ABSTRACT
Cholera remains an important but neglected public health threat, affecting the health of the poorest populations and imposing substantial costs on public health systems. Cholera can be eliminated where access to clean water, sanitation, and satisfactory hygiene practices are sustained, but major improvements in infrastructure continue to be a distant goal. New developments and trends of cholera disease burden, the creation of affordable oral cholera vaccines (OCVs) for use in developing countries, as well as recent evidence of vaccination impact has created an increased demand for cholera vaccines. The global OCV stockpile was established in 2013 and with support from Gavi, has assisted in achieving rapid access to vaccine in emergencies. Recent WHO prequalification of a second affordable OCV supports the stockpile goals of increased availability and distribution to affected populations. It serves as an essential step toward an integrated cholera control and prevention strategy in emergency and endemic settings.
Introduction
Vibrio cholerae (serotypes O1 and O139) is a highly transmissible bacterium, which can cause a rapidly dehydrating, watery diarrheal disease known as cholera. Cholera is endemic in over 50 countries with an estimated 2 million cases and 100,000 deaths per year and a population at risk of approximately 1.5 billion.Citation1 Cholera outbreaks are frequent and prolonged in endemic areas with recurrent seasonal patterns. Epidemics also occur in non-endemic areas, initiated by exogenous introduction of V. cholerae, often associated with complex emergencies that result in the breakdown of infrastructure or population displacement. Although cholera affects any age group, children under 5 y of age are at higher risk of contracting cholera in endemic settings.Citation2
The disease occurs when V. cholerae colonizes the small intestine and secretes the 2-subunit cholera toxin. The B subunit of the toxin binds to the epithelial cell surface, whereas the A subunit triggers a biochemical cascade causing watery diarrhea which may lead to severe dehydration.Citation3 Following clinical disease, an immune response occurs both to the toxin and to the bacterial cell wall. The most important protective antigen, especially for vaccine protection, is most likely the O antigen of the cell wall lipopolysaccharide (LPS).Citation4 In endemic areas, asymptomatic V. cholerae infections range from 40 to 80% and can present as mild diarrhea.Citation5 Since vibrios have a mucosal port of entry, oral vaccination is the preferable administration route since it can successfully elicit locally produced intestinal antibodies and stimulate immunologic memory.Citation6 Furthermore, oral vaccines present the advantage of not requiring delivery by staff trained for injections during mass campaigns and also eliminate the risk of needle stick injuries.Citation7 Though easier to administer than its predecessors, challenges in the field include: achieving high coverage when given in the recommended dosing regimen to mobile populations (2 doses in a 2–6 week interval), volume constraints, and its cold chain requirement during storage and transport (2–8°C).
Fluid replacement serves as the primary treatment for cholera. The mainstay of fluid resuscitation is oral rehydration solution (ORS) if the patient can tolerate intake by mouth, or IV fluids followed by ORS for severe dehydration.Citation8 Oral zinc in children under 5 y of age, although not essential for treatment, has been found to be important in assisting with regeneration of the intestinal epithelium and has shown to reduce duration and severity of diarrheal episodes, as well as possibly preventing future episodes of diarrhea for up to 3 months.Citation9-11 Antimicrobial therapy as an adjunct to fluid resuscitation has been shown to decrease the duration and volume of diarrhea by approximately 50% in cholera patients with severe dehydration.Citation12,13 In addition, antibiotics also contribute to stopping the excretion of vibrio more rapidly, reducing the risk of transmission when the patient returns to the community.
Successful control and elimination of cholera in developing countries are directly related to improvements in hygiene and availability of clean drinking water, as was seen with the curbing of the Latin American cholera epidemic in the 1990s.Citation14,15 Cholera remains a persistent problem in many resource limited settings where poverty, political instability, natural calamities, or security conditions make implementation of appropriate surveillance and control activities challenging.Citation16
Available OCVs
WHO prequalified oral cholera vaccines (OCVs) currently available on the global market include Dukoral (SBL Vaccin, Sweden), Shanchol (Shantha Biotechnics Ltd, India), and Euvichol (Eubiologics, South Korea). All of these require a 2 dose regimen for maximum protection. Vaccine prequalification is an activity led by the World Health Organization (WHO) intended to ensure that vaccines purchased by UN procurement agencies meet WHO recommendations for quality, safety, and efficacy.Citation17 Only prequalified OCVs can be included in the global OCV stockpile.Citation18 Current projections of OCV supply are based on planned replenishment into the stockpile, which is divided into an emergency stock (outbreaks and humanitarian emergencies) and non-emergency reserve (endemic hotspots). An OCV (mORC-Vax, Vabiotech) is currently being produced in Vietnam and one (Cholvax, Incepta Vaccine Ltd.) is under development in Bangladesh, where clinical trials are being initiated at the time of writing. However, neither is WHO prequalified and they are currently intended for national use only.
