ABSTRACT
Background: Intussusception has been identified as a rare adverse event following rotavirus immunization. We sought to determine the incidence of intussusception among infants in Canada both before and after introduction of rotavirus immunization programs. Methods: We used Canadian Institute for Health Information (CIHI) Discharge Abstract Database (DAD) to identify infants under 1 y of age who were admitted to a Canadian hospital, which the exception of Quebec, which does not submit data to CIHI, with a diagnosis of intussusception (ICD-10 code K56.1, and ICD-9 code 560) between January 1st, 2003 and December 31, 2013. We compared rates of intussusception hospitalization before and after rotavirus vaccine program introduction. Rates were adjusted for calendar year, age (in months), sex and region using Poisson regression models. Denominator data for infants under 1 year, stratified by age in months, were obtained from Statistics Canada. Results: Annual intussusception hospitalization rates ranged from 20-30 per 100,000 infants over the study period, with no evidence of a trend over time. Intussusception hospitalization rates were highest in infants 4 to <8 months and lowest in those under 2 months or between 10 and <12 months. Males had higher rates than females both overall and within each age group. The rate of intussusception hospitalization after rotavirus vaccine program introduction was 22.4 (95% CI: 18.3, 27.4) compared to 23.4 (95% CI: 21.5, 25.4) per 100,000 before program introduction. Conclusions: We have described baseline intussusception hospitalization rates for infants in Canada and have found no evidence of a change in rate after implementation of routine rotavirus immunization programs.
Introduction
Intussusception is a condition wherein a section of intestine slides into a distal segment of the intestine (often referred to as “telescoping”), creating an obstruction. It is one of the leading causes of acute intestinal obstruction in infants and young children, with higher incidence in males.Citation1,2 A recent literature review reported the mean incidence across studies of intussusception among children <1 year was 74 per 100,000 infant years, with wide variability across countries and regions. For example, incidence was about 30 per 100,000 in North American studies and exceeded 100 per 100,000 in studies from a number of other countries (e.g., Australia, Hong Kong, Japan, and Israel).Citation3 Accurate estimates of intussusception incidence are not available for many countries, and in the vast majority of childhood cases no clear cause is identified.Citation1,2
In 1999, the world's first vaccine to protect against rotavirus infection, RotaShield®, was withdrawn from the market in the United States (US) less than one year after its introduction due to an identified association suggesting a 20- to 30-fold increase in relative risk of intussusception for infants who received RotaShield® and attributable risk of between 10 and 20 excess cases per 100,000 vaccinated infants.Citation4 Associations between intussusception and newer rotavirus vaccines were also reported, namely for RotarixTM in Mexico,Citation5,6 Brazil,Citation5 Australia,Citation7,8 and the US,Citation9 and RotaTeq® in the USCitation10,11 and Australia.Citation7,8 However, the estimates of increased risk were much lower than those reported for RotaShield®, with relative risks of <10 and attributable risks in the range of 1 to 5 additional intussusception cases per 100,000 infants vaccinated. It is not clear whether the increased risk reflects an absolute increase in intussusception in infancy or earlier occurrence among infants in whom it would have occurred later in infancy in the absence of immunization.Citation8 To our knowledge, no studies assessing risk of intussusception after rotavirus immunization using data from Canada have been published to date.
While the World Health Organization's Global Advisory Committee on Vaccine Safety has concluded that, based on the available data, both RotaTeq® and Rotarix™ exhibit acceptable safety profiles where a small but significant increased risk of intussusception is outweighed by the benefit of the vaccine, they recommend that post-licensure safety surveillance be undertaken in any country introducing the vaccine.Citation12,13 Canada's National Advisory Committee on Immunization (NACI) has also highlighted the importance of safety surveillance post-implementation.Citation14 The 2 rotavirus vaccines available in Canada, RotaTeq® and Rotarix™, were licensed in 2006 and 2008 respectively. Passive vaccine safety surveillance is conducted by local and provincial/territorial public health agencies in Canada, who then report nationally to the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS), enabling national surveillance of intussusception following rotavirus vaccines. Active surveillance of pediatric intussusception cases is conducted through the Immunization Monitoring Program-Active (IMPACT). These data are also included in CAEFISS reports.Citation15
The objective of our study was to apply a case-finding algorithm developed and validated in Ontario, CanadaCitation16 to national health administrative data (with the exception of Quebec, which does not submit data to CIHI DAD) to determine the incidence of intussusception among infants less than one year of age, both before and after introduction of rotavirus immunization programs. This would allow us to establish an estimate of the background rate of intussusception.
