ABSTRACT
We investigated the long-term immune profiles of dose-sparing, AS03-adjuvanted vaccines compared to a traditional high-dose, unadjuvated influenza vaccine formulation. BALB/c mice received 2 IM injections of influenza A/Uruguay/716/2007 (H3N2) split vaccine antigen: high-dose (HD) (3 µg hemagglutinin (HA)/dose) or low-dose (LD) formulations (0.03 µg or 0.003 µg HA) with AS03 and were followed to 34 weeks post-boost (pb). We examined serologic responses, spleen and bone marrow (BM) HA-specific antibody-secreting cells (ASCs) by ELISpot, influenza-specific cytokine/chemokine production in re-stimulated splenocytes by multiplex ELISA, and antigen-specific CD4+ T cells that express cytokines (IL-2, IFNγ, TNFα and IL-5) by flow cytometry. All formulations elicited robust serum antibody titers that persisted for at least 34 weeks. The number of antigen-specific ASCs in the spleen and BM were higher in the 2 LD +AS03 groups, but despite having fewer ASCs, the average spot size in the HD-unadjuvanted group was larger at later time-points, suggesting greater antibody production per cell. Striking differences in the long-term profiles induced by the different vaccine formulations may contribute to these different ASC profiles. The HD-unadjuvanted vaccine elicited strong Th2 cytokines during the first 6 weeks pb but LD+AS03 groups generated broader, more durable responses at later timepoints. Finally, the 0.03 µg HA+AS03 group generated the greatest number of antigen-specific CD4+ T cells and the highest percentage of poly-functional cells that expressed 2 or more cytokines. Although all of the tested vaccines induced durable antibody responses, we show that different vaccine formulations (dose-sparing, adjuvant) generate distinct long-term immune profiles. Furthermore, our data suggest that the different profiles may be generated through unique mechanisms.
Introduction
During the 2009–2010 H1N1 influenza pandemic, adjuvanted, dose-sparing vaccines were widely used. The vaccine that ∼41% of all Canadians receivedCitation1 (Arepanrix™, GSK Vaccines, Mississauga, ON, Canada) consisted of an oil-in-water emulsion adjuvant (AS03) containing squalene and α-tocopherol with 25% of the H1N1 strain-specific antigen (Ag) used in routine inactivated seasonal influenza vaccines.Citation2,3 Although this formulation met the serologic licensure criteria,Citation4 we observed unusual antibody avidity responses in some immunologically naïve children.Citation5 Although this formulation met the serologic licensure criteria, we observed unusual antibody avidity responses in some immunologically naïve children. In these children, avidity rapidly increases after the first dose of vaccine but then wanes after the second dose. This observation raised questions about both the quality and durability of the immune response elicited by the dose-sparing, AS03-adjuvanted formulation.
To investigate this question, we first developed a BALB/c mouse model of exaggerated low-dose vaccination with adjuvant. This previous work found that even extremely low doses of Ag administered with AS03 could generate a humoral response equivalent to that induced by a higher (‘standard’) dose (3 µg HA) but with much broader activation of cytokines.Citation6 This work suggested that both Ag and adjuvant dose in AS03-adjuvanted formulations could strongly influence the quality and quantity of immune responses elicited. We next sought to investigate the durability of the responses induced in our very low-dose vaccine model. We were particularly interested in the impact of AS03, which induces the local and transient activation of innate responses, leading to recruitment of antigen presenting cells (APCs), increased antigen uptake by APCs, and enhanced adaptive responses against co-administered antigens.Citation7,8
The objective of this study was therefore to compare the long-term (8 month) evolution of humoral and cellular responses induced in BALB/c mice by ‘standard’ (high Ag dose, unadjuvanted) versus AS03-adjuvanted, very low-dose vaccination. Robust and durable antibody responses were generated in all groups but the highest responses were consistently seen in the group that received 1/100th of the standard Ag dose+AS03. The AS03-adjuvanted, low-dose formulations elicited more antibody secreting cells (ASCs), much broader, mixed influenza-specific cytokine responses, and generated greater numbers of antigen-specific CD4+ T cells.
Results
Serum antibody levels are maintained long-term following LD-AS03-adjuvanted or HD-unadjuvanted vaccination
Mice were immunized with a ‘standard’ dose of 3 μg split vaccine antigen based on hemagglutinin (HA) content (termed ‘high-dose’ or HD) or with 2 low-dose (LD) formulations 0.03 μg HA+AS03, and 0.003 μg HA+AS03.
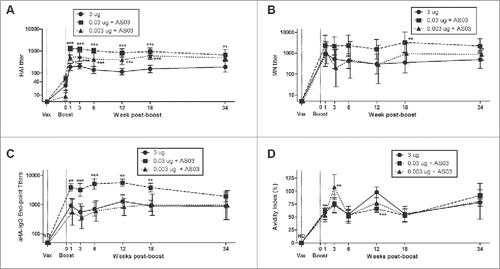
All vaccine formulations generated robust and durable serum antibody titers detectable by HAI, MN and endpoint ELISA assays (). Mice vaccinated with 0.03 μg HA+AS03 generated the highest titers in these 3 assays at all time-points. The mice vaccinated with the lowest-dose+AS03 generally had intermediate serologic response between the 0.03 μg HA+AS03 and the HD-unadjuvanted groups. Similar to what we observed in the short-term study,Citation6 high HA-specific antibody avidity was present by 1 week pb in all groups and remained elevated throughout the 34 week study ().
Figure 1. Long-term serum antibody response in mice after 2 immunizations of unadjuvanted A/Uruguay H3N2 split vaccine or AS03-adjuvanted dose-sparing vaccines. BALB/c mice were immunized intramuscularly on days 0 and 21. Serum samples were collected before each immunization and at indicated time-points post-boost. HAI titers (A), MN titers (B), anti-HA IgG endpoint titers (C) and antibody avidity indices (D) against homologous virus was determined from individual mice. Differences between low-dose adjuvanted groups to 3 µg HA only at each time-point are indicated; *, P < 0.05, **, P ≤ 0.01, ***, P ≤ 0.001. Data represent geometric means and 95% confidence intervals. (A) and (C) depict 8–16 mice per group. (B) and (D) depict 5–8 and 8–10 mice per groups, respectively.