Killed whole cell vaccine with cholera toxin B subunit
Dukoral contains a mixture of the recombinant B subunit of cholera toxin plus killed strains of V. cholerae O1 representing serotypes Inaba and Ogawa and biotypes El Tor and Classical. The vaccine does not contain any of the cholera toxin A subunit and is free of its toxic effects. Because the heat labile toxin (LT) of E. coli cross reacts with cholera toxin, this vaccine has been shown to provide short term cross protection against diarrhea caused by enterotoxigenic E. coli.Citation19 The vaccine requires 2 doses for adults and 3 doses for children below 5 y of age. The vaccine requires co-administration of a buffer to prevent degradation of the toxin subunit. In Bangladesh, a placebo controlled randomized controlled trial (RCT) in 90,000 individuals aged 2 y and above demonstrated 85% efficacy for 6 months following vaccination and 50% efficacy of 3 y for older children and adults.Citation20 In Beira, Mozambique, mass vaccination was feasible and effective in preventing cholera in a population with a 20–30% seroprevalence of HIV.Citation21 More than 14,000 people received at least one dose and a case control study demonstrated 78% protection against cholera and 89% protection against cholera with severe dehydration. Of note, all strains isolated in this evaluation were El Tor variants that produced a modified form of the classical cholera toxin, representing a newer, dominant, and more severe variety of cholera. Though WHO prequalified, it has mainly been used as a travelers' vaccine due to limited production and price. In addition the fact that it required a buffer to be dissolved in potable water made it logistically impractical in mass vaccination campaigns.
Modified killed whole cell only vaccines
On the basis of the encouraging findings of the use of WC-rCTB vaccine, the technology for production of the oral killed whole cell vaccine was transferred from Sweden to Vietnam in the 1980s. The Vietnamese government commenced local development of an inexpensive oral O1 serogroup whole cell only vaccine in the 1980s. This vaccine was similar in composition to WC-rCTB except it lacked the B subunit toxin. It was shown to be safe and conferred 66% protection against cholera during an epidemic which occurred 8–10 months following vaccination in an open field trial.Citation22 The vaccine was made into a bivalent formulation (O1 and O139) and was licensed as mORC-Vax in 1997. Because the vaccine lacks the B subunit of the cholera toxin, it does not require co-administration with an oral buffer. Over 20 million doses were used in Vietnam's public health programs. Unfortunately, this vaccine was not suitable for WHO prequalification as the Vietnamese national regulatory authority (NRA) was not assessed as WHO functional, a condition for pre-qualification. International scientists worked together to improve vaccine constituents and the manufacturing process. In particular, the International Vaccine Institute (IVI) played a key role in the improvements in formulation and standardization of the vaccine and in facilitating the technology transfer of the modified vaccine to India, which had a functional NRA approved by WHO. The target group for OCVs are individuals aged 1 y and above (except Dukoral which is indicated for 2 y and above).Citation23 OCVs are only being supplied to populations deemed to be at high risk of cholera. In these situations, based on a risk-benefit analysis, the Global Task Force on Cholera Control (GTFCC) considered that there are considerable benefits, and very few risks, from including pregnant women in a vaccine campaign,Citation24 especially given the lack of observed adverse events following vaccination in this group and the fact that they are at higher risk for severe disease and death.
The resulting vaccine, Shanchol, was prequalified by WHO in 2011 and shown to be well tolerated and highly immunogenic in Vietnam, India, and Ethiopia.Citation27-29 Shanchol has conferred 67% protection in a double blind randomized placebo controlled trial in more than 67,000 children and adults in Kolkata, India.Citation30 However, levels of protection were not uniform across all age groups. Young children aged one to 5 y, were significantly less protected with a cumulative efficacy of 42% over 5 y. Similarly, an efficacy trial of a single dose of Shanchol conducted in Bangladesh showed evidence of protection only in persons who were vaccinated as older children (≥5 years of age) or adults. The vaccine protective efficacy was 40% (95% confidence interval [CI], 11 to 60%) against all cholera episodes, 63% (95% CI, 24 to 82%) against severely dehydrating cholera episodes, and 63% (95% CI, −39 to 90%), 56% (95% CI, 16 to 77%), and 16% (95% CI, −49% to 53%) against all cholera episodes among persons vaccinated at the age of 5 to 14 years, 15 or more years, and 1 to 4 years, respectively.Citation31 A large community based feasibility and effectiveness trial with over 267,000 participants in Bangladesh showed that in real life settings in a highly mobile urban community, a population based vaccination program achieving moderate coverage in hyperendemic settings could substantially reduce the burden of disease and greatly contribute to long term cholera control.Citation32 When comparing responses between one and 2 vaccine doses, investigators from Kolkata found no increase in seroconversion (4-fold rise in serum vibriocidal antibodies), following a second dose as compared to those after the first dose.Citation33 Interestingly, this may be directly related to the amount of natural exposure and pre-existing antibodies since higher seroconversion rates were noted following a second dose in comparatively less endemic areas in Haiti and Ethiopia.Citation29,34,35 This suggests that there may be important geographical differences in immunological response in areas of varying cholera exposure. A phase 3 placebo RCT assessing a single dose of Shanchol in over 200,000 individuals from the hyperendemic setting of Bangladesh found an efficacy of 40% against all cholera cases and 60% protective against cholera cases with severe dehydration over a 6 month period.Citation31 A recent study in Haiti showed that HIV-infected individuals developed somewhat lower but still appreciable serum vibriocidal antibody responses, compared with those in HIV-uninfected individuals, and that, among HIV-infected persons, the magnitude of these responses varied inversely with CD4 lymphocyte counts.Citation36
Euvichol is the second affordable OCV which resulted from the further development of the Vietnamese vaccine. Euvichol was prequalified by the WHO in late 2015. It has the same formulation as Shanchol (the 2 vaccines are essentially identical) and clinical studies have demonstrated immunological non-inferiority, as to Shanchol.Citation37 The entry of Euvichol into the market is expected to significantly increase vaccine availability and use. Similar non-inferiority evaluations are underway for another formulation of WC-OCV, Cholvax (Incepta, Bangladesh). Once complete, the aim is to dramatically increase production capacity, enabling vaccination of large populations at risk throughout Bangladesh.