These estimates are important for 2 reasons. Firstly, the descriptive epidemiology of intussusception across Canada establishes a benchmark for background risk of intussusception over time including the time period prior to the introduction of publicly funded rotavirus vaccine programs in Canadian jurisdictions. A good understanding of the background intussusception incidence is important for effective vaccine safety surveillance in order to identify potential safety signals. Secondly, this analysis allows for an examination of whether there was a change in the epidemiology following the introduction of the immunization programs.
Results
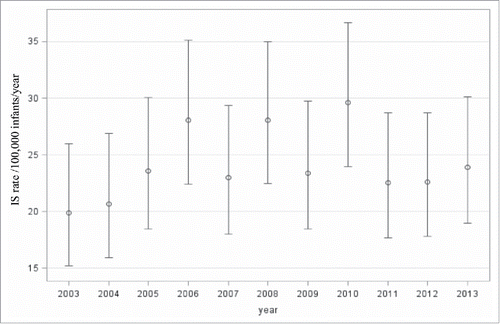
Annual incidence of intussusception ranged from about 20 per 100,000 infants in 2003 to 30 per 100,000 infants in 2010, adjusting for age group in months, sex and region. There was no evidence of a clear trend over the study period (p = 0.296) ()
Figure 1. Overall annual intussusception admission rates in Canadian infants by year and 95% confidence limits adjusted for age group, sex and region 2003-2013.
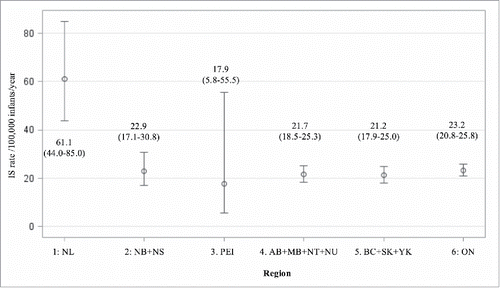
The overall annual rates of intussusception admissions in infants were very similar across all the major regions, ranging from 21 per 100,000 in Region 5:(BC+SK+YK) to 23 per 100,000 in Region 6:(Ontario). The intussusception rate was markedly higher for NL (61 per 100,000), and slightly lower for PEI (18 per 100,000), but both these provinces had extremely wide confidence intervals due to small sample size ().
Figure 2. Annual intussusception admissionrates and 95% confidence intervals in infants by region adjusted for age group, sex and year 2003-2013.
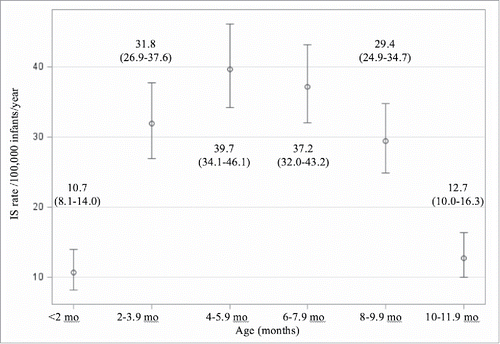
The adjusted annual incidence of intussusception admissions among infants varied strongly by age group in months (p < 0.0001), with the highest incidence occurring between 4 and 8 months of age, and the lowest incidence in infants under 2 months and between 10-12 months (). Incidence was higher in males compared to females both overall and in each age category. Sex-specific intussusception admission rates among infants adjusted for age group, calendar year and region were 18.7 (95% CI 16.5-21.3) for females and 30.6 (95% CI 27.5-33.9) for males per 100,000 infants for a relative incidence for females vs. males of 0.61 (95% CI 0.53-0.71, p < 0.0001).
