ABSTRACT
Rotavirus is the leading cause of severe and dehydrating diarrhea in children aged under 5 years. We undertook this hospital-based surveillance study to examine the possible relationship between the severity of diarrhea and the various G-group rotaviruses circulating in India. Stool samples (n = 2,051) were systematically collected from 4,711 children aged <5 years admitted with severe acute gastroenteritis to 12 medical school centers from April 2011 to July 2012. Rotavirus testing was undertaken using a commercially available enzyme immunoassay kit for the rotavirus VP6 antigen (Premier Rotaclone Qualitative ELISA). Rotavirus positive samples were genotyped for VP7 and VP4 antigens by reverse-transcription polymerase chain reaction at a central laboratory. Of the stool samples tested for rotavirus antigen, 541 (26.4%) were positive for VP6 antigen. Single serotype infections from 377 stool samples were compared in terms of gastroenteritis severity. Among those with G1 rotavirus infection, very severe diarrhea (Vesikari score ≥ 16) was reported in 59 (33.9%) children, severe diarrhea (Vesikari score 11–15) in 104 (59.8%), moderate (Vesikari score 6–10) and mild diarrhea (Vesikari score 0–5) in 11 (6.3%). Among those with G2 infection, very severe diarrhea was reported in 26 (27.4%) children, severe diarrhea in 46 (48.4%), and moderate and mild diarrhea in 23 (24.2 %). Among those with G9 infection, very severe diarrhea was reported in 47 (54.5%) children, severe diarrhea in 29 (33.6%), and moderate and mild diarrhea in 10 (11.9%). Among those with G12 infection, very severe diarrhea was reported in 9 (40.9%) children and severe diarrhea in 13 (59.1%). The results of this study indicate some association between rotavirus serotypes and severity of gastroenteritis.
Introduction
Rotavirus infection (mostly caused by Group A viruses) is a major, vaccine-preventable disease in humans worldwide. Although the infection is ubiquitous in all ages, it is most significant, and often quite severe, in infants and young children. The burden of severe rotavirus illness and death is greatest in children from countries with low socio-economic status, with more than 80% of rotavirus-related deaths estimated to occur in Asia and sub-Saharan Africa.Citation1
India, in particular, has a large population at risk of clinically significant rotavirus gastroenteritis: of the 1.2 billion population, 11% are aged <5 years. In 2008, diarrhea attributable to rotavirus infection resulted in 453,000 deaths (95% CI 420,000–494,000) worldwide in children aged <5 years, representing 37% of deaths attributable to diarrhea and 5% of all deaths in children aged <5 years.Citation2 Five countries accounted for more than half of all deaths attributable to rotavirus infection: Democratic Republic of the Congo, Ethiopia, India, Nigeria, and Pakistan, with India alone accounting for 22% of deaths (98,621 deaths).Citation2
Rotavirus Serotypes are specified by 2 outer capsid proteins, VP4 and VP7.Citation3 The protease-sensitive protein VP4 defines P genotypes and the glycoprotein VP7 defines the G genotypes.Citation4 As the 2 genes that determine G-types and P-types can be passed on separately to progeny viruses, different combinations are found.Citation5
Of the 24 G genotypes and 33 P genotypes described to date, 12 G and 15 P genotypes are known to infect humans.Citation6,7 Genotype G1P[8], G2P[4], G3P[8], G4P[8] and G9P[8] cause over 90% of rotavirus disease worldwide. Genotype G9 strains were initially identified in the USA and Japan in 1983–1984.Citation8,9 The G9 strains re-emerged in the early to mid-1990s, and the global prevalence has increased, such that G9 in combination with P[8], P[4] and P[6] have been detected in over 55 countries in Europe, Asia, Africa, South and North America, and represent the dominant genotype in some regions over the past decade.Citation10,11
However, G1 has remained the predominant strain in India, while G9 and G12 are emerging as new strains.Citation12,13 Interestingly, recent studies have indicated that the G9 strain is mainly confined to the eastern part of India.13Current available data indicates fluctuations in rotavirus G/P-type that also vary by geography and season, as well as the emergence of unusual types, such as G5, G6, G8, G9, G12, and P[6] for reasons that are poorly understood. This makes accurately predicting serotype circulation and understanding the clinical implications of the serotype changes a challenge.Citation11
A pentavalent vaccine containing 5 separate viruses that expressed either human G1, 2, 3, or 4 VP7s, and a human P(8) VP4 on the bovine WC3 backbone was developed and licensed after extensive clinical evaluation, by Merck Pharmaceuticals as RotaTeq®.Citation14 Using a wild-type human rotavirus isolate P1A[8]G1 strain which represented the most common human rotavirus VP7 and VP4 antigens and attenuated by multiple tissue culture passages, a monovalent oral rotavirus vaccine was developed by GlaxoSmithKline Biologicals, as Rotarix®.Citation15 Both Rotarix and RotaTeq vaccines have since demonstrated the efficacy in developing countries, leading to the WHO recommendation for their widespread introduction.Citation16
At the time of this study both Rotarix and Rotateq were available in the private market in India and had not been included in the Indian National Immunization Schedule. The subjects involved in this study were from suburban and rural population and depended upon the study hospitals to provide vaccination in compliance with the Indian National Immunization Schedule. None of the available Rotavirus vaccines was being used in the study population.
