ABSTRACT
Neisseria meningitidis serogroup B is the most prevalent cause of invasive meningococcal disease in Europe and members of laboratories working on meningococci are at risk due to frequent handling. Recommendation for anti-meningococcal vaccination among these workers has been recently updated upon the licensure in Europe of Bexsero® vaccine. We tested the immunogenicity and safety of this vaccine among adults laboratory staff using the recommended schedule of 2 doses at 5 weeks interval. The vaccine was well tolerated in spite of frequent local side effects and all participants reported at least one side effect after each dose. Immunogenicity was evaluated 6 weeks and one year after the second dose. All participants showed increase in their bactericidal titers against the components of the vaccine 6 weeks after the second dose, however titers declined significantly one year later.
Introduction
The first meningococcal vaccine targeting serogroup B (Bexsero®) was recently licensed in Europe and unlike for serogroups A, C, Y and W, is not capsular polysaccharide-based. Indeed, the chemical structure of Neisseira meningitidis (Nm) serogroup B (NmB) capsule mimics human polysaccharides on the neural cells making it unsuitable for vaccine development.Citation1
Bexsero® is a multicomponent vaccine based on protein antigens and is the first vaccine to be developed using the reverse vaccinology approach.Citation2 It contains 4 highly immunogenic components: 3 recombinant proteins (fHbp, NadA and NHBA) and the outer membrane vesicle (OMV) of the Men-ZB® vaccine containing the major outer membrane protein, PorA P1.4.Citation3
Staffs in laboratories working on meningococci are at 65 to 184 times of higher risk to develop invasive meningococcal disease (IMD) than the general population.Citation4,5 Twenty-two cases were so far reported as meningococcal laboratory acquired infection and half of them were due to NmB.Citation6 Accordingly, all laboratory members who will be working with Nm are advised to be vaccinated using one of the conjugate quadrivalent vaccines against serogroups A, C, Y and W. This recommendation was updated in France upon the licensure of Bexsero® for laboratory staff. We report here the results of a one-year serological study among laboratory workers who received this vaccine.
Results
A total of 14 laboratory workers were offered the Bexsero® vaccine. Twelve of them volunteered to receive the vaccine with 11 subjects who were fully vaccinated with the proposed schedule of 1+1 as one participant left the laboratory before the second dose. Eight participants provided 2 blood samples before and 6 weeks after the second dose and 7 participants provided a blood sample one year after the second dose.
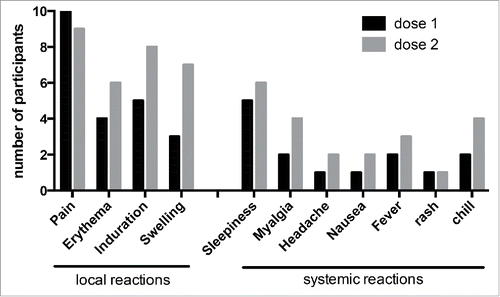
Self-reporting of side effects were collected from 10 participants after the first dose and from 9 participants for the second dose during 7 days after each dose. All participants reported at least one side effect after each injection. We observed after the first dose that all vaccinees reported local reactions on injection site and sleepiness for 50% of participants. After the second dose, the side effects tend to increase particularly at the injection site (pain and swelling, 100% and 88% respectively) and systemic reaction such as fever (33%), myalgia and chill (50% each) ().
Before the first dose 38% to 50% of the participants had protective bactericidal titers of ≥ 4 against at least one of the vaccine antigens (). Six weeks after the second dose, titers of ≥ 4 against the reference strains harbouring matching fHbp, NadA or PorA P1.4 were observed in all participants, while 88% showed titers against NHBA antigen. Indeed, one participant did not reach protective titer after 2 doses for NHBA when tested against the strain NGH38. All the participants with basal hSBA titres of at least 4 before vaccination, showed a 4-fold increase of hSBA titers against the corresponding vaccine antigen. Geometric means of hSBA titers at baseline and after 2 doses showed significant increase for each of the vaccine components (p-values < 0.05) ().
One
Table 1. Immune response to Bexsero® by hSBA against 4 representative strains harboring Bexsero® antigens and against clinical isolates.
We also took advantage of these sera to test the coverage of 6 meningococcal clinical isolates by the Bexsero®. We therefore tested pre- and post-vaccination (6 weeks after the second dose) pooled sera to explore hSBA titers against 4 clusters of IMD. The tested isolates harboured the same fHbp variant as the one included in the vaccine but did not match PorA P1.4 present in the vaccine (data for NHBA and NadA were not available). The six clinical isolates of these clusters were all predicted to be covered by the Bexsero® as hSBA titers using post-vaccination sera were all ≥ 4. Four fold increases in hSBA titers were also observed when a titer ≥ 4 was observed using the pool of pre-vaccination sera ().