Figure 3. Annual intussusception admission rates and 95% confidence intervals in infants by age group in months, adjusted for calendar year, sex and region 2003-2013*. *P < 0.0001 for a differential effect by age group.
Annual intussusception rates among infants before and after program introduction adjusted for age group, sex and calendar year were 23.4 per 100,000 infants (95% CI 21.5-25.4) before program introduction and 22.4 per 100,000 infants (95% CI 18.3-27.4) after program introduction for a relative incidence of intussusception after vs. before program introduction of 0.96 (95% CI 0.78, 1.18, p = 0.69). Therefore, there was no evidence to suggest any change in the adjusted intussusception rates after rotavirus vaccine program introduction. Sensitivity analyses limiting the age of intussusception incidence to infants 2 to 8 months, and then further limiting it to infants 2 to 6 months and 2 to 4 months did not change the conclusions of the comparisons by year or comparing pre- and post- program introduction (Appendix ).
Discussion
In this study, we reported a reasonably stable and consistent incidence of intussusception admissions across Canada between 2003 and 2013. Pooled annual intussusception admission rates in infants fluctuated between 20 and 30 cases per 100,000 infants between 2003 and 2013, with no evidence of an increasing or decreasing trend over time, and no evidence of a change in intussusception incidence after the introduction of universal rotavirus immunization programs. Rates of intussusception in infants across regions for all years combined were also similar, varying between 21 and 23 cases per 100,000 person-years, with the exception of NL, where much higher rates were observed. The higher rate in NL could be due to chance in this small provincial sample, differences in coding and reporting practices in NL, or genuinely higher incidence. Regional variation has been observed in other countries.Citation17 Further study including primary data collection would be necessary to verify and confirm the reasons for these higher rates.
We also showed that the highest incidence in the first year of life occurs between 4 and 8 months of age, which is consistent with the findings in other studies.Citation1,6,10,18-20 Our estimates for intussusception incidence in Ontario agreed with those reported in our previous validation study in Ontario,Citation16 and we observed similar rates across most other Canadian jurisdictions. The Canadian rates we observed were also similar to those reported in the US,Citation21,22 but were much lower than those reported in AsianCitation23-26 and Western European countries,Citation1,19,27,28 as well as Australia.Citation18 These differences might be explained though a combination of practice and healthcare utilization patterns, ethnic differences, or differences in techniques of case ascertainment and disease identification algorithm properties.
Although we had relatively limited follow-up time available for analysis after implementation of rotavirus immunization programs, we found no evidence of an increase in admission rates for infant intussusception across Canadian jurisdictions. This remained true after limiting the age of intussusception cases to smaller age cohorts in infancy which corresponds to the time when infants would receive the series, as well as the first dose (i.e., 2 to 8, 2 to 6 months and 2 to 4 months), in which we would expect to see a safety signal associated with the vaccine, if it were to exist, since this coincides with the time period in which the vaccine would be given. Our findings are reassuring given that other studies have reported slight, but statistically significant increases in intussusception following program implementation.Citation5-8,10 Although Canada does not have a national immunization registry and we were not able to report on vaccine coverage in those jurisdictions with rotavirus immunization programs, Wilson and co-authors estimated that in the province of Ontario, coverage during the first year of the program was approximately 87%.Citation29
There have been 9 cases of intussusception reported to CAEFISS as adverse events following rotavirus immunization by provinces included in our study after the implementation of their rotavirus immunization programs and until December 2013 (personal communication, Dr Robert Pless, Public Health Agency of Canada, Dec 16, 2015). Cases in this passive surveillance system were required to be reported within 42 d of administration of rotavirus vaccine, though reporting of events was voluntary for health care providers, and then only if they were aware that the adverse event occurred. The total birth cohort in the 3 jurisdictions reporting cases over this time period was 527,155 infants.Citation29 If we use the range of background rates we identified (i.e., between 19.9 and 29.6 per 100,000 infants per year), and consider that cases are only reported within a 42-day rather than 365-day period, we would expect within this birth cohort between 12 and 18 cases of intussusception reported based on the background rates. Although the number of cases would be lower as we do not have 100% vaccine coverage, these numbers suggest that no vaccine safety signal has been identified through the Canadian passive vaccine safety surveillance system.