Modified killed whole cell vaccine with cholera toxin B subunit
A new killed OCV is under development by researchers at University of Goteborg and Hilleman Laboratories. The simplified production approach focuses on using one genetically engineered El Tor Hikojima strain that undergoes a single inactivation method. Inclusion of a recombinant low cost cholera toxin with the B subunit in the new formulation would avoid the need for buffer co-administration, strict cold chain requirements, and would offer short-term cross protection against enterotoxigenci (ETEC) diarrhea.Citation19 This modified WC-rCTB candidate has been shown to be immunogenic in mice.Citation38 If shown to be safe and protective in humans, this option could result in lower manufacturing costs and substantially reduce OCV prices. Future work on a new oral mucosal adjuvant for a second generation version may further potentiate intestinal immune responses, leading to improved long-term protection among all age groups.Citation39 A summary of key characteristics and clinical trial data for currently available and candidate killed OCVs is presented in .
Table 1. Killed oral cholera vaccines.
Global oral cholera vaccine stockpile
Achievements
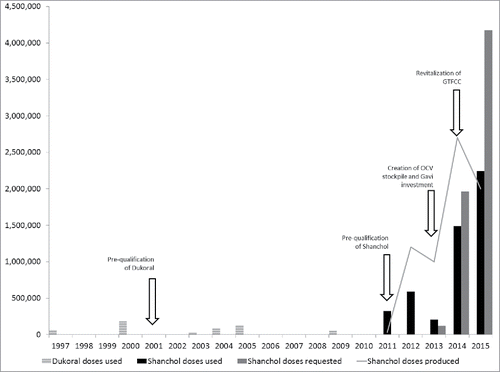
The global OCV stockpile was created in 2013 as a mechanism to encourage vaccine use for underserved populations and encourage the change from low demand, low production, high unit costs, and inequitable distribution, to an increased demand and production, lower unit costs, and greater equity of distribution. Since its creation, global interest in cholera has been boosted. The availability of OCVs that are safe, effective, and easy to administer, and have the ability to afford herd protection at a population level are important accomplishments of this public health intervention.Citation41,42 While vaccine alone will not eliminate disease, it provides a new tool in the armoury of cholera response measures. OCV could act as a trigger mechanism to mobilize endemic countries and partners to develop and implement multisectoral cholera control programmes. In this sense the experience in Haiti, whose 2010 cholera outbreak was one of the triggers to establish the stockpile, is emblematic: the country went from a scenario where virtually no OCV was available to respond to the outbreak, to being able to integrate it into its regular cholera control efforts.Citation43-45 Since the creation of the stockpile, there have been over 5 million doses deployed in humanitarian crises, outbreaks, and endemic settingsCitation46 (). Because of limited supply, OCV for emergency use is released after review of country applications by the International Coordinating Group (ICG), composed of UNICEF, Médecins Sans Frontières (MSF), The International Federation of Red Cross and Red Crescent Societies (IFRC), and WHO.
It is notable that 2015 was the first year in which OCV requests exceeded supply. While immediate availability has been a major reason behind increased interest, other important factors include the observed feasibility of mass OCV campaigns and ability to confer protection to underserved populations in complex situations.Citation47-49 The increased use and experience with OCV adds multidimensional values to the established control measures. In the past, OCV and traditional measures of cholera response (water, sanitation, hygiene), surveillance, community education, and access to treatment had been viewed as competitive, if not mutually exclusive. With greater awareness, they are being considered complementary, if not synergistic.Citation32 The December 2015 WHO prequalification of Euvichol, is expected to significantly increase current global OCV production,Citation50 as will further development and production of the Bangladesh modified WC only vaccine, Cholvax. Currently non-inferiority immunogenicity studies are underway for Cholvax, with the aim of licensure and eventual WHO prequalification planned for the coming years.
Challenges
Diarrheal diseases are responsible for killing over 700,000 children every year, representing the second leading cause of death in children under 5 y of age.Citation51 Unfortunately, oral vaccines against enteric infections have been less immunogenic and efficacious when given to those living in less developed countries, especially in young children.Citation52,53 When evaluated in the highly endemic setting of Kolkata, the modified WC only OCV also exhibited lower efficacy in children under 5 y (42%). However, it should be noted that more cases were prevented by vaccination in this age group than any other age group due to the higher incidence of disease in this group: 10.5/1000 (1–4 years), 5.5/1000 (5–14 years), 3.1/1000 (≥15 years). Though the rationale is not completely understood, key factors associated with poor oral vaccine performance in children in developing countries appear to be related to the intestinal environment of these populations. Specific factors that need to be evaluated further include protein energy and micronutrient malnutrition, maternal antibody interference, concomitant parasitic infections, and intestinal mucosal damage due to environmental enteropathy.Citation54 Also, the suggestively lower protective effects seen in this age group could reflect a lesser degree of pre-existing natural anti-cholera immunity in young children compared to older strata of the population, which have had more chance to be naturally exposed to cholera throughout their lives. It is also worth mentioning that most studies do not rule out a degree of protection in young children but rather seem underpowered to detect the extent of this effect, highlighting the need to conduct specific evaluations of vaccine effects in the younger strata of the population. Furthermore estimates of vaccine efficacy or effectiveness refer to direct vaccine effects and tend not to include indirect ones (i.e. the level of protection conferred to non-immune individuals who live in a vaccinated population). Even if OCV is less immunogenic in children, it may be that these will benefit from indirect protection (i.e., herd immunity) by living in areas where older strata of the population are protected.