Discussion
In this study we report proficient immune response to several NmB strains among laboratory workers vaccinated with Bexsero® vaccine. The vaccine was safe in spite of high proportion of local side effects consistently with previous reports.Citation7,8,9
Our data also showed that depending on the NmB strain tested, between 38 to 50% of subjects had at baseline hSBA titers ≥4, which is presumably due to exposure to meningococci through pharyngeal carriage. However, this baseline was significantly increased 6 weeks after the second dose against all vaccine components except for NHBA in one participant.
In spite of the small number of participants, our results are similar to those previously described among adult population although these studies used a 3 doses schedule at 0, 2 and 6 months.Citation7,8,9 Our study further evaluate the persistence of the immune response one year after the second dose and showed that hSBA geometric mean titers significantly dropped for almost all vaccine antigens (except for NadA) but still remained protective, as already reported 6 months after the third dose in the 3 doses schedule.Citation7 Moreover, one year after vaccination 3 participants showed only protection against NadA in agreement with recently reported data.Citation10 nadA encoding gene is present in 35% of French MenB but only 0.6% of NmB isolates are predicted to be covered only by this vaccine antigen on the basis of their levels of expression of NadA antigen.Citation11
These results are promising for the protection of laboratory staff working with Nm, in combination with ACYW vaccination and good practices in handling meningococci.Citation6 A 2-doses scheme may be suitable. Nevertheless; a booster dose after at least one year should be proposed in order to ensure longer protection for laboratory staff handling N. meningitidis.
In addition to laboratory workers, the Bexsero® vaccine is recommended in France for at high risk persons such as those with complement deficiencies and to control NmB outbreaks if they are covered by the vaccine.Citation12 Our data also provide a general and direct method to show strain coverage using pooled sera (as those from this study) to predict coverage by the Bexsero® of clinical isolates involved in outbreaks by comparing titers before and after vaccination (6 weeks after the second dose). The use of pooled sera from vaccinated infants and toddlers was shown to give correlated results as those obtained using individual sera.Citation13 However hSBA titers may be overestimated when using pooled sera from vaccinated adults due to immunity induced by natural exposure and larger antibody repertoire.
Our data are also in agreement with those showing that coverage predicted by hSBA is larger than that predicted by MATS assays.Citation14 In spite of a decline in seroprotection after one year post vaccination, targeted vaccination to control NmB outbreak may preclude clonal expansion of virulent isolates.
Methods
Subjects
According to the updated recommendation, Bexsero® vaccination was offered at voluntary basis to laboratory workers of the French National Reference Laboratory. No known contraindication to vaccination was recorded and all participants signed informed consent forms.
Vaccine
Bexsero® was provided in prefilled syringes for intramuscular injection and contains 50 μg of each of fHbp, NHBA, NadA and 25 μg of OMV from the strain NmB NZ98/254. Each dose contains also 1.5 mg aluminum hydroxide, 3.25 mg NaCl, 10 mM histidine and water up to 0.5 ml.
Vaccine schedule
Each participant received 2 doses of Bexsero® at 5 weeks interval. Both injections were given intramuscularly into the deltoid of the non-dominant arm. Blood samples were collected before the first dose at baseline, 6 weeks and one year after the second dose.
Safety
Post-injection reactions and systemic effects were solicited by self-reporting by each participant after each injection.
Immunogenicity
Immune responses to vaccination were assessed by serum bactericidal assay using exogenous human complement as a common external source of complement (hSBA). The previously described reference strains for hSBA assay (H44/76 and 5/99) were used in our assay in order to attribute the observed bactericidal activity to fHbp or NadA respectively,Citation15 the reference strain for the antigen NHBA (NGH38) in the Meningococcal Antigen Typing System (MATS) assayCitation11 were selected to run the assays in order to attribute the observed bactericidal activity to NHBA antigen. The strain LNP24349 B:7–2,4:cc162:F5-9 that harbours PorA P1.4 was also selected to determine the effect of PorA P1.4 antigen and further because it harbours fHbp gene belonging to variant 2, nadA gene is absent and the level of expression of NHBA gene is lower than the protective bactericidal threshold, PBT, as determined by MATS.Citation11 hSBA titers of at least 4 are considered to be correlated with the protection. Each serum was tested individually against each strain. Additionally, the sera were pooled (pre and post vaccination) in order to perform hSBA against outbreak isolates received at the National Reference Center (LNP27783 B:7–2,13–2:cc41/44:F1-5, LNP27896 B:7,16:cc32:F3-3, LNP27899 B:7,16:cc32:F3-3, LNP27931 B:7–1,1:cc865:F1-6, LNP27942 B:19,15–1:NA:F1-5, LNP27943 B:19,15–1:NA:F1-5). Over the year 2015, clonal complex cc41/44 accounted for 21% of the French NmB invasive isolates and clonal complex cc32 accounted for 20%. The isolates of cc865 and unassigned isolates represented 1.6% and 1.2% respectively.