Both Rotateq and Rotarix have demonstrated heterotypic protection in the efficacy studies but the level of protection is lower than that seen for the homotypic strains, therefore it is important to keep a track of new emerging strains and severity associated with them.
As there are still a number of new rotavirus vaccines under development globally, the results from this study may help in understanding the need for inclusion of rotavirus strains depending upon the severity of clinical implications associated with particular serotypes.
Earlier, we reported an estimate of the burden of rotavirus gastroenteritis in children aged <5 years, taking into account rotavirus positivity, genotypic distribution and seasonality in different regions of India.Citation13 One of the objectives of this surveillance was to categorize rotavirus-associated diarrhea by clinical severity and associated serotypes. Given the epidemiological variability in rotavirus G-type distribution, and the spectrum of clinical manifestation of rotavirus disease, we examined the possible relationship between epidemiologic and clinical features of rotavirus gastroenteritis and G types.
Results
Stool samples (n = 2051) were systematically collected from 4,711 children aged <5 years and were tested for VP6 antigen. Of the 541 rotavirus VP6 antigen positive stool samples, the predominant rotavirus genotypes reported were G1 (38%), G2 (18%), G9 (18%) and G12 (9%). A large number of children (25%) had mixed infections. In order to directly compare the G type strains, and eliminate any bias in reporting, all mixed and non-typeable samples were excluded. A total of 377 positive stool samples were included in our analysis. Median of maximum number of stools collected was 9 in any 24 hours.
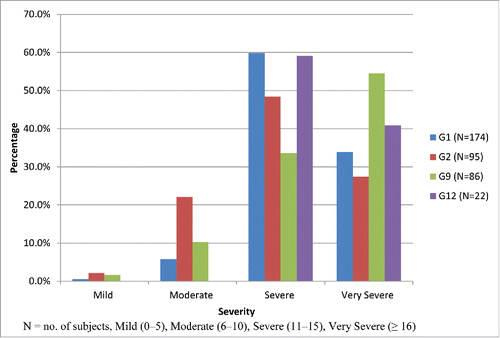
shows the distribution of rotavirus strains by diarrhea severity. G1-specific diarrhea was reported in 174 children, with very severe diarrhea reported in 59 (33.9%), severe diarrhea in 104 (59.8%), moderate diarrhea in 10 (5.8%) and mild diarrhea in 1 (0.5%). G2-specific diarrhea was reported in 95 subjects, with very severe diarrhea reported in 26 (27.4%) children, severe diarrhea in 46 (48.4%), moderate diarrhea in 21 (22.1%) and mild diarrhea in 2 (2.1%). G9-specific diarrhea was reported in 86 subjects, with very severe diarrhea reported in 47 (54.5%) children, severe diarrhea in 29 (33.6%), moderate diarrhea in 9 (10.3%) and mild diarrhea in 1 (1.6%). G12-specific diarrhea was reported in 22 children, with very severe diarrhea reported in 9 (40.9%), severe diarrhea in 13 (59.1%); there were no cases of moderate or mild diarrhea. These differences are confirmed with the observed median of the Vesikari score per genotype (p-value = 0.0002). Diarrhea due to G3 strain was not seen, and the G4 strain was found from one child, therefore neither of these strains were considered for direct comparison.
Figure 1. Reported rotavirus strains and associated diarrhea severity (based on Vesikari grading scale).
compares the demographic details of the infants who experienced G1, G2, G9 and G12 gastroenteritis, as well as their clinical characteristics. There was significant difference in the mean age at hospitalization of the children. The greatest number of children reporting vomiting for more than 3 days was in the G9 group (58.8%), followed by G12 (40.9%) and G2 (26.3%), while the lowest number of subjects was in G1 group (24.7%). Dehydration was relatively higher in the G12 (90.9%) and G9 groups (88.4%) compared with the 2 other groups, G1 (78.7) and G2 (64.2%). High number of loose stools in 24h is more frequent in subjects with G1. The association between genotype and high number of loose stools in 24h, vomiting for more than 3 days and Veskari score is confirmed by the multivariate approach, once adjusting on the age effect.