A strength of our study was that we used a validated algorithm to identify cases of intussusception. Our study was also population-based, using the CIHI DAD which has virtually complete coverage for admissions to acute care facilities (>99.9%) in the years studied.Citation30 A limitation of our study was that the ICD-10 code-based algorithm we used to identify infant intussusception cases was limited to identifying infants who were hospitalized. There is some evidence to suggest that relying on hospitalization data will underestimate intussusception incidence if cases are managed in the emergency room or short stay units.Citation31,32 In one study occurring in 12 pediatric tertiary care centers in Canada in 2008/09, 25.2% of intussusception cases were managed in a short stay unit or the emergency department. The number of cases managed in this way likely varied across hospitals due to differences in hospital and physician practices.Citation31 The algorithm that we developed in our previous work included only CIHI DAD admissions data, and was validated with chart review conducted at a large pediatric hospital in Ottawa as the reference standard. Algorithms using emergency room and outpatient data (from the National Ambulatory Care Reporting System) were also evaluated but were found to have worse performance characteristics compared to the CIHI DAD algorithm.Citation16 We acknowledge that the validation study was based on data from one pediatric hospital in one province, and may not be representative of practices elsewhere. Although the ICD-10 code-based algorithm we employed was validated in an Ontario population, it is possible that its performance characteristics differ in different Canadian jurisdictions due to practice patterns, data abstraction practices, data quality and other causes. However, we are reassured by the consistency in incidence estimates across provinces (except NL). Finally, we did not have access to individual-level immunization data, so were not able to assess if any of the infants hospitalized with intussusception were vaccinated, or if vaccinated, when in relation to immunization the intussusception occurred. However, we would have been able to discern an overall increase in intussusception hospitalization in the post-program period, if this had occurred.
In conclusion, we report the incidence of intussusception before and after implementation of rotavirus vaccine programs in a number of Canadian jurisdictions. In the limited post-implementation data available to date, there was no evidence of an increase incidence following implementation of universal rotavirus immunization programs. All though our study was not powered to find modest increases in risk, it was well powered to detect larger effects that would be clinically important in the context of the established benefits of rotavirus immunization. Ongoing surveillance will continue to provide more post-implementation follow-up time however this preliminary assessment provides some assurance that a safety signal is not present. Our estimates of background incidence rates of intussusception across most Canadian provinces and territories, including those with and without publicly funded immunization programs, will help inform public health decision making about the risk/benefit analysis of rotavirus in Canada and will help ensure continued confidence in the vaccine.
Methods
Study design
We conducted a retrospective cohort study using Canadian population-based health administrative data covering the period from January 1, 2002 to December 31, 2013. This study was approved by both the Ottawa Health Science Network Research Ethics Board, and Public Health Ontario's Ethics Review Board.
Study population
We included all infants admitted to a Canadian hospital with a diagnosis of intussusception between January 1st, 2002 and December 31, 2013. We have defined infants as persons under one year of age. We identified hospital admissions using the Canadian Institute for Health Information (CIHI) Discharge Abstract Database (DAD). The investigators had access to the entire database available in the CIHI DAD, which was derived from the full source population of patients admitted to Canadian hospitals. Hospitals are mandated to report all admissions to CIHI, and hence coverage approaches 100%.Citation30 The province of Quebec was excluded from this analysis as Quebec does not contribute data to the CIHI DAD.
We identified intussusception cases using our validated case-finding algorithm.Citation16 This algorithm was developed by determining the accuracy of combinations of diagnostic, procedural, and billing codes for the identification of intussusception, in comparison to reference standard cases identified through a chart review of patients from the Children's Hospital of Eastern Ontario. Through a systematic process of trial and error, the algorithm that maximized positive predictive value while maintaining a high sensitivity was selected. The final case-finding algorithm included any occurrence of International Classification of Diseases (ICD)-9 code 560 or ICD-10 code K56.1 listed on the DAD discharge abstract.Citation16 This method was validated in Ottawa, Ontario and found to have good sensitivity (89.3%), specificity (>99.9%), positive predictive value (PPV; 72.4%), and negative predictive value (NPV; >99.9%).Citation16 Canada implemented ICD-10 during 2002 and 2003, so in those years, both coding systems were used in Canadian hospitals. After 2003, ICD-10 coding was used exclusively.