LD-AS03-adjuvanted vaccines generated more long-term plasma cells and memory B cells in the bone marrow (BM) than HD-unadjuvanted vaccine
We used ELISpot assays to count and measure the spot size of influenza-specific antibody secreting cells (ASCs or plasma cells) in spleen and bone marrow (BM) () as well as memory B cell-derived ASCs (termed memory ASCs) following ex vivo stimulation (; Fig. S1). Vaccination with 0.03 μg HA+AS03 rapidly generated large numbers of influenza HA-specific plasma cells and memory ASCs in the spleen compared to the HD-unadjuvanted and the 0.003 μg HA+AS03 groups (). These latter groups also exhibited somewhat slower kinetics. The induction of plasma cells and memory ASCs in the BM was also greatest in the 0.03 μg HA+AS03 group but numbers rose progressively over the 34 week period pb (; Fig. S1C). ASCs and memory ASCs also accumulated over time in the BM of the lowest dose animals (0.003 μg HA+AS03) but with numbers intermediate between the 0.03 μg HA+AS03 and the HD-unadjuvanted groups. In general, 5–10X more influenza-specific plasma cells and memory ASCs were detected in the BM of all groups compared to those detectable in spleens, particularly at the later time-points after vaccination ().
Figure 2. Generation and long-term maintenance of influenza HA-specific antibody secreting cells (ASCs) after 2 immunizations of unadjuvanted A/Uruguay H3N2 split vaccine or AS03-adjuvanted dose-sparing vaccines. BALB/c mice were immunized intramuscularly on days 0 and 21. Splenocytes and bone marrow (BM) cells were isolated from individual mice at indicated time-points post-boost to investigate HA-specific IgG antibody secreting cells (ASCs) by ELISpot. The number (A) and spot size (B) of ASCs in splenocytes. The number (C) and spot size (D) of ASCs in BM cells. Representative ELISpot wells of cells isolated at12 weeks post-boost are shown in (E) and (F). Differences between low-dose adjuvanted groups to 3 µg HA only at each time-point are indicated; *, P < 0.05, **, P ≤ 0.01, ***, P ≤ 0.001. Data represent means and standard errors, and depict 5–8 mice per group.
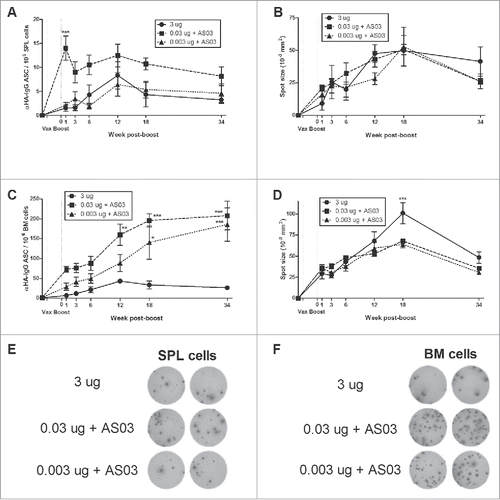
Figure 3. Generation and long-term maintenance of influenza HA-specific memory B cell-derived antibody secreting cells (ASCs) after 2 immunizations of unadjuvanted A/Uruguay H3N2 split vaccine or AS03-adjuvanted dose-sparing vaccines. BALB/c mice were immunized intramuscularly on days 0 and 21. Splenocytes and bone marrow (BM) cells were isolated from individual mice at indicated time-points post-boost. Cells were stimulated ex vivo with CpG and human IL-2 to quantify the number and spot size of HA-specific memory B cell-derived ASCs (memory ASCs) by ELISpot. The number (A) and spot size (B) of memory ASCs in splenocytes. The number (C) and spot size (D) of memory ASCs in BM cells. Representative ELISpot wells of cells isolated at 34 weeks post-boost are shown in (E) and (F). Differences between low-dose adjuvanted groups to 3 µg HA only at each time-point are indicated; *, P < 0.05, **, P ≤ 0.01, ***, P ≤ 0.001. Data represent means and standard errors, and depict 5–8 mice per group.
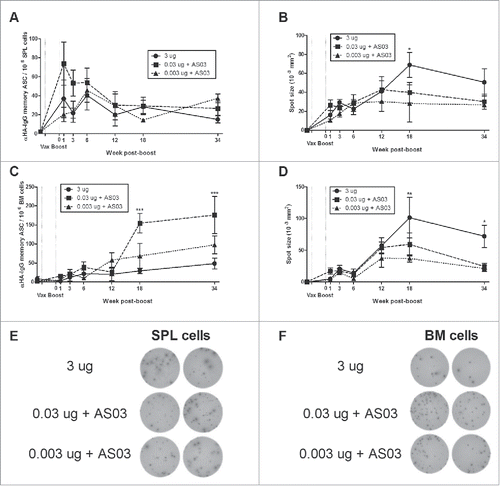
The mean spot size in an ELISpot assay is correlated with the amount of antibody secreted per cell.Citation9 Although HD-unadjuvanted vaccine tended to generate fewer plasma cells and memory ASCs, this group tended to have larger spot sizes at later time-points, particularly in the BM (; Fig. S1D).
LD-AS03-adjuvanted and HD-unadjuvanted vaccines generate similar influenza-specific lymphoproliferation but different long-term cytokine profiles
To investigate cellular immune responses, we investigated the capacity of Ag-stimulated splenocytes to proliferate and produce cytokines/chemokines at different time-points after vaccination. Interestingly, both of the LD-adjuvanted groups had higher levels of Ag-specific proliferation at 1 week pb (Fig. S2) but stimulation-indices were comparable across groups at most later time-points.
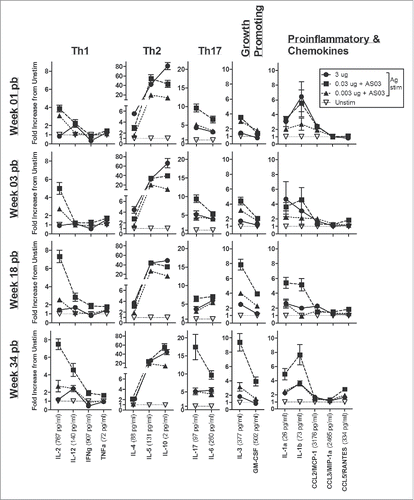
Despite the similarity in Ag-driven splenocyte proliferation across groups, the vaccines tested induced very different cytokine/chemokine response patterns. The overall long-term cytokine profiles are shown in with cytokine levels presented as fold-increases from the unstimulated control samples. Graphs of individual cytokines/chemokines at all time-points are also shown in Fig. S3. We found that the LD-AS03-adjuvanted vaccines established a long-term pattern of Ag-specific cytokine/chemokine secretion that was quantitatively greater and qualitatively broader than the HD-unadjuvanted formulation. The overall pattern of response in the HD-unadjuvanted group tended to favor Th2-type (IL-4 and IL-5) and regulatory (IL-10) cytokines in the first weeks pb as well as the chemokine CCL2/MCP-1 at later time-points (; Fig. S3). In contrast, the LD-adjuvanted formulations, and particularly the 0.03 µg HA+AS03 group, generated a much broader, mixed antigen-specific cytokine/chemokine response pattern, that tended to become more exaggerated at later time-points (). This response included higher levels of Th1 (IL-2, IFNγ, IL-12), Th17 (IL-6, IL-17), growth-promoting (IL-3, GM-CSF) and pro-inflammatory cytokines/chemokines (IL-1α, IL-1β, CCL3/MIP-1α and CCL5/RANTES), as well as secretion of Th2 cytokines (IL-4, IL-5 and IL-10) (; Fig. S3).