Improved knowledge of immune responses to V. cholerae can provide important insight and understanding behind ideal dosing strategies. Robust immune responses have been detected 7 d following the first dose in a highly endemic region.Citation55 Increases in seroconversion rates following a second dose are likely related to cholera incidence and pre-existing immunity in a given population. Populations in areas with increased natural boosting may exhibit an earlier and stronger immune response following a single dose. Studies evaluating vibriocidal responses have found no significant differences in rates of seroconversion between 2 and 4 week schedules in hyperendemic areas.Citation56 Hence, defining a strategy for endemic areas may involve the administration of the first dose (primer) followed by subsequent doses (boosters) at a regular interval thereafter based on characteristics of the epidemiological setting. Because pre-existing immunity likely plays a substantial role in the magnitude and duration of protection, potential strategies for national programs implementation would need to be evaluated in clinical field trials immunologically representative of each region.
Monitoring and evaluation (M+E) after vaccination is essential to demonstrate that OCV reduces the impact of cholera and has a role in its prevention and control, combined with classical control measures. In this sense, the stockpile becomes a ‘data- generator’ and the GTFCC, revitalized by WHO in 2014, serves as a platform where donors and partners work together to demonstrate the public health potential of a coordinated effort against cholera. Since the creation of the stockpile, many M+E activities have been completed or are underway to assess coverage, acceptability, feasibility, safety, efficacy/effectiveness, and especially impact and cost effectiveness of OCVs. With regards to the latter, conducting economic analyses on the immediate costs on the individuals and the health systems and on the long term societal costs due to loss of life time productivity is key. In a recent campaign in South Sudan, the cost of a fully immunized individual was estimated at USD$5.6, including the vaccine (66%), storage and transport (10%), and delivery (24%).Citation58 However, costs can vary depending on the contexts and on the strategy used and more data is currently being analysed from campaigns conducted so far. Through transfer of technology and partnerships between governmental, nongovernmental, academic, and manufacturing partners, increased access to affordable OCV has become a reality. Nevertheless, continued efforts to develop delivery and production strategies are needed to further identify best practices, which simplify delivery, increase affordability and equitable access to underserved populations.
Funding and commercial issues for developing country markets
The global OCV stockpile was created on the principle that vaccines have a role in the prevention and control of cholera when used in conjunction with accessible health care and improvements in water and sanitation. In November 2013, the Gavi board approved a US$115 million contribution over 5 y (2013–2018) to support the utilization of a global stockpile strategy, to optimize delivery of OCV in epidemic and endemic settings. In addition to increasing access to OCV, the Gavi support aims to generate a body of evidence describing the public health impact of OCV use in mass campaigns. This evidence will be used to inform a further Gavi investment decision. The key objectives of the Gavi investment include:
Breaking the current cycle of low demand, low supply, significantly increasing global OCV production and availability
Reducing impact of cholera in outbreak, humanitarian crisis and endemic settings
Strengthening the evidence base by demonstrating public health potential of OCV campaigns.
Increasing equitable access to underserved populations
Cholera vaccination will be reconsidered by the Gavi board in 2018 for ongoing long-term support, based on information gathered during the investment period, notably on impact and cost. Between the 2 modified WC OCVs that are currently prequalified by WHO (Shanchol and Euvichol), approximately 6 million doses will be available in 2016. The stockpile has also been established on the understanding that currently the number of doses is limited relative to the need for vaccine and that mechanisms should be established to ensure equitable access for the populations most exposed to the risk of cholera, resulting in an emergency stock to be readily available to respond to outbreaks and humanitarian crises and a non-emergency reserve to be used to control endemic cholera ().
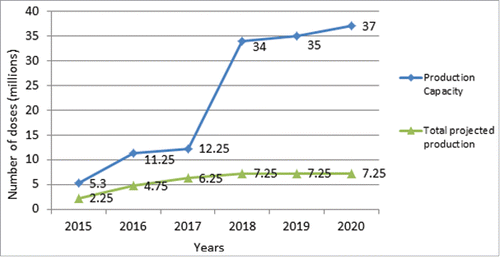
The introduction of OCV as a novel public health intervention requires careful project management to balance insecure vaccine supply against an increasing demand, which can outstrip a finite vaccine stock, especially in the event of a large outbreak or humanitarian crisis which by nature are predictably un-predictable. In the current investment period for the OCV stockpile, Shanchol production will remain at approximately 2 million doses per year. Current projections for Euvichol production are for 3–4 million doses per year, with a maximum production capacity of up to 25 million doses per year (). The Bangladesh modified WC only OCV could add another 20–40 million doses, initially only for the in-country market, but, if eventually prequalified, also for the international one. Though forecast demand for oral cholera vaccine has increased, the international global health community should remain cautiously optimistic until vaccine production meets projected demand.