We counted only the first occurrence of intussusception under 1 y of age for each child to capture only incident cases. Of note, the validation study documented increased PPV of the algorithm to 77.1% for children under 1 y of age, and 79.8% when cases were identified after 2002 with the ICD-10 code.Citation16
We obtained population size for infants under the age of one year, stratified by jurisdiction (province/territory) and age in months from Canadian census data collected by Statistics Canada. The 2001, 2006 and 2011 Canadian censuses were used, and inter-censal population size estimates were imputed using linear interpolation/extrapolation.
Provinces and territories, and immunization program implementation
Canadian Provinces and Territories vary tremendously in population size from 13.5 million people in Ontario to 145,000 people in Prince Edward Island (PEI) in 2013.Citation31 Publicly funded immunization programs are implemented at the provincial/territorial level. Although as of February 3 2016, 10 Canadian jurisdictions had implemented rotavirus immunization programs, only those with programs implemented before the end of the study period were defined as having a vaccine program (). All jurisdictions use RotarixTM vaccine. Three jurisdictions (New Brunswick, Nova Scotia, and Nunavut) did not have publicly funded programs as of February 2016. Four jurisdictions (Alberta, Manitoba, Northwest Territories, and Newfoundland and Labrador) implemented programs, but post-implementation follow-up time was not available during our study period for 3 and was only available for 3 months of the study period for one. Therefore, for the purposes of this analysis, all 7 were defined as not having a rotavirus program. Five jurisdictions (British Columbia, Saskatchewan, Ontario, PEI and the Yukon) had rotavirus immunization programs that began between 2010 and 2012, allowing for post-implementation follow-up time to be observed and as such were defined as having a program in our analysis.
Table 1. Publicly Funded Rotavirus programs in Canada, excluding Quebec.
Statistical analysis
We calculated annual crude incidence rates of intussusception per 100,000 infants (under one year of age) overall and according to region, calendar year, age group (in months) and sex subgroups. We calculated rates using all jurisdictions in the years before any rotavirus immunization programs were implemented. To address small numbers of observed intussusception cases in several subgroup strata, jurisdictions were pooled into regions according to geography and program implementation as follows:
Region 1: NL: Program implemented but insufficient post-implementation follow-up time
Region 2: NB+NS: No programs within study period*
Region 3: PEI: Program within study period
Region 4: AB+MB+NT+NU: No programs within study period
Region 5: BC+SK+YK: Programs within study period
Region 6: Ontario: Program within study period
*Although NS does not have a publicly funded RV program, the Halifax metro area (which accounts for half the population of NS) did have a universal rotavirus program for approximately 2 y during the study period, although the vaccine coverage achieved was low, never exceeding 40%.Citation32
Due to observed heterogeneity, NL was reported separately in the results. In addition, PEI was not included in a pooled region since it had a rotavirus program with available follow-up, whereas the other Eastern provinces either had no program or insufficient post-implementation follow-up to evaluate.
We used Poisson regression to model trends in intussusception admission rates with model terms for subgroups of interest. The dependent variable was the number of events in each stratum, and the offset parameter was the total number of infants in each stratum. Annual incidence of intussusception admission was calculated overall, by subgroup (defined above) and according to implementation of rotavirus vaccine programs. Overall models included terms adjusting for age group (in months), sex, calendar year and region. Intussusception admission rates reported in subgroups of interest are adjusted for the other factors listed above.
To compare intussusception admission rates in periods before and after rotavirus program introduction, we defined pre- and post-periods specifically for each province and territory based on the date of program initiation (), and then pooled the data from all pre-periods and all post-periods across the jurisdictions. We classified the entire observation time for jurisdictions having no rotavirus program as “pre-period” for the pre- versus post- analysis.