Table 1. Rotavirus serotypes associated demographic and clinical characteristics.
Discussion
Our study shows that children hospitalized with G1 serotype infection generally had less severe diarrhea than those with the new emerging strains, G9 and G12. These data are consistent with studies from 3 Latin American countries, which indicate that serotype G9 infection is associated with more-severe disease than serotype G1 infection.Citation17-19 Similar trends were reported in studies conducted in the United Kingdom, which showed that the proportion of G9 strains (P[6] and P[8]) was significantly higher among patients admitted to the hospital than those treated at the community level.Citation18 Another hospital-based study conducted in London found a higher proportion of cases requiring intravenous rehydration among those infected with the G9 strain, compared with other common G types.Citation19 In contrast, while a study from Indonesia reported greater clinical severity with G2 serotype among hospitalized subjects due to rotavirus gastroenteritis,Citation20 2 studies conducted in Italy and the United States did not find differences in disease severity between patients with G9 infection and patients with infection due to other G types.Citation21,22 Difference in severity may be traced back to the origin of G9 and G12 strains, which goes back to mid-nineties in Europe and United states while it is more recent in India.Citation10-13
Differences in disease severity may be caused not only by variation in virulence, but by the introduction of new strains. When a rotavirus strain is newly introduced into a community, more frequent and more-severe cases are anticipated than those associated with the common rotavirus strains because of a lack of maternal antibodies, and lack of prior exposure to this specific rotavirus type. Clinical determinants, such as duration of vomiting and dehydration, are more frequently observed with G9 and G12 strains, which may contribute to the higher severity associated with these strains.
One limitation of this analysis is that the surveillance protocol was set up to capture only acute hospitalized gastroenteritis cases, which only represents the trends observed in children who were already more sick than those who may have been either managed in the community, or who may not have been sick enough to require hospitalization. This limitation suggested the need for a further research involving both hospitalized and non-hospitalized cases of rotavirus gastroenteritis. In addition, mixed infections and non-typable samples, which form a major part of the overall disease burden, were not considered for clinical severity comparisons.
In summary, G9 and G12 serotype associated rotavirus gastroenteritis infections are on the increase and represent 2 important emerging serotypes in children younger than 5 years in India.Citation12,13 Our findings suggest that rotavirus gastroenteritis infections caused by G9 and G12 (emerging serotypes) are associated with clinically more-severe disease than infections with G1 and G2 serotypes, which are still the most common serotypes seen in the Indian population.Citation13
This study also highlights the importance of including the epidemiologically emerging rotavirus strains in future multivalent rotavirus vaccines. This would help to make the vaccines regionally more specific, broaden the scope of vaccine coverage and enhance the ability of the vaccine to provide a secured homotypic protective response to the potentially severe serotypes.
Patients and methods
Study centers and duration
The surveillance study was conducted to capture the severity and strains of rotavirus gastroenteritis in children aged <5 years at 12 medical school centers in India, from April 2011 to July 2012. ()
Inclusion criteria
Children <5 years of age presenting with severe acute gastroenteritis (defined by the passage of ≥3 looser than normal stools, with or without vomiting during the preceding 24 hour period) and requiring hospitalization for at least 6 hours were eligible for inclusion in this study. An approved, informed consent statement, for obtaining stool samples, was obtained from the parents/legally acceptable representatives of the children, as per local ethical and regulatory requirements. Upon obtaining consent, the children were included in the study and a stool sample obtained. Children older than 60 months, and those younger than 60 months but not requiring hospitalization for at least 6 hours, or whose parents did not consent for stool sampling were not included in the study.
Clinical assessment
Parameters for clinical assessment of diarrhea severity included: time of onset, duration and maximum number of episodes of diarrhea and vomiting, intensity of fever and dehydration. These parameters were recorded in a case report form. Severity of diarrhea was assessed using the Vesikari scoring system.Citation23 As per the Vesikari score, a grade of 0–5 was considered as mild, 6–10 as moderate, 11–15 as severe and >16 as very severe.