Figure 4. Unadjuvanted A/Uruguay H3N2 split vaccine and AS03-adjuvanted dose-sparing vaccines generate different antigen-specific cytokine/chemokine profiles over time. BALB/c mice were immunized intramuscularly on days 0 and 21, and splenocytes were isolated from individual mice at indicated time-points post-boost. Cells were stimulated ex vivo with media (unstimulated background) or with A/Uruguay H3N2 split vaccine and culture supernatant was collected after 72 hours. The concentrations of indicated cytokines/chemokines in supernatants were determined using Q-Plex™ Mouse Cytokine - Screen (16-plex) multiplex ELISA. Cytokine/chemokine profiles at 1, 3, 18 and 34 weeks post-boost are shown. For each cytokine/chemokine, concentrations are shown as the fold increase from “Unstim,” which represents the average concentration of unstimulated samples for all groups at all time-points. The concentration of “Unstim” background is indicated in brackets next to cytokine/chemokine names. Individual cytokine/chemokine concentrations at all time-points are shown in Fig S3. Data represent means and standard errors, and depict 5–8 mice per group.
LD-AS03-adjuvanted vaccines generated more poly-functional antigen-specific CD4+ T cells than HD-unadjuvanted vaccine
Antigen-specific CD4+ T cells in splenocytes, or cells that responded to antigen stimulation ex vivo by producing cytokines, were detected at short and long-term time points by flow cytometry (). With the 3 different vaccine formulations, the majority of responding cells were single positive for one of the 4 cytokines. At early (3 weeks pb) and late (18 weeks pb) time-points, the largest numbers of single positive cells expressed IFNγ, followed by IL-5, TNFα and IL-2, respectively (). The 0.003 µg HA +AS03 group tended to produce the most single positive CD4+ T cells. Smaller numbers of poly-functional T cells were also detectable (). Specifically, the 0.03 µg HA+AS03 group generated the most antigen-specific CD4+ T cells double positive for IL-2 and TNFα (), or IL-2 and IL-5 (), or triple positive for IL-2, TNFα and IL-5 (). No quadruple positive CD4+ T cells were detected in any group.
Figure 5. Long-term generation of antigen-specific CD4+ T cells after 2 immunizations of unadjuvanted A/Uruguay H3N2 split vaccine or AS03-adjuvanted dose-sparing vaccines. BALB/c mice were immunized intramuscularly on days 0 and 21, and splenocytes were isolated from individual mice at indicated time-points post-boost. Cells were stimulated ex vivo with A/Uruguay H3N2 split vaccine and co-stimulatory antibodies, then analyzed by flow cytometry for CD4+ T cells that produced a combination of IL-2, IFNγ, TNFα, or IL-5 cytokines. The gating strategy is described in Fig. S4. Frequency of CD4+ T cells expressing all combinations of IL-2, IFNγ, TNFα, or IL-5 cytokines at week 3 and 18 post-boost (A). The frequency of IL-2+TNFa+ double positive (B), IL-2+IL-5+ double positive (C) or IL-2+TNFa+IL-5+ triple positive (D) CD4+ T cells. The distribution of CD4+ T cell subsets based on the number of cytokines expressed (E). The size of the pie chart is also representative of the magnitude of the total response. For (B), (C), and (D), differences between low-dose adjuvanted groups to 3 µg HA only at each time-point are indicated; *, P < 0.05, **, P ≤ 0.01, ***, P ≤ 0.001. Data represent mean and standard errors, and depicts 5–8 mice per group.
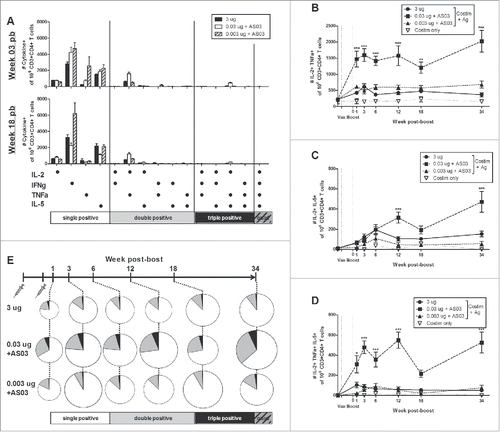
We categorized the cells according to the number of cytokines produced, and used pie charts to better observe the trends of the antigen-specific T cell response. We also used the size of the individual pie charts to represent the total number of responding T cells. We show that the HD-unadjuvanted group had detectable antigen-responsive CD4+ T cells at all time-points, including a small percentage (14.4% ± 4.4; mean ± standard deviation) that were poly-functional, or expressing 2 or more of the cytokines investigated (). The 0.03 µg HA+AS03 group tended to have the largest number of both antigen-responsive T cells (8084 ± 1743; mean per one million CD3+CD4+ T cells ± standard deviation) and poly-functional T cells (29.9% ± 4.8) at all time-points when compared to the HD-unadjuvanted (5549 ± 1596) group. Finally, the 0.003 µg HA+AS03 group tended to have an intermediate level of responding CD4+ T cells (7225 ± 2697), but generated fewer poly-functional cells (12.8% ± 4.8) (). These results suggest that antigen quantity may play an important role in the generation of poly-functional T cells.
Similarly to our previous results, at 3 weeks post-boost, more antigen-specific CD8+ T cells were detected in the LD-adjuvanted compared to HD-unadjuvanted groups, but no major differences were observed at later timepoints (Fig. S5).
Discussion
Despite widespread use during the 2009–10 H1N1 pandemic, there is relatively little published pre-clinical information about the use of AS03 as a dose-sparing adjuvant for influenza vaccination strategies.Citation6,10-12 The pandemic experience created the unusual situation in which much of what we know about the immunological impact of AS03 is derived from human studies. This body of work has demonstrated that AS03-adjuvanted, dose-sparing formulations can induce strong serum antibody responses that persist for at least 12 months in healthy children,Citation13-17 adultsCitation18-23 and the elderly,Citation24-26 and that influenza-specific, poly-functional CD4+ T cells can be detected in human PBMC samples for at least 6 months after vaccination.Citation27-29
We have previously shown that AS03 can induce powerful humoralCitation6,12 and cellular responsesCitation6 to influenza antigens over a surprisingly wide range of Ag doses (100- to 1000-fold lower than a ‘standard’ 3 µg HA dose in mice) at 3 weeks after a booster immunization The objective of this study was to determine the persistence of these responses up to 34 weeks after LD-AS03-adjuvanted vs. HD-unadjuvanted vaccination. We observed the same trends for AS03 effects seen at 3 weeks post-boost (i.e. strengthened humoral and broadened cellular responses) persisted or even more pronounced for up to ∼8 months after vaccination. Both HD-unadjuvanted and LD-adjuvanted vaccines elicited high serum antibody titers that persisted for at least 34 weeks, but the highest titers were detected in the 0.03 µg HA+AS03 group.