Future directions in OCV implementation research
The growing body of vaccine effectiveness and economic data is contributing to inform larger investments in integrated control strategies, including surveillance, WASH, and OCV measures. Recently, countries have become more willing to report cholera, which is a significant change from the past. The addition of a new WHO prequalified (December 2015) affordable OCV to the global market has significant implications in addressing worldwide un-met demand, doubling the current supply to 6 million doses in 2016, with the potential for further increased production in the future. Although this expected increase in production will presumably allow for more preventive use in non-emergency settings, the flexible mechanisms between the emergency stock and the non-emergency reserve ensure that priority will be given to emergencies if needed ().
However, even with successful deployments to areas of need and planned increases in production, it will probably remain difficult to secure and deliver enough vaccine to all high risk regions for the next few years at least. For those reasons, it is imperative to maximize monitoring and evaluation of future OCV campaigns to demonstrate how best to use OCV in each of the following contexts:
Emergency settings:
○ Vaccination in a humanitarian crisis affecting large numbers of people in cholera endemic areas with population displacement due to war, civil unrest, or natural disasters.
○ Vaccination in response to a cholera outbreak to prevent its spread.
Non-emergency settings:
○ Vaccination to reduce incidence of disease in highly endemic hotspots.
Understanding how to utilize a finite resource to obtain optimal impact cost-effectively through the evaluation of current and alternative strategies is essential to improve cholera prevention and control strategies.
Unconventional approaches can also serve to improve success and even decrease cost. Recent data has shown that WC-OCV is stable at elevated temperatures, with no significant change in safety or immunogenicity profiles.Citation40 This allows for novel approaches to be explored, to increase access to the highest risk, hardest to reach, and most vulnerable populations. Alternative vaccination strategies, such as the use of a single dose regimen and extended dosing intervals could offer increased benefit to populations, where traditional 2 round campaigns may be unfeasible. Similarly measuring the feasibility, coverage, and impact of modified second dose delivery approaches, utilizing community distribution and self-administration in high risk, mobile populations, such as fishermen, refugees, and internally displaced people is also being explored.
More studies are needed to estimate indirect effects and measure the impact of OCV on cholera epidemiology at the population level in addition to the individual level. In addition, continued efforts must be focused on innovative vaccine research and development of new adjuvants for improved temperature sensitivity and mucosal immune responses, which could provide insight to longer duration of protection with a single dose and improved efficacy in younger children. Additional research on the benefits of supplementary interventions (e.g. concomitant micronutrient supplementation) and delivery schedules (e.g., increasing the delay between the first and the second dose) may also improve immunization of children.Citation58 Furthermore, delivering OCVs together with routine childhood immunizations could make the introduction of cholera vaccines in endemic areas easier.Citation58 Finally, given the potential for a single dose strategy with longer term protection, several live OCVs, such as the recently US FDA approved Vaxchora (Paxvax, USA),Citation59 are currently in the product pipeline and the focus of important discussions for future vaccination policies.
Conclusion
For too long the approach to cholera has been a combination of chronic neglect or preparedness and response to outbreaks. OCV is an important public health tool to decrease cholera morbidity and mortality. Increasing the availability and distribution of OCV to affected populations represents an important step forward toward the implementation of a successful multisectoral cholera prevention and control strategy.
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest.
References
- Ali M, Nelson AR, Lopez AL, Sack DA. Updated Global Burden of Cholera in Endemic Countries. PLoS Negl Trop Dis 2015; 9(6):e0003832; PMID:26043000; http://dx.doi.org/10.1371/journal.pntd.0003832
- Deen JL, von Seidlein L, Sur D, Agtini M, Lucas ME, Lopez AL, Kim DR, Ali M, Clemens JD. The high burden of cholera in children: comparison of incidence from endemic areas in Asia and Africa. PLoS Neglected Tropical Diseases [electronic resource] 2008; 2(2):e173; PMID:18299707