Stool specimen collection
Approximately 5 ml of stool sample was collected from every alternate children if available, in stool containers either on the day of presentation to hospital or within 48 hours of hospital admission to avoid confounding from hospital-acquired infections. All the stool specimens were stored at −20°C until testing, and sufficient care was taken to avoid freeze–thaw cycles.
Detection of rotavirus
All the stool samples were tested for rotavirus VP6 antigen using a commercial enzyme immunoassay kit (Premier Rotaclone Qualitative EIA, Meridian Bioscience Inc., Cincinnati, USA) at the respective study centers, in duplicate, and with appropriate controls. Rotavirus VP6 antigen positive stool samples were sent for genotyping at a Central Laboratory at the Department of Gastrointestinal Sciences, Christian Medical College, Vellore, India.
Strain surveillance and characterization
Genotyping was performed using methods that have been previously published and considered standard.Citation13,23,Citation24
Statistical analysis
Diarrheal hospital log books, case report forms and genotype result reports were used to generate the data for analysis. All logs and forms were scrutinized for completeness, and the data were entered into Excel 2012 (Microsoft, Redmond, WA, USA). Analysis was performed using QuickCalcs, version 5 (GraphPad Software, Inc., La Jolla, CA, USA). Stool samples were collected as continuous variable. Tests of proportion, Chi-squared and Fisher exact tests were applied for qualitative data, and Wilcoxon rank-sum test for quantitative data. A multinomial logistic regression was used as a multivariate approach. P values <0.05 were considered to be statistically significant.
Ethics
The study was conducted according to The Code of Ethics of the World Medical Association (Declaration of Helsinki), ICH-GCP guidelines issued by the Central Drugs Standard Control Organization, India and the Indian Council of Medical Research ethical guidelines. Independent Ethics Committee/Institutional Review Board clearance was obtained before initiation of the study at each center. The study was registered in the Indian Clinical Trial Registry under number CTRI/2012/03/002475.
Disclosure of potential conflicts of interest
All the authors except Tarun Saluja, Mandeep S. Dhingra, Rajendra Prasad, Annick Moureau and Badri N. Patnaik were the Principal Investigators of the study at their respective study sites. All the Principal Investigators declared that they had no financial interests in the funder but received research grant to undertake the study. Tarun Saluja and Rajendra Prasad are employed by Shantha Biotechnics Private Limited and were involved in planning, analyzing and interpreting the study. Annick Moureau and Mandeep S. Dhingra are employed by Sanofi Pasteur. Annick Moureau was involved in the interpretation of data. Mandeep S. Dhingra was involved in planning, analyzing and interpreting the study. Badri N. Patnaik is employed by Shantha Biotechnics Private Limited and was involved in analyzing and interpreting the study.
Acknowledgments
We are grateful to the subjects who volunteered to participate in this research study.
Funding
This study was funded by a research grant from Shantha Biotechnics Private Limited (A part of the Sanofi group).
References
- Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis 2006; 12:304-6; PMID:16494759; http://dx.doi.org/10.3201/eid1202.050006
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136-41; PMID:22030330; http://dx.doi.org/10.1016/S1473-3099(11)70253-5
- O'Ryan M. The ever-changing landscape of rotavirus serotypes. Pediatr Infect Dis J 2009; 28:S60-2; PMID:19252426; http://dx.doi.org/10.1097/INF.0b013e3181967c29
- Patton JT. Rotavirus diversity and evolution in the post-vaccine world. Discov Med 2012; 13:85-97; PMID:22284787
- Desselberger U, Wolleswinkel-van den Bosch J, Mrukowicz J, Rodrigo C, Giaquinto C, Vesikari T. Rotavirus types in Europe and their significance for vaccination. Pediatr Infect Dis J 2006; 25:S30-41; PMID:16397427; http://dx.doi.org/10.1097/01.inf.0000197707.70835.f3
- Maes P, Matthijnssens J, Rahman M, Van Ranst M. RotaC: a web-based tool for the complete genome classification of group A rotaviruses. BMC Microbiol 2009; 9:238; PMID:19930627; http://dx.doi.org/10.1186/1471-2180-9-238
