention; 20 Jul 2016. http://www.cdc.gov/vaccines/vac-gen/side-effects.htm
- Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc 2007; 2(9):2049-56; PMID:17853860; http://dx.doi.org/10.1038/nprot.2007.296
- Tukhvatulin AI, Dzharullaeva AS, Tukhvatulina NM, Shcheblyakov DV, Shmarov MM, Dolzhikova IV, Stanhope-Baker P, Naroditsky BS, Gudkov AV, Logunov DY, et al. Powerful complex immunoadjuvant based on synergistic effect of combined TLR4 and NOD2 activation significantly enhances magnitude of humoral and cellular adaptive immune responses. PLoS One 2016 11(5):e0155650; PMID:27187797; http://dx.doi.org/10.1371/journal.pone.0155650