One of the most interesting observations in this study was the striking difference in ELISpot results across the different vaccine formulations. The LD-adjuvanted vaccines generated the most influenza-specific ASCs and memory ASCs, corresponding to plasma and memory B cells, respectively. The 0.03 µg HA+AS03 group had generated large numbers of ASCs and memory ASCs that were elevated by 1 week pb and remained high throughout the 8-month study. In contrast, the HD-unadjuvanted vaccine and the lowest dose with adjuvant (0.003 µg HA+AS03) groups tended to produce a relatively poor splenic ASC response at all time-points, which peaked at 6–12 weeks pb. These trends confirm and extend our splenocyte observations with LD+AS03 formulations at 3 weeks pb.Citation6
In apparent contradiction to the numbers of ASCs detected by ELISpot in response to the different vaccines, ELISpot size tended to be greatest in the HD-unadjuvanted animals at later timepoints. The size of spots in the ELISpot assay is thought to reflect the amount of antibody secreted by each cell.Citation9 Surprisingly given the differences in absolute ASC numbers and antibody levels outlined above, the ASCs and memory ASCs generated by the HD-unadjuvanted group tended to have larger spot sizes than the LD-adjuvanted groups after 12 weeks pb. On the other hand, at the earlier timepoints (up to 6 weeks pb), the LD-adjuvanted groups tended to have larger spot sizes but significant differences were not observed. This result is in line with our previous study that investigated responses at 3 weeks pb and found that the spot sizes of splenic ASCs and memory ASCs in the LD-adjuvanted groups were larger than in the HD-unadjuvated group.Citation6 However, the long-lived plasma cells and memory B cells detected in the BM of the HD-unadjuvanted group clearly had larger spot sizes at 18 and 34 weeks pb. These data suggest that the HD-unadjuvanted formulation induces fewer ASCs but that each of these cells secretes more antibodies, compared to the LD-adjuvanted formulations.
Although the quantification of antigen-specific ASCs by ELISpot is a frequently used measure of vaccine-induced immune responses, the analysis of ASC spot size is less common. However, ASC spot size was investigated following vaccination of a novel measles vaccine in mice,Citation30 and ASC spot size was found to vary following Streptococcus pneumoniae polysaccharide vaccination in mice with the presence or absence of a protein conjugate.Citation31 In the latter study, Taillardet et al. demonstrated that the thymus-dependent, tetanus toxoid-polysaccharide conjugate formulation generated BM plasma cells with greater antibody secretion compared to a thymus-independent polysaccharide vaccine given with CpG adjuvant.Citation31 That study concluded that plasma cells activated by the different types of vaccines were functionally different. Finally, although not strictly comparable to our mouse study, it is nonetheless interesting that Kim et al. have recently reported that a high-dose of influenza antigen induces a striking effect on the pattern of immune responses in elderly subjects.Citation32 For example, Kim et al. observed an enhanced early plasmablast response in the high-dose compared to standard dose vaccine recipients. Our unexpected ELISpot results therefore raise the possibility that the HD-unadjuvanted and LD-adjuvanted formulations may use very different mechanisms (for example, by direct activation or indirect means, such as inflammatory signals) to generate and maintain long-term serum antibody responses.
While both patterns of response observed in the ELISpot assays were able to maintain strong long-term antibody production, the HAI, MN and ELISA titers in the LD-adjuvanted groups were significantly higher at the later time-points, possibly as a result of the larger numbers of long-lived plasma and memory B cells induced. Although the animals in the current study were not challenged with an infectious virus or a late vaccine boost, the levels of antibody produced during a recall response are correlated with both the number of activated antibody secreting B cells and the secretion rate of these cells.Citation33 It will therefore be of considerable interest to know if there are major differences in a late secondary recall response following initial immunization with the HD-unadjuvanted and LD-adjuvanted formulations. Our next study focuses on the level of protection against homologous and heterologous challenge infections.
One possible explanation for these different patterns of response may have been revealed in the cytokine/chemokine production profiles of the influenza antigen re-stimulated splenocytes. Previously, we showed that at 3 weeks pb the HD-unadjuvanted vaccine generated a strong but narrowly-focused Th2 response, while the LD-adjuvanted vaccines elicited a broader response with the production of a wide range of cytokines/chemokines.Citation6 In this study, we show that these trends are amplified in the long-term and that the different vaccine formulations generated unique long-term cytokine/chemokine response patterns.
The early Th2-focused response seen in the HD-unadjuvanted group was associated with overall lower numbers of ASCs but greater antibody production per ASC than the LD-adjuvanted groups, and was clearly sufficient to induce an effective B cell response with robust, long-term serum antibody titers. In contrast, splenocytes isolated from the LD-adjuvanted vaccine groups induced a much broader, mixed response including Th2, Th1 and Th17-type cytokines which is consistent with mixed Th1/Th2 responses seen in earlier studies.Citation6,10 This broader response may have contributed to the long-term maintenance of higher serum antibodies titres and the generation of more ASCs overall even though each individual ASC produced less antibody. The mixed cytokine response generated by the LD-adjuvanted groups may have also promoted the higher frequency of antigen-specific T cells that we observed by flow cytometry.
The LD-adjuvanted and HD-unadjuvanted formulations also generated different patterns of responding T cells. Overall, the LD-adjuvanted formulations, specifically the 0.03 μg HA+AS03 group, produced more antigen-responsive CD4+ T cells compared to the HD-unadjuvanted group at both early and later time-points, which is consistent with our previous work.Citation6 At all time-points, the majority of the responding cells in all the groups were single positive although smaller numbers of poly-functional cells were also present at all time-points. The 0.03 µg HA+AS03 group generated the largest proportional of poly-functional CD4+ T cells, but reduction of the antigen dose (0.003 µg HA+ AS03) reduced the proportional of poly-functional cells. The 0.003 µg HA+AS03 group also tended to have the highest frequency of single positive cells. These observations suggest that there may be limits on the degree of dose sparing possible with adjuvanted vaccines after which aspects of the immune response are affected. Since serum antibody levels were essentially equivalent with LD-adjuvanted compared to HD-unadjuvanted formulations in our studies, reliance on serum HAI titers for licensure of dose-sparing influenza formulations (± adjuvants) could potentially miss major changes in the immune responses induced. Furthermore, the large differences in antigen-specific CD4+ T cell profiles generated by the LD-adjuvanted and HD-unadjuvanted vaccines raise the possibility that these formulations drive immune responses by very different mechanisms.