- Sanchez J, Holmgren J. Virulence factors, pathogenesis and vaccine protection in cholera and ETEC diarrhea. Curr Opin Immunol 2005; 17(4):388-98; PMID:15963708; http://dx.doi.org/10.1016/j.coi.2005.06.007
- Jonson G, Osek J, Svennerholm AM, Holmgren J. Immune mechanisms and protective antigens of Vibrio cholerae serogroup O139 as a basis for vaccine development. Infect Immun 1996; 64(9):3778-85; PMID:8751929
- Nelson EJ, Harris JB, Glenn Morris J, Calderwood SB, Camilli A. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat Rev Micro 2009; 7(10):693-702; http://dx.doi.org/10.1038/nrmicro2204
- Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med 2005; 11(4 Suppl):S45-53; PMID:15812489; http://dx.doi.org/10.1038/nm1213
- Levine MM. Can needle-free administration of vaccines become the norm in global immunization? Nat Med 2003; 9(1):99-103; PMID:12514720; http://dx.doi.org/10.1038/nm0103-99
- Harris JB, LaRocque R, Qadri F, Ryan ET, Calderwood SB. Cholera. Lancet 2012; (379):2466-76; PMID:22748592; http://dx.doi.org/10.1016/S0140-6736(12)60436-X
- Roy SK, Hossain MJ, Khatun W, Chakraborty B, Chowdhury S, Begum A, Mah-e-Muneer S, Shafique S, Khanam M, Chowdhury R. Zinc supplementation in children with cholera in Bangladesh: randomised controlled trial. BMJ 2008; 336(7638):266-8; PMID:18184631; http://dx.doi.org/10.1136/bmj.39416.646250.AE
- Bhandari N, Mazumder S, Taneja S, Dube B, Agarwal RC, Mahalanabis D, Fontaine O, Black RE, Bhan MK. Effectiveness of zinc supplementation plus oral rehydration salts compared with oral rehydration salts alone as a treatment for acute diarrhea in a primary care setting: a cluster randomized trial. Pediatrics 2008; 121(5):e1279-85; PMID:18450870; http://dx.doi.org/10.1542/peds.2007-1939
- Lukacik M, Thomas RL, Aranda JV. A meta-analysis of the effects of oral zinc in the treatment of acute and persistent diarrhea. Pediatrics 2008; 121(2):326-336; PMID:18245424; http://dx.doi.org/10.1542/peds.2007-0921
- Nelson EJ, Nelson DS, Salam MA, Sack DA. Antibiotics for both moderate and severe cholera. N Engl J Med 2011; 364(1):5-7; PMID:21142691; http://dx.doi.org/10.1056/NEJMp1013771
- Lindenbaum J, Greenough WB, Islam MR. Antibiotic therapy of cholera. Bull World Health Organ 1967; 36(6):871-83; PMID:4865453
- Koo D, Traverso H, Libel M, Drasbek C, Tauxe R, Brandling-Bennett D. Epidemic cholera in Latin America, 1991–1993: implications of case definitions used for public health surveillance. Bull Pan Am Health Organ 1996; 30(2):134-43; PMID:8704754
- Anh DD, Lopez AL, Tran HTM, Cuong NV, Thiem VD, Ali M, Deen JL, von Seidlein L, Sack DA. Oral cholera vaccine development and use in Vietnam. PLoS medicine 2014; 11(9):e1001712; PMID:25180511; http://dx.doi.org/10.1371/journal.pmed.1001712
- WHO. Revitalizing control efforts for cholera. Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations 2015; 90(40):530-4.
- Dellepiane N, Wood D. Twenty-five years of the WHO vaccines prequalification programme (1987–2012): lessons learned and future perspectives. Vaccine 2015; 33(1):52-61; PMID:24300593; http://dx.doi.org/10.1016/j.vaccine.2013.11.066
- Martin S, Lopez AL, Bellos A, Deen J, Ali M, Alberti K, Anh DD, Costa A, Grais RF, Legros D. Post-licensure deployment of oral cholera vaccines: a systematic review. Bull World Health Organ 2014; 92(12):881-93; PMID:25552772; http://dx.doi.org/10.2471/BLT.14.139949
- Clemens JD, Sack DA, Harris JR, et al. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J Infect Dis 1988; 158(2):372-7; PMID:3042876; http://dx.doi.org/10.1093/infdis/158.2.372
- Clemens JD, Sack DA, Harris JR, Van Loon F, Chakraborty J, Ahmed F, Rao MR, Khan MR, Yunus M, Huda N. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet 1990; 335(8684):270-3; PMID:1967730; http://dx.doi.org/10.1016/0140-6736(90)90080-O
- Lucas MES, Deen JL, von Seidlein L, Wang XY, Ampuero J, Puri M, Ali M, Ansaruzzaman M, Amos J, Macuamule A, et al. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med 2005; 352(8):757-67; PMID:15728808; http://dx.doi.org/10.1056/NEJMoa043323
- Trach DD, Clemens JD, Ke NT, Thuy HT, Son ND, Canh DG, Hang PV, Rao MR. Field trial of a locally produced, killed, oral cholera vaccine in Vietnam. Lancet 1997; 349(9047):231-5; PMID:9014909; http://dx.doi.org/10.1016/S0140-6736(96)06107-7
- WHO. Cholera vaccines: WHO position paper. Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations 2010; 85(13):117-28.
- WHO. Technical Note: Evidence of the risks and benefits of vaccinating pregnant women with WHO pre-qualified cholera vaccines during mass vaccination campaigns. 2016; http://www.who.int/cholera/vaccines/Risk_Benefits_vaccinating_pregnant_women_Technical_Note_13Jan2016.pdf?ua=1. Accessed March 20, 2016, 2016.