- Patel MM, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J 2011; 30:S1-5; PMID:21183833; http://dx.doi.org/10.1097/INF.0b013e3181fefa1f
- Clark HF, Hoshino Y, Bell LM, Groff J, Hess G, Bachman P, Offit PA. Rotavirus isolate WI61 representing a presumptive new human serotype. J Clin Microbiol 1987; 25:1757-62; PMID:2443534
- Nakagomi T, Ohshima A, Akatani K, Ikegami N, Katsushima N, Nakagomi O. Isolation and molecular characterization of a serotype 9 human rotavirus strain. Microbiol Immunol 1990; 34:77-82; PMID:1691433; http://dx.doi.org/10.1111/j.1348-0421.1990.tb00994.x
- Gentsch JR, Laird AR, Bielfelt B, Griffin DD, Banyai K, Ramachandran M, Jain V, Cunliffe NA, Nakagomi O, Kirkwood CD, et al. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J Infect Dis 2005; 192(Suppl 1):S146-59; PMID:16088798; http://dx.doi.org/10.1086/431499
- Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol 2005; 15:29-56; PMID:15484186; http://dx.doi.org/10.1002/rmv.448
- Kang G, Arora R, Chitambar SD, Deshpande J, Gupte MD, Kulkarni M, Naik TN, Mukherji D, Venkatasubramaniam S, Gentsch JR, et al. Multicenter, hospital-based surveillance of rotavirus disease and strains among indian children aged. J Infect Dis 2009; 200(Suppl 1):S147-53; PMID:19817593; http://dx.doi.org/10.1086/605031
- Saluja T, Sharma SD, Gupta M, Kundu R, Kar S, Dutta A, Silveira M, Singh JV, Kamath VG, Chaudhary A, et al. A multicenter prospective hospital-based surveillance to estimate the burden of rotavirus gastroenteritis in children less than five years of age in India. Vaccine 2014; 32(Suppl 1):A13-9; PMID:25091667; http://dx.doi.org/10.1016/j.vaccine.2014.03.030
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. Rotavirus Efficacy and Safety Trial (REST) study team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354(1):23-33; PMID:16394299; http://dx.doi.org/10.1056/NEJMoa052664
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11-22; PMID:16394298; http://dx.doi.org/10.1056/NEJMoa052434
- WHO. Rotavirus vaccines: an update. Weekly Epidemiological Record 2009; 84:533-40; PMID:20034143
- Linhares AC, Verstraeten T, Wolleswinkel-van den Bosch J, Clemens R, Breuer T. Rotavirus serotype G9 is associated with more-severe disease in Latin America. Clin Infect Dis 2006; 43:312-4; PMID:16804845; http://dx.doi.org/10.1086/505493
- Iturriza-Gomara M, Green J, Brown DW, Ramsay M, Desselberger U, Gray JJ. Molecular epidemiology of human group A rotavirus infections in the United Kingdom between 1995 and 1998. J Clin Microbiol 2000; 38:4394-401; PMID:11101570
- Cubitt WD, Steele AD, Iturriza M. Characterisation of rotaviruses from children treated at a London hospital during 1996: emergence of strains G9P2A[6] and G3P2A[6]. J Med Virol 2000; 61:150-4; PMID:10745248; http://dx.doi.org/10.1002/(SICI)1096-9071(200005)61:1%3c150::AID-JMV24%3e3.0.CO;2-W
- Wardana OP, Athiyyah AF, Budiono, Soedirham O, Soegijanto S. The role of rotavirus genotype in the severity of acute diarrhea in children under 5 years old at Surabaya, Indonesia. J Hum Virol Retrovirol 2015; 2:00039. DOI: 10.15406/jhvrv.2015.02.00039
- Arista S, Vizzi E, Ferraro D, Cascio A, Di Stefano R. Distribution of VP7 serotypes and VP4 genotypes among rotavirus strains recovered from Italian children with diarrhea. Arch Virol 1997; 142:2065-71; PMID:9413515; http://dx.doi.org/10.1007/s007050050224
- Clark HF, Lawley DA, Schaffer A, Patacsil JM, Marcello AE, Glass RI, Jain V, Gentsch J. Assessment of the epidemic potential of a new strain of rotavirus associated with the novel G9 serotype which caused an outbreak in the United States for the first time in the 1995–1996 season. J Clin Microbiol 2004; 42:1434-8; PMID:15070985; http://dx.doi.org/10.1128/JCM.42.4.1434-1438.2004
- Ruuska T, Vesikari T. A prospective study of acute diarrhoea in Finnish children from birth to 2 1/2 years of age. Acta Paediatr Scand 1991; 80:500-7; PMID:1872172; http://dx.doi.org/10.1111/j.1651-2227.1991.tb11893.x
- Iturriza-Gomara M, Kang G, Gray J. Rotavirus genotyping: keeping up with an evolving population of human rotaviruses. J Clin Virol 2004; 31:259-65; PMID:15494266; http://dx.doi.org/10.1016/j.jcv.2004.04.009