Since RotarixTM is recommended at 2 and 4 months of age, with completion of the second dose by 8 months of age, and the latency period between immunization and intussusception onset is not thought to be long,Citation4,11 we conducted sensitivity analyses in order to focus on the most relevant time periods after immunization. We modeled rates of intussusception in infants aged 2 to 8 months, 2 to 6 months, and 2 to 4 months. We then repeated our analysis comparing time periods before and after rotavirus program introduction and comparing rates of intussusception across years to look for trends.
What's known on this subject
Intussusception has been identified as a rare adverse event associated with a previous rotavirus vaccine, which was discontinued. Newer vaccines are also associated with a smaller increased risk. Intussusception surveillance is a necessary component of any population rotavirus immunization program.
What this study adds
We established the background rate of intussusception in Canadian provinces and territories to inform ongoing surveillance, and demonstrated that there has been no increase in incidence of intussusception following introduction of population rotavirus immunization programs in several Canadian provinces to date.
Abbreviations
| CIHI | = | Canadian Institute for Health Information |
| DAD | = | Discharge Abstract Database |
| ICD | = | International Classification of Disease |
| US | = | United States |
| NPV | = | Negative Predictive Value |
| PPV | = | Positive Predictive Value |
| NL | = | Newfoundland and Labrador |
| NB | = | New Brunswick |
| NS | = | Nova Scotia |
| AB | = | Alberta |
| MB | = | Manitoba |
| NT | = | Northwest Territories |
| NU | = | Nunavut |
| YK | = | Yukon |
| ON | = | Ontario |
| PEI | = | Prince Edward Island |
| CI | = | Confidence Interval |
| NACI | = | National Advisory Committee on Immunization |
| CAEFISS | = | Canadian Adverse Events Following Immunization Surveillance System |
| IMPACT | = | Immunization Monitoring Program-Active |
Disclosure of potential conflicts of interest
The authors have no conflicts of interest relevant to this article to disclose.
Author contributions
Dr. Deeks conceptualized, designed and executed the study, interpreted the results, drafted the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Dr. Hawken conceptualized, designed and executed the study, carried out all data preparation and statistical analysis, interpreted the results, drafted the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Ms. Ducharme, Dr. Rosella, Dr. Benchimol Dr Langley, Dr. Wilson, Dr. Crowcroft, Dr. Halperin, Dr. Desai, Dr. Naus, Dr. Sanford and Dr. Mahmood provided important scientific input into the conduct of the study and interpretation of results, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Acknowledgments
Parts of this material are based on data and information provided by the Canadian Institute for Health Information. However, the analyses, conclusions, opinions and statements expressed herein are those of the authors and not those of the Canadian Institute for Health Information.
Eric Benchimol was supported by a New Investigator Award from the Canadian Institutes of Health Research, the Canadian Association of Gastroenterology, and Crohn's and Colitis Canada. Laura Rosella is supported by Tier 2 Canada Research Chair in Population Health Analytics. Joanne Langley is supported by the CIHR-GSK Chair in Pediatric Vaccinology at Dalhousie University.
Funding
This study was funded by the Canadian Immunization Research Network, through a grant from the Canadian Institutes of Health Research.
References
- Buettcher M, Baer G, Bonhoeffer J, Schaad UB, Heininger U. Three-year surveillance of intussusception in children in Switzerland. Pediatrics 2007; 120(3):473-80; PMID:17766518; http://dx.doi.org/10.1542/peds.2007-0035
- Cera S. Intestinal Intussusception. Clin Colon Rectal Surg 2008; 21(2):106-13; PMID:20011406; http://dx.doi.org/10.1055/s-2008-1075859
- Jiang J, Jiang B, Parashar U, Nguyen T, Bines J, Patel MM. Childhood intussusception: a literature review. PLoS One 2013; 8(7):e68482
- Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, Zanardi LR, Setia S, Fair E, LeBaron CW, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med 2001; 344(8):564-72; PMID:11207352; http://dx.doi.org/10.1056/NEJM200102223440804
- Patel MM, López-Collada VR, Bulhões MM, De Oliveira LH, Márquez AB, Flannery B, Esparza-Aguilar M, Montenegro Renoiner EI, Luna-Cruz ME, Sato HK, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med 2011; 364(24):2283-92; PMID:21675888; http://dx.doi.org/10.1056/NEJMoa1012952
- Velázquez FR, Colindres RE, Grajales C, Hernández MT, Mercadillo MG, Torres FJ, Cervantes-Apolinar M, DeAntonio-Suarez R, Ortega-Barria E, Blum M, et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr Infect Dis J 2012; 31(7):736-44; http://dx.doi.org/10.1097/INF.0b013e318253add3
- Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, Booy R, Bines JE. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine 2011; 29(16):3061-6; PMID:21316503; http://dx.doi.org/10.1016/j.vaccine.2011.01.088
- Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, Bines J, McIntyre PB. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia's National Immunization Program. Clin Infect Dis 2013; 57(10):1427-34; http://dx.doi.org/10.1093/cid/cit520
- Weintraub ES, Baggs J, Duffy J, Vellozzi C, Belongia EA, Irving S, Klein NP, Glanz JM, Jacobsen SJ, Naleway A, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med 2014; 370(6):513-9; PMID:24422678; http://dx.doi.org/10.1056/NEJMoa1311738
- Yen C, Tate JE, Steiner CA, Cortese MM, Patel MM, Parashar UD. Trends in intussusception hospitalizations among US infants before and after implementation of the rotavirus vaccination program, 2000-2009. J Infect Dis 2012; 206(1):41-8; PMID:22539812; http://dx.doi.org/10.1093/infdis/jis314
- Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, Selvam N, Selvan M, Lee GM, Nguyen M. Intussusception risk after rotavirus vaccination in US infants. N Engl J Med 2014; 370(6):503-12; PMID:24422676; http://dx.doi.org/10.1056/NEJMoa1303164
- World Health Organization. Rotavirus vaccine and intussusception 2010 [Available from: http://www.who.int/vaccinesafety/committee/topics/rotavirus/rotarixandrotateq/Dec2010/en/
- World Health Organization. Update on intussusception following rotavirus vaccine administration 2014 [Available from: http://www.who.int/vaccine_safety/committee/topics/rotavirus/rotarix_and_rotateq/dec_2013/en/
- National Advisory Committee on Immunization. Updated Statement on the use of Rotavirus Vaccines 2010 [Available from: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/10vol36/acs-4/index-eng.php
- Public Health Agency of Canada. Vaccine Safety Monitoring 2015 [Available from: http://www.phac-aspc.gc.ca/im/vs-sv/mon-surv-eng.php
- Ducharme R, Benchimol EI, Deeks SL, Hawken S, Fergusson DA, Wilson K. Validation of diagnostic codes for intussusception and quantification of childhood intussusception incidence in Ontario, Canada: a population-based study. J Pediatr 2013; 163(4):1073-9.e3; PMID:23809052; http://dx.doi.org/10.1016/j.jpeds.2013.05.034
- Samad L, Cortina-Borja M, Bashir HE, Sutcliffe AG, Marven S, Cameron JC, Lynn R, Taylor B. Intussusception incidence among infants in the UK and Republic of Ireland: a pre-rotavirus vaccine prospective surveillance study. Vaccine 2013; 31(38):4098-102; PMID:23871447; http://dx.doi.org/10.1016/j.vaccine.2013.06.084