Both the LD-adjuvanted and HD-unadjuvanted groups developed high titres of influenza-specific antibodies that persisted for at least 8 months after vaccination. However, there were also important differences in the responses generated by these different formulations. Although adjuvant-induced inflammation can sometimes promote short- rather than long-term immunity,Citation34,35 we found that the LD-AS03-adjuvanted formulations were the most effective at inducing long-term memory responses in our mouse model. These formulations elicited very different antigen-specific cytokine/chemokine profiles and patterns of antigen-specific CD4+ T cells in re-stimulated splenocytes compared to the HD-unadjuvanted vaccine. Our results confirm the dose-sparing potential of AS03 that has already been demonstrated in the human experience with the AS03-adjuvated pH1N1 vaccineCitation13,15,16,18,19,24,26 and provide reassurance that this response does not appear to come at the expense of long-term memory. However, there is likely an optimal level of any given antigen (+ adjuvant) that will elicit and maintain a multi-faceted immune response. A more complete understanding of the long-term immune responses will be useful in the generation of more effective vaccines that optimize both antigen dose and adjuvant use.
Materials & methods
Vaccine, adjuvant and mouse immunizations
Monovalent influenza A/Uruguay/716/2007 (H3N2) detergent-split inactivated vaccine antigen and AS03 were manufactured by GSK Vaccines.Citation6,36 AS03B is an Adjuvant System containing α-tocopherol and squalene in an o/w emulsion (5.93 mg tocopherol). The AS03B used for the studies described herein are equivalent to 1/10th of the adult human dose, and hereafter referred to as AS03. The detergent-split virion vaccines were ad-mixed with AS03 by gentle inversion preceding each immunization. Vaccine injection volume was the same in all groups (50 μl). Three vaccine formulations were used as previously describedCitation6: a ‘standard’ dose of 3 μg split vaccine antigen based on hemagglutinin (HA) content (termed ‘high-dose’ or HD) and 2 low-dose (LD) formulations 0.03 μg HA+AS03, and 0.003 μg HA+AS03. No AS03-alone control group was included since our previous work has demonstrated that antigen-specific immune responses are not generated.Citation6,36 Eight to 10 week-old female BALB/c mice (Charles River Laboratories) were immunized by injection into the gastrocnemius muscle on days 0 and 21 (0.5 CC syringe with 28G needle); 35 mice were immunized with each vaccine formulation. Before each immunization and at various intervals post-boost (pb), blood was collected from the left lateral saphenous vein. At 1, 3, 6, 12, 18, and 34 weeks pb, 5 to 8 mice were sacrificed to more thoroughly assess humoral and cellular responses. Serum samples, splenocytes and bone marrow (BM) cells were collected from each mouse and processed individually as described below. All procedures were carried out in accordance with guidelines of the Canadian Council on Animal Care, as approved by the Animal Care Committee of McGill University.
Antibody titer measurement
Hemagglutination inhibition (HAI) and microneutralization (MN) assays were performed as previously described.Citation6,36
ELISA protocols to determine HA-specific IgG end-point titers and antibody avidity were also performed as previously describedCitation6,36 but with modifications for avidity analysis. Briefly, different urea concentrations were tested and 2 M urea (15 min at RT) was found to reduce ∼50% of the optical density (OD) of most samples. Serum samples were diluted at 1/50 or more to yield a final OD between 0.1 and 1.5. The avidity index (AI) was calculated as (OD after 2 M urea incubation) / (total OD without urea) X 100%.
Isolation of splenocytes and bone marrow (BM) cells
Spleens were collected and processed individually for each mouse. Splenocytes were isolated as previously describedCitation6,36 and re-suspended in RPMI supplemented with 10% FBS, 1mM penicillin/streptomycin (all from Wisent) and 0.5 mM β-mercaptoethanol (Sigma) (complete RPMI, cRPMI). At sacrifice, the femurs and tibias of each mouse were collected in Hank's balanced salt solution without calcium and magnesium (HBSS) (Wisent). BM plugs were flushed from the bones using cRPMI and 21G needles, passed through a 70μm cell strainer (BD Biosciences), treated with ACK lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM Na2EDTA; pH 7.2), then washed with HBSS and re-suspended in cRPMI.
ELISpot assays
Influenza HA-specific antibody secreting cells (ASCs) in splenocytes and BM cells were determined using the ELISpotPlus for Mouse IgG kit (MabTech Inc.) following Protocol I using biotinylated Ag as previously described.Citation6 Plates were read using a CTL series 3B ImmunoSpot analyzer (CTL Analyzers LLC) with ImmunoSpot 4.0.3 software supplied by the manufacturer. To enumerate memory B-cell-derived ASCs, splenocytes and BM cells were poly-clonally stimulated to differentiate memory B cells into ASCs detectable by ELISpot. Cells were stimulated for 5 days ex vivo with CpG and human IL-2 as previously describedCitation6 and modified fromCitation37-39 to generate memory B cell-derived plasma cells before seeding onto ELISpot plates as described above. In addition, at 12, 18 and 34 weeks pb, cells were also stimulated ex vivo for 2 days with R848 TLR-agonist and recombinant mouse IL-2 (both provided by the ELISpotPlus for Mouse IgG kit; MabTech Inc) according to the manufacturer's protocol, and then seeded onto ELISpot plates as described above (Fig. S1). For each sample, cells (10Citation4 to 10Citation6 per well) were added to 2 to 4 wells of ELISpot plates. Cells were also cultured without stimulation for 2 or 5 days and were included as negative controls on ELISpot plates, and were at baseline (data not shown). Results are described as the number of HA-specific IgG ASCs or memory ASCs per 10Citation6 splenocytes or BM cells. Spot sizes were measured as 10−3 mm2 according to the ImmunoSpot software.
Splenocyte stimulation and quantitation of cytokines in supernatant
Splenocytes isolated at each time-point were stimulated ex vivo with cRPMI (unstimulated) or 2.5 μg/ml homologous split vaccine antigen (based on HA content) as previously described.Citation6,36 Supernatants were collected at 72 h and stored at −80°C until cytokine analysis. The concentrations of 16 cytokines and chemokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-17, MCP-1/CCL2, IFNγ, TNFα, MIP-1α/CCL3, GM-CSF and RANTES/CCL5) were determined using Q-Plex™ Mouse Cytokine - Screen (16-plex) multiplex ELISA following the manufacturer's guidelines (Quansys Biosciences). Ag-stimulated samples for each mouse were run as singlets. Unstimulated samples were pooled for each group and run as singlets. Since cytokine concentrations of the unstimulated samples were very similar for all vaccine formulation, they were grouped as a general “Unstimulated” control. To investigate global cytokine profiles, cytokine concentrations are shown as the fold increase from “Unstimulated.” Actual concentrations of all cytokines/chemokines at all time-points are also shown in Fig. S3.