- Ciglenecki I, Bichet M, Tena J, Mondesir E, Bastard M, Tran NT, Antierens A, Staderini N. Cholera in pregnancy: outcomes from a specialized cholera treatment unit for pregnant women in Leogane, Haiti. PLoS Negl Trop Dis 2013 Aug 15; 7(8):e2368; http://dx.doi.org/10.1371/journal.pntd.0002368
- Khan AI, Chowdhury F, Leung DT, Larocque RC, Harris JB, Ryan ET, Calderwood SB, Qadri F. Cholera in pregnancy: Clinical and immunological aspects. Int J Infect Dis 2015; 39:20-4; PMID:26283553; http://dx.doi.org/10.1016/j.ijid.2015.08.006
- Anh D, Canh D, Lopez A, Thiem VD, Long PT, Son NH, Deen J, von Seidlein L, Carbis R, Han SH, et al. Safety and immunogenicity of a reformulated Vietnamese bivalent killed, whole-cell, oral cholera vaccine in adults. Vaccine 2007; 25(6):1149-55; PMID:17055622; http://dx.doi.org/10.1016/j.vaccine.2006.09.049
- Mahalanabis D, Lopez AL, Sur D, Deen J, Manna B, Kanungo S, von Seidlein L, Carbis R, Han SH, Shin SH, et al. A randomized, placebo-controlled trial of the bivalent killed, whole-cell, oral cholera vaccine in adults and children in a cholera endemic area in Kolkata, India. PLoS ONE 2008; 3(6):e2323; PMID:18523643; http://dx.doi.org/10.1371/journal.pone.0002323
- Desai SN, Akalu Z, Teshome S, Teferi M, Yamuah L, Kim DR, Yang JS, Hussein J, Park JY, Jang MS, et al. A randomized, placebo-controlled trial evaluating safety and immunogenicity of the killed, bivalent, whole-cell oral cholera vaccine in Ethiopia. Am J Trop Med Hyg 2016; 21(2):194–201; PMID:26681205
- Bhattacharya SK, Sur D, Ali M, Kanungo S, You YA, Manna B, Sah B, Niyogi SK, Park JK, Sarkar B, et al. Five year efficacy of a bivalent killed whole-cell oral cholera vaccine in Kolkata, India: a cluster-randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2013; 13(12):1050-6; PMID:24140390; http://dx.doi.org/10.1016/S1473-3099(13)70273-1
- Qadri F, Wierzba TF, Ali M, Chowdhury F, Khan AI, Saha A, Khan IA, Asaduzzaman M, Akter A, Khan A, et al. Efficacy of a Single-Dose, Inactivated Oral Cholera Vaccine in Bangladesh. N Engl J Med 2016; 374(18):1723-1732; PMID:27144848; http://dx.doi.org/10.1056/NEJMoa1510330
- Qadri F, Ali M, Chowdhury F, Khan AI, Saha A, Khan IA, Begum YA, Bhuiyan TR, Chowdhury MI, Uddin MJ, et al. Feasibility and effectiveness of oral cholera vaccine in an urban endemic setting in Bangladesh: a cluster randomised open-label trial. Lancet 2015; 386(10001):1362-71; PMID:26164097; http://dx.doi.org/10.1016/S0140-6736(15)61140-0
- Kanungo S, Paisley A, Lopez A, Bhattacharya M, Manna B, Kim DR, Han SH, Attridge S, Carbis R, Rao R, et al. Immune responses following one and two doses of the reformulated, bivalent, killed, whole-cell, oral cholera vaccine among adults and children in Kolkata, India: a randomized, placebo-controlled trial. Vaccine 2009; 27(49):6887-93; PMID:19761838; http://dx.doi.org/10.1016/j.vaccine.2009.09.008
- Charles RC, Hilaire IJ, Mayo-Smith LM, Teng JE, Jerome JG, Franke MF, Saha A, Yu Y, Kováč P, Calderwood SB, et al. Immunogenicity of a Killed Bivalent (O1 and O139) Whole Cell Oral Cholera Vaccine, Shanchol, in Haiti. PLoS Negl Trop Dis 2014; 8(5):e2828; PMID:24786645; http://dx.doi.org/10.1371/journal.pntd.0002828
- Desai SN, Akalu Z, Teferi M, Manna B, Teshome S, Park JY, Yang JS, Kim DR, Kanungo S, Digilio L. Comparison of immune responses to a killed bivalent whole cell oral cholera vaccine between endemic and less endemic settings. Trop Med Int Health 2016; 21(2):194-201; http://dx.doi.org/10.1111/tmi.12641
- Ivers LC, Charles RC, Hilaire IJ, Mayo-Smith LM, Teng JE, Jerome JG, Rychert J, LaRocque RC, Xu P, Kovácˇ P, et al. Immunogenicity of the bivalent oral cholera vaccine Shanchol in Haitian adults with HIV infection. J Infect Dis 2015; 212(5):779–83; PMID:25722294
- Baik YO, Choi SK, Olveda RM, Espos RA, Ligsay AD, Montellano MB, Yeam JS, Yang JS, Park JY, Kim DR, et al. A randomized, non-inferiority trial comparing two bivalent killed, whole cell, oral cholera vaccines (Euvichol vs Shanchol) in the Philippines. Vaccine 2015; 33(46):6360-5; PMID:26348402; http://dx.doi.org/10.1016/j.vaccine.2015.08.075