- Justice F, Carlin J, Bines J. Changing epidemiology of intussusception in Australia. J Paediatr Child Health 2005; 41(9-10):475-8; PMID:16150062; http://dx.doi.org/10.1111/j.1440-1754.2005.00686.x
- Kolsen Fischer T. Intussusception in Early Childhood: a cohort study of 1.7 million children. Pediatrics 2004; 114(3):782-5; PMID:15342854; http://dx.doi.org/10.1542/peds.2004-0390
- Bines J. Intussusception and rotavirus vaccines. Vaccine 2006; 24(18):3772-6; PMID:16099078; http://dx.doi.org/10.1016/j.vaccine.2005.07.031
- Tate JE, Simonsen L, Viboud C, Steiner C, Patel MM, Curns AT, Parashar UD. Trends in intussusception hospitalizations among US infants, 1993-2004: implications for monitoring the safety of the new rotavirus vaccination program. Pediatrics 2008; 121(5):e1125-32; PMID:18450856; http://dx.doi.org/10.1542/peds.2007-1590
- Parashar UD, Holman RC, Cummings KC, Staggs NW, Curns AT, Zimmerman CM, Kaufman SF, Lewis JE, Vugia DJ, Powell KE, et al. Trends in intussusception-associated hospitalizations and deaths among US infants. Pediatrics 2000; 106(6):1413-21; PMID:11099597; http://dx.doi.org/10.1542/peds.106.6.1413
- Nakagomi T, Takahashi Y, Arisawa K, Nakagomi O. A high incidence of intussusception in Japan as studied in a sentinel hospital over a 25-year period (1978-2002). Epidemiol Infect 2006; 134(1):57-61; PMID:16409651; http://dx.doi.org/10.1017/S0950268805004644
- Noguchi A, Nakagomi T, Kimura S, Takahashi Y, Matsuno K, Koizumi H, Watanabe A, Noguchi H, Ito T, Ohtsuka M, et al. Incidence of intussusception as studied from a hospital-based retrospective survey over a 10-year period (2001-2010) in Akita Prefecture, Japan. Japanese J Infect Dis 2012; 65(4):301-5; PMID:22814151; http://dx.doi.org/10.7883/yoken.65.301
- Tran LAT, Yoshida LM, Nakagomi T, Gauchan P, Ariyoshi K, Anh DD, Nakagomi O, Thiem VD. A high incidence of intussusception revealed by a retrospective hospital-based study in Nha Trang, Vietnam between 2009 and 2011. Trop Med Health 2013; 41(3):121-7; PMID:24155653; http://dx.doi.org/10.2149/tmh.2013-09
- Jo DS, Nyambat B, Kim JS, Jang YT, Ng TL, Bock HL, Kilgore PE. Population-based incidence and burden of childhood intussusception in Jeonbuk Province, South Korea. Int J Infect Dis 2009; 13(6):e383-8; PMID:19362503; http://dx.doi.org/10.1016/j.ijid.2009.01.011
- Kohl LJ, Streng A, Grote V, Koletzko S, Liese JG. Intussusception-associated hospitalisations in southern Germany. Euro J Pediatr 2010; 169(12):1487-93; PMID:20640443; http://dx.doi.org/10.1007/s00431-010-1248-x
- Jenke AC, Klaassen-Mielke R, Zilbauer M, Heininger U, Trampisch H, Wirth S. Intussusception: incidence and treatment-insights from the nationwide German surveillance. J Pediatr Gastroenterol Nutr 2011; 52(4):446-51; PMID:21415671; http://dx.doi.org/10.1097/MPG.0b013e31820e1bec
- Wilson SE, Rosella LC, Wang J, Le Saux N, Crowcroft NS, Harris T, Bolotin S, Deeks SL. Population-level impact of ontario's infant rotavirus immunization program: evidence of direct and indirect effects. PLoS One 2016; 11(5):e0154340; PMID:27168335; http://dx.doi.org/10.1371/journal.pone.0154340
- Canadian Institute for Health Information. Data Quality Documentation, Discharge Abstract Database—Multi-Year Information2012. Available from: https://www.cihi.ca/en/dad_multi-year_en.pdf
- Statistics Canada. Canadian Population by year, by province and territory (April 1st, 2016). Available from: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo02a-eng.htm
- Sanford C, Langley JM, Halperin SA, Zelman M, Program MMURV. A universal infant rotavirus vaccine program in two delivery models: Effectiveness and adverse events following immunization. Hum Vaccin Immunother 2015; 11(4):870-4; PMID:25746110; http://dx.doi.org/10.1080/21645515.2015.1012028
Appendix
Table A1. Sensitivity Analysis: Estimated IS Rates before vs. after RV Program Introduction 1) Overall, 2) In 2−<8 month-olds, 3) In 2−<6 month-olds, 4) In 2−<4 month-olds, 2003-2013.