Statistical methods
Results were computed as geometric mean of titers with their associated Clopper Pearson 95% confidence intervals (CI). Results were also expressed as percentage of subjects with hSBA titer ≥4 that is correlated with protection and as a 4-fold increase when baseline titers were ≥4 (for the samples after 6 weeks of the second dose).Citation16
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 1983; 2:355-7; PMID:6135869; http://dx.doi.org/10.1016/S0140-6736(83)90340-9
- Rappuoli R. Reverse vaccinology, a genome-based approach to vaccine development. Vaccine 2001; 19:2688-91; PMID:11257410; http://dx.doi.org/10.1016/S0264-410X(00)00554-5
- Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 2012; 30(Suppl 2):B87-97; PMID:22607904; http://dx.doi.org/10.1016/j.vaccine.2012.01.033
- Sejvar JJ, Johnson D, Popovic T, Miller JM, Downes F, Somsel P, Weyant R, Stephens DS, Perkins BA, Rosenstein NE. Assessing the risk of laboratory-acquired meningococcal disease. J Clin Microbiol 2005; 43:4811-4; PMID:16145146; http://dx.doi.org/10.1128/JCM.43.9.4811-4814.2005
- Boutet R, Stuart JM, Kaczmarski EB, Gray SJ, Jones DM, Andrews N. Risk of laboratory-acquired meningococcal disease. J Hosp Infect 2001; 49:282-4; PMID:11740877; http://dx.doi.org/10.1053/jhin.2001.1084
- Borrow R, Findlow J, Gray S, Taylor S, Kaczmarski E. Safe laboratory handling of Neisseria meningitidis. J Infect 2014; 68:305-12; PMID:24440738; http://dx.doi.org/10.1016/j.jinf.2014.01.003
- Toneatto D, Ismaili S, Ypma E, Vienken K, Oster P, Dull P. The first use of an investigational multicomponent meningococcal serogroup B vaccine (4CMenB) in humans. Hum Vaccin 2011; 7:646-53; PMID:21904120; http://dx.doi.org/10.4161/hv.7.6.15482
- Kimura A, Toneatto D, Kleinschmidt A, Wang H, Dull P. Immunogenicity and safety of a multicomponent meningococcal serogroup B vaccine and a quadrivalent meningococcal CRM197 conjugate vaccine against serogroups A, C, W-135, and Y in adults who are at increased risk for occupational exposure to meningococcal isolates. Clin Vaccine Immunol 2011; 18:483-6; PMID:21177912; http://dx.doi.org/10.1128/CVI.00304-10
- Findlow J, Bai X, Findlow H, Newton E, Kaczmarski E, Miller E, Borrow R. Safety and immunogenicity of a four-component meningococcal group B vaccine (4CMenB) and a quadrivalent meningococcal group ACWY conjugate vaccine administered concomitantly in healthy laboratory workers. Vaccine 2015; 33:3322-30; PMID:26025807; http://dx.doi.org/10.1016/j.vaccine.2015.05.027
- McQuaid F, Snape MD, John TM, Kelly S, Robinson H, Houlden J, Voysey M, Toneatto D, Kitte C, Dull PM, et al. Persistence of bactericidal antibodies to 5 years of age after immunization with serogroup B meningococcal vaccines at 6, 8, 12 and 40 months of age. Pediatr Infect Dis J 2014; 33:760-6; PMID:24722351; http://dx.doi.org/10.1097/INF.0000000000000327
- Vogel U, Taha MK, Vazquez JA, Findlow J, Claus H, Stefanelli P, Caugant DA, Kriz P, Abad R, Bambini S, et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis 2013; 13:416-25; PMID:23414709; http://dx.doi.org/10.1016/S1473-3099(13)70006-9
- Lecocq H, Parent du Chatelet I, Taha MK, Levy-Bruhl D, Dervaux B. Epidemiological impact and cost-effectiveness of introducing vaccination against serogroup B meningococcal disease in France. Vaccine 2016; 34: 2240-2250; PMID:27002504
- Budroni S, Kleinschmidt A, Boucher P, Medini D. Pooled-sera hSBA titres predict individual seroprotection in infants and toddlers vaccinated with 4CMenB. Vaccine 2016; 34:2579-84; PMID:27083425; http://dx.doi.org/10.1016/j.vaccine.2016.04.009
- Frosi G, Biolchi A, Lo Sapio M, Rigat F, Gilchrist S, Lucidarme J, Findlow J, Borrow R, Pizza M, Giuliani MM, et al. Bactericidal antibody against a representative epidemiological meningococcal serogroup B panel confirms that MATS underestimates 4CMenB vaccine strain coverage. Vaccine 2013; 31:4968-74; PMID:23954380; http://dx.doi.org/10.1016/j.vaccine.2013.08.006
- Giuliani MM, Biolchi A, Serruto D, Ferlicca F, Vienken K, Oster P, Rappuoli R, Pizza M, Donnelly J. Measuring antigen-specific bactericidal responses to a multicomponent vaccine against serogroup B meningococcus. Vaccine 2010; 28:5023-30; PMID:20493284
- Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection–serum bactericidal antibody activity. Vaccine 2005; 23:2222-7; PMID:15755600; http://dx.doi.org/10.1016/j.vaccine.2005.01.051