Splenocyte stimulation, intracellular staining and flow cytometry (FC) analysis
Protocols were modified from Moris et. al.Citation27 and as previously describedCitation6 to analyze influenza-specific T cell responses. Splenocytes were seeded in 96-well U-bottom plates (BD Biosciences) at 106 cells in 200 µl total per well. Splenocytes from each mouse were stimulated in singlet with anti-mouse CD28 (37.51) and CD49d (HMa2) antibodies (both from BD Biosciences) at 2 µg/ml final and 2.5 µg/ml of A/Uruguay H3N2 vaccine antigen based on HA content (“Costim+Ag”-stimulated) in cRPMI. As negative controls, pooled splenocytes for each group were incubated with anti-mouse CD28 and CD49d antibodies (“Costim only,” background). After 2 hrs at 37°C+5% CO2, GolgiPlug (BD Biosciences) was added according to manufacturer's protocol to all samples and incubated for an additional 12 hrs. As positive controls, pooled splenocytes for each group were incubated with costimulatory antibodies and PMA+ionomycin (2.5 µg/ml and 5 µg/ml final, respectively) and GolgiPlug for 12 hrs. Control samples were stained and analyzed as described below (data not shown). After incubation, cells were transferred to V-bottom plates (BD Biosciences) for FC staining.
Overall, cells were stained for viability, then CD3, CD4 and CD8 on the surface and intracellularly for IL-2, IL-5, IFNγ and TNFα. Antibodies used were as follows: Mouse BD Fc Block (2.4G2), V500 anti-mouse CD4 (RM4-5), PerCP-Cy5.5 anti-mouse CD8a (53–6.7), PE anti-mouse IFNγ (XMG1.2) and APC anti-mouse/anti-human IL-5 (TRFK5) (all from BD Biosciences); fixable viability dye eFluor780, FITC anti-mouse CD3e (145–2C11), PE-Cy7 anti-mouse IL-2 (JES6-5H4), and eFluor450 anti-mouse TNFα (MP6-XT22) (all from eBioscience).
Cells were stained with fixable viability dye eFluor 780 following manufacturer's protocol, then incubated with Mouse BD Fc Block (1 µl/sample) for 10 mins, and surface stained with anti-CD3e, anti-CD4, and anti-CD8a antibodies (each at 0.5 µl/sample) for 20 mins on ice. After washing, cells were resuspended in 100 µl IC Fixation buffer (eBioscience) and store at 4°C for 48 hrs. Cells were permeabilized with Permeabilization buffer (eBioscience) and then intracellularly stained with anti-IL-2, anti-IL-5, anti- IFNγ and anti-TNFα (each at 1 µl/sample) for 45 mins on ice. After washing, data was acquired on a FACSCanto II flow cytometer using FACSDiva software (Becton Dickinson). Singly-stained compensation controls were prepared with OneComp eBeads (eBioscience) for all antibodies with the exception of V500 anti-mouse CD4 and fixable viability dye eFluor 780, which were prepared with splenocytes. Automatic compensation calculations were performed in FACSDiva. Data analysis was performed using FlowJo software (Tree Star).
The flow cytometry gating strategy is shown in Fig. S4. We analyzed CD4+ and CD8+ T cells that produce a combination of IL-2, IFNγ, TNFα and IL-5 cytokines. Results are expressed as the number of cytokine positive cell per one million cells. The background (cells stimulated with anti-CD28 and anti-CD49d antibodies only, “Costim only”) is the average signal from all groups.
Statistically analysis
Serologic data were log transformed before analysis. Differences between curves were calculated by regular 2-way analysis of variance (ANOVA) with Bonferroni post-test to compare all possible pairs of groups at all time-points. All analyses were performed using GraphPad Prism 5.0 software. P values <0.05 were considered statistically significant.
Trademark disclosure
Arepanrix is a trademark of the GSK group of companies.
Abbreviations
| ASCs | = | antibody-secreting cells |
| Ag | = | antigen |
| BM | = | bone marrow |
| HAI | = | hemagglutination inhibition |
| HA | = | hemagglutinin |
| HD | = | high-dose |
| LD | = | low-dose |
| MN | = | microneutralization |
| pb | = | post-boost |
Disclosure of potential conflicts of interest
EB and CPM are, or were at the time of the study, employees of the GSK group of companies. CPM owns stock in GSK and is listed as an inventor on patents owned by GSK. The remaining authors declare no commercial or financial conflict of interest.
Author contributions
Author contributions: KKY and BJW designed the study and experiments. KKY, AB and VB performed experiments under the supervision of KKY. KKY and BJW analyzed the data and wrote the paper with input from EB and CPM.
KHVI_A_1241360_Supplementary_material.zip
Download Zip (885.2 KB)Acknowledgments
We thank A. Ricciardi and J. Gupta for technical assistance, D.S. Burt for critical review of the manuscript, and N. Bernard for providing the ELISpot plate reader. We thank U. Krause (GSK Vaccines) for editorial assistance and coordinating the review of the manuscript, and A. Morasse (GSK Vaccines) for FACS data review. We also thank the scientists at GSK Vaccines involved in manuscript revision. Data included in this paper was previously presented in part at Immunology 2013 - Centennial Annual Meeting of the American Association of Immunologists (AAI), May 2013, Honolulu, Hawaii (Abstract 123.13).
Funding
This work was supported by grants from Canadian Institutes of Health Research (CIHR) (#34469), the Public Health Agency of Canada/CIHR Influenza Research Network (PCIRN) and the Ministère de l’économie, de l'innovation et des exportations of Québec (the last with Genome Québec oversight). KKY received a PCIRN fellowship.