- Lebens M, Karlsson SL, Kallgard S, Blomquist M, Ekman A, Nygren E, Holmgren J. Construction of novel vaccine strains of Vibrio cholerae co-expressing the Inaba and Ogawa serotype antigens. Vaccine 2011; 29(43):7505-13; PMID:21807059; http://dx.doi.org/10.1016/j.vaccine.2011.06.121
- Larena M, Holmgren J, Lebens M, Terrinoni M, Lundgren A. Cholera toxin, and the related nontoxic adjuvants mmCT and dmLT, promote human Th17 responses via cyclic AMP-protein kinase A and inflammasome-dependent IL-1 signaling. Journal of immunology (Baltimore, Md. : 1950) 2015; 194(8):3829-39; PMID:25786687; http://dx.doi.org/10.4049/jimmunol.1401633
- Saha A, Khan A, Salma U, Jahan N, Bhuiyan TR, Chowdhury F, Khan AI, Khanam F, Muruganandham S, Reddy Kandukuri S, et al. The oral cholera vaccine Shanchol when stored at elevated temperatures maintains the safety and immunogenicity profile in Bangladeshi participants. Vaccine 2016; 34(13):1551-8; PMID:26896684; http://dx.doi.org/10.1016/j.vaccine.2016.02.020
- Ali M, Emch M, von Seidlein L, Yunus M, Sack DA, Rao M, Holmgren J, Clemens JD. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet 2005; 366(9479):44-49; PMID:15993232; http://dx.doi.org/10.1016/S0140-6736(05)66550-6
- Ali M, Sur D, You YA, Kanungo S, Sah B, Manna B, Puri M, Wierzba TF, Donner A, Nair GB, et al. Herd Protection by a Bivalent Killed Whole-Cell Oral Cholera Vaccine in the Slums of Kolkata, India. Clin Infect Dis 2013; 56(8):1123-31; PMID:23362293; http://dx.doi.org/10.1093/cid/cit009
- Binagwaho A, Nyatanyi T, Nutt CT, Wagner CM. Disease outbreaks: support for a cholera vaccine stockpile. Nature 2012; 487(7405):39; PMID:22763542; http://dx.doi.org/10.1038/487039c
- Pape JW, Rouzier V. Embracing oral cholera vaccine–shifting response to cholera. N Engl J Med 2014; 370(22):2067-9; PMID:24869720; http://dx.doi.org/10.1056/NEJMp1402837
- Severe K, Rouzier V, Anglade SB, Bertil C, Joseph P, Deroncelay A, Mabou MM, Wright PF, Guillaume FD, Pape JW. Effectiveness of Oral Cholera Vaccine in Haiti: 37-Month Follow-Up. Am J Trop Med Hyg 2016; 94(5):1136-42; PMID:26928838; http://dx.doi.org/10.4269/ajtmh.15-0700
- Nogareda F. Oral cholera vaccine campaigns 2013–2015: monitoring andevaluation. World Health Organization OCV Monitoring and Evaluation Working Group Meeting. Paper presented at: World Health Organization OCV Monitoring and Evaluation Working Group Meeting2015; Geneva.
- Luquero FJ, Grout L, Ciglenecki I, Sakoba K, Traore B, Heile M, Diallo AA, Itama C, Page AL, Quilici ML, et al. Use of Vibrio cholerae Vaccine in an Outbreak in Guinea. N Engl J Med 2014; 370(22):2111-20; PMID:24869721; http://dx.doi.org/10.1056/NEJMoa1312680
- Ivers LC, Hilaire IJ, Teng JE, Almazor CP, Jerome JG, Ternier R, Boncy J, Buteau J, Murray MB, Harris JB, et al. Effectiveness of reactive oral cholera vaccination in rural Haiti: a case-control study and bias-indicator analysis. The Lancet Global Health 2015; 3(3):e162-e168; PMID:25701994; http://dx.doi.org/10.1016/S2214-109X(14)70368-7
- Abubakar A, Azman AS, Rumunu J, Ciglenecki I, Helderman T, West H, Lessler J, Sack DA, Martin S, Perea W, et al. The First Use of the Global Oral Cholera Vaccine Emergency Stockpile: Lessons from South Sudan. PLoS Med 2015; 12(11):e1001901; PMID:26576044; http://dx.doi.org/10.1371/journal.pmed.1001901
- Desai SN, Pezzoli L, Martin S, Costa A, Rodriguez C, Legros D, Perea W. A second affordable oral cholera vaccine: implications for the global vaccine stockpile. The Lancet. Global health 2016; 4(4):e223-224; PMID:27013303
- WHO. Diarrhoeal disease fact sheet 2013. 2016.
- Pasetti M, Simon J, Sztein M, Levine M. Immunology of gut mucosal vaccines. Immunol Rev 2011; 239(1):125-148; PMID:21198669; http://dx.doi.org/10.1111/j.1600-065X.2010.00970.x
- Qadri F, Bhuiyan TR, Sack DA, Svennerholm AM. Immune responses and protection in children in developing countries induced by oral vaccines. Vaccine 2013; 31(3):452-60; PMID:23153448; http://dx.doi.org/10.1016/j.vaccine.2012.11.012
- Levine MM. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol 2010; 8(129); PMID:20920375
- Saha A, Chowdhury MI, Khanam F, Bhuiyan MS, Chowdhury F, Khan AI, Khan IA, Clemens J, Ali M, Cravioto A, et al. Safety and immunogenicity study of a killed bivalent (O1 and O139) whole-cell oral cholera vaccine Shanchol, in Bangladeshi adults and children as young as 1 year of age. Vaccine