References
- Statistics Canada (2010). Canadian Community Health Survey: H1N1 Vaccinations. Statistics Canada http://www.statcan.gc.ca/daily-quotidien/100719/dq100719b-eng.htm
- GlaxoSmithKline Inc (2009). Arepanrix H1N1 [package insert]. Mississauga, ON, Canada
- Leroux-Roels G. Prepandemic H5N1 influenza vaccine adjuvanted with AS03: a review of the pre-clinical and clinical data. Expert Opin Biol Ther 2009; 9:1057-71; PMID:19555313; http://dx.doi.org/10.1517/14712590903066695
- Health Canada (2010). Product Information Leaflet Arepanrix™ H1N1 AS03-Adjuvanted H1N1 Pandemic Influenza Vaccine - Version 4 approved 20 April 2010. Health Canada http://www.hc-sc.gc.ca/dhp-mps/prodpharma/legislation/interimorders-arretesurgence/prodinfo-vaccin-eng.php
- Yam KK, Gupta J, Brewer A, Scheifele DW, Halperin S, Ward BJ. Unusual Patterns of IgG Avidity in Some Young Children following Two Doses of the Adjuvanted Pandemic H1N1 (2009) Influenza Virus Vaccine. Clin Vaccine Immunol 2013; 20:459-67; PMID:23345582; http://dx.doi.org/10.1128/CVI.00619-12
- Yam KK, Gupta J, Winter K, Allen E, Brewer A, Beaulieu E, Mallett CP, Burt DS, Ward BJ. AS03-Adjuvanted, Very-Low-Dose Influenza Vaccines Induce Distinctive Immune Responses Compared to Unadjuvanted High-Dose Vaccines in BALB/c Mice. Front Immunol 2015; 6:207; PMID:25972874; http://dx.doi.org/10.3389/fimmu.2015.00207
- Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, Planty C, Elouahabi A, Harvengt P, Carlsen H et al. Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 2011; 29:2461-73; PMID:21256188; http://dx.doi.org/10.1016/j.vaccine.2011.01.011
- Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity 2010; 33:492-503; PMID:21029960; http://dx.doi.org/10.1016/j.immuni.2010.10.002
- Bromage E, Stephens R, Hassoun L. The third dimension of ELISPOTs: quantifying antibody secretion from individual plasma cells. J Immunol Methods 2009; 346:75-9; PMID:19465022; http://dx.doi.org/10.1016/j.jim.2009.05.005
- Baz M, Samant M, Zekki H, Tribout-Jover P, Plante M, Lanteigne AM, Hamelin ME, Mallett C, Papadopoulou B, Boivin G. Effects of different adjuvants in the context of intramuscular and intranasal routes on humoral and cellular immune responses induced by detergent-split A/H3N2 influenza vaccines in mice. Clin Vaccine Immunol 2012; 19:209-18; PMID:22190392; http://dx.doi.org/10.1128/CVI.05441-11
- Ann J, Samant M, Rheaume C, Dumas C, Beaulieu E, Morasse A, Mallett C, Hamelin ME, Papadopoulou B, Boivin G. Adjuvanted inactivated influenza A(H3N2) vaccines induce stronger immunogenicity in mice and confer higher protection in ferrets than unadjuvanted inactivated vaccines. Vaccine 2014; 32:5730-9; PMID:25173481; http://dx.doi.org/10.1016/j.vaccine.2014.08.029
- Mallett CP, Beaulieu E, Joly MH, Baras B, Lu X, Liu F, Levine MZ, Katz JM, Innis BL, Giannini SL. AS03-adjuvanted H7N1 detergent-split virion vaccine is highly immunogenic in unprimed mice and induces cross-reactive antibodies to emerged H7N9 and additional H7 subtypes. Vaccine 2015; 33:3784-7; PMID:26100923; http://dx.doi.org/10.1016/j.vaccine.2015.06.053
- Poder A, Simurka P, Li P, Roy-Ghanta S, Vaughn D. An observer-blind, randomized, multi-center trial assessing long-term safety and immunogenicity of AS03-adjuvanted or unadjuvanted H1N1/2009 influenza vaccines in children 10–17 years of age. Vaccine 2014; 32:1121-9; PMID:24252703; http://dx.doi.org/10.1016/j.vaccine.2013.11.031
- Nolan T, Izurieta P, Lee BW, Chan PC, Marshall H, Booy R, Drame M, Vaughn DW. Heterologous prime-boost vaccination using an AS03B-adjuvanted influenza A(H5N1) vaccine in infants and children. J Infect Dis 2014; 210:1800-10; PMID:24973461; http://dx.doi.org/10.1093/infdis/jiu359
- Langley JM, Reich D, Aggarwal N, Connor D, Lebel MH, Gupta A, Garfield H, Li P, Madan A, Vaughn DW. Randomized, multicenter trial of a single dose of AS03-adjuvanted or unadjuvanted H1N1 2009 pandemic influenza vaccine in children 6 months to. Pediatr Infect Dis J 2012; 31:848-58; PMID:22801094; http://dx.doi.org/10.1097/INF.0b013e31825e6cd6
- Saitoh A, Nagai A, Tenjinbaru K, Li P, Vaughn DW, Roman F, Kato T. Safety and persistence of immunological response 6 months after intramuscular vaccination with an AS03-adjuvanted H1N1 2009 influenza vaccine: an open-label, randomized trial in Japanese children aged 6 months to 17 years. Hum Vaccin Immunother 2012; 8:749-58; PMID:22495117; http://dx.doi.org/10.4161/hv.19684
- Gilca V, De SG, Hamelin ME, Boivin G, Ouakki M, Boulianne N, Sauvageau C, Dionne M, Gilca R, Skowronski D. Antibody persistence and response to 2010–2011 trivalent influenza vaccine one year after a single dose of 2009 AS03-adjuvanted pandemic H1N1 vaccine in children. Vaccine 2011; 30:35-41; PMID:22063386; http://dx.doi.org/10.1016/j.vaccine.2011.10.062
- Yang WH, Dionne M, Kyle M, Aggarwal N, Li P, Madariaga M, Godeaux O, Vaughn DW. Long-term immunogenicity of an AS03-adjuvanted influenza A(H1N1)pdm09 vaccine in young and elderly adults: an observer-blind, randomized trial. Vaccine 2013; 31:4389-97; PMID:23856331; http://dx.doi.org/10.1016/j.vaccine.2013.07.007
- Ikematsu H, Nagai H, Kawashima M, Kawakami Y, Tenjinbaru K, Li P, Walravens K, Gillard P, Roman F. Characterization and long-term persistence of immune response following two doses of an AS03A-adjuvanted H1N1 influenza vaccine in healthy Japanese adults. Hum Vaccin Immunother 2012; 8:260-6; PMID:22426369; http://dx.doi.org/10.4161/hv.18469
- Yang PC, Yu CJ, Chang SC, Hsieh SM, Drame M, Walravens K, Roman F, Gillard P. Safety and immunogenicity of a split-virion AS03A-adjuvanted A/Indonesia/05/2005 (H5N1) vaccine in Taiwanese adults. J Formos Med Assoc 2012; 111:333-9; PMID:22748624; http://dx.doi.org/10.1016/j.jfma.2011.02.006
- Langley JM, Risi G, Caldwell M, Gilderman L, Berwald B, Fogarty C, Poling T, Riff D, Baron M, Frenette L et al. Dose-sparing H5N1 A/Indonesia/05/2005 pre-pandemic influenza vaccine in adults and elderly adults: a phase III, placebo-controlled, randomized study. J Infect Dis 2011; 203:1729-38; PMID:21606531; http://dx.doi.org/10.1093/infdis/jir172
- Leroux-Roels I, Roman F, Forgus S, Maes C, De BF, Drame M, Gillard P, van der Most R, Van MM, Hanon E et al. Priming with AS03 A-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non-randomised extension of a double-blind randomised primary study. Vaccine 2010; 28:849-57; PMID:19835828; http://dx.doi.org/10.1016/j.vaccine.2009.10.017
- Risi G, Frenette L, Langley JM, Li P, Riff D, Sheldon E, Vaughn DW, Fries L. Immunological priming induced by a two-dose series of H5N1 influenza antigen, administered alone or in combination with two different formulations of AS03 adjuvant in adults: Results of a randomised single heterologous booster dose study at 15 months. Vaccine 2013; 31:436-7; PMID:23387064; http://dx.doi.org/10.1016/j.vaccine.2012.11.002
- Ferguson M, Risi G, Davis M, Sheldon E, Baron M, Li P, Madariaga M, Fries L, Godeaux O, Vaughn D. Safety and long-term humoral immune response in adults after vaccination with an H1N1 2009 pandemic influenza vaccine with or without AS03 adjuvant. J Infect Dis 2012; 205:733-44; PMID:22315336; http://dx.doi.org/10.1093/infdis/jir641
- Ikematsu H, Tenjinbaru K, Li P, Madan A, Vaughn D. Evaluation of immune response following one dose of an AS03A-adjuvanted H1N1 2009 pandemic influenza vaccine in Japanese adults 65 years of age or older. Hum Vaccin Immunother 2012; 8:1119-25; PMID:22854661; http://dx.doi.org/10.4161/hv.21081
- Nicholson KG, Abrams KR, Batham S, Clark TW, Hoschler K, Lim WS, Medina MJ, Nguyen-Van-Tam JS, Read RC, Warren FC et al. Immunogenicity and safety of a two-dose schedule of whole-virion and AS03A-adjuvanted 2009 influenza A (H1N1) vaccines: a randomised, multicentre, age-stratified, head-to-head trial. Lancet Infect Dis 2011; 11:91-101; PMID:21168369; http://dx.doi.org/10.1016/S1473-3099(10)70296-6
- Moris P, van der Most R, Leroux-Roels I, Clement F, Drame M, Hanon E, Leroux-Roels GG, Van MM. H5N1 influenza vaccine formulated with AS03A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol 2011; 31:443-54; PMID:21174144; http://dx.doi.org/10.1007/s10875-010-9490-6
- Gillard P, Caplanusi A, Knuf M, Roman F, Walravens K, Moris P, Drame M, Schwarz TF. An assessment of prime-boost vaccination schedules with AS03A -adjuvanted prepandemic H5N1 vaccines: a randomized study in European adults. Influenza Other Respir Viruses 2013; 7:55-65; PMID:22405557; http://dx.doi.org/10.1111/j.1750-2659.2012.00349.x
- Van Damme P, Kafeja F, Bambure V, Hanon E, Moris P, Roman F, Gillard P. Long-term persistence of humoral and cellular immune responses induced by an AS03A-adjuvanted H1N1 2009 influenza vaccine: an open-label, randomized study in adults aged 18–60 years and older. Hum Vaccin Immunother 2013; 9:1512-22; PMID:23571166; http://dx.doi.org/10.4161/hv.24504
- Bergen MJ, Pan CH, Greer CE, Legg HS, Polo JM, Griffin DE. Comparison of the immune responses induced by chimeric alphavirus-vectored and formalin-inactivated alum-precipitated measles vaccines in mice. PLoS One 2010; 5:e10297; PMID:20421972; http://dx.doi.org/10.1371/journal.pone.0010297
- Taillardet M, Haffar G, Mondiere P, Asensio MJ, Gheit H, Burdin N, Defrance T, Genestier L. The thymus-independent immunity conferred by a pneumococcal polysaccharide is mediated by long-lived plasma cells. Blood 2009; 114:4432-40; PMID:19767510; http://dx.doi.org/10.1182/blood-2009-01-200014
- Kim JH, Talbot HK, Mishina M, Zhu Y, Chen J, Cao W, Reber AJ, Griffin MR, Shay DK, Spencer SM et al. High-dose influenza vaccine favors acute plasmablast responses rather than long-term cellular responses. Vaccine 2016; 34:4594-601; PMID:27473306; http://dx.doi.org/10.1016/j.vaccine.2016.07.018
- Henn AD, Rebhahn J, Brown MA, Murphy AJ, Coca MN, Hyrien O, Pellegrin T, Mosmann T, Zand MS. Modulation of single-cell IgG secretion frequency and rates in human memory B cells by CpG DNA, CD40L, IL-21, and cell division. J Immunol 2009; 183:3177-87; PMID:19675172; http://dx.doi.org/10.4049/jimmunol.0804233
- Amsen D, Backer RA, Helbig C. Decisions on the road to memory. Adv Exp Med Biol 2013; 785:107-20; PMID:23456843; http://dx.doi.org/10.1007/978-1-4614-6217-0_12
- Alter G, Sekaly RP. Beyond adjuvants: Antagonizing inflammation to enhance vaccine immunity. Vaccine 2015; 33 Suppl 2:B55-9; PMID:26022570; http://dx.doi.org/10.1016/j.vaccine.2015.03.058
- Yam KK, Gupta J, Allen EK, Burt KR, Beaulieu E, Mallett CP, Burt DS, Ward BJ. Comparison of AS03 and Alum on immune responses elicited by A/H3N2 split influenza vaccine in young, mature and aged BALB/c mice. Vaccine 2016; 34:1444-51; PMID:26873056; http://dx.doi.org/10.1016/j.vaccine.2016.02.012
- Morel S, Denoel P, Godfroid F, Cortvrindt C, Vanderheyde N, Poolman J. Induction of Bordetella pertussis-specific immune memory by DTPa vaccines. Vaccine 2011; 29:3449-55; PMID:21382483; http://dx.doi.org/10.1016/j.vaccine.2011.02.062
- Giannini SL, Hanon E, Moris P, Van MM, Morel S, Dessy F, Fourneau MA, Colau B, Suzich J, Losonksy G et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006; 24:5937-49; PMID:16828940; http://dx.doi.org/10.1016/j.vaccine.2006.06.005
- Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 2002; 298:2199-202; PMID:12481138; http://dx.doi.org/10.1126/science.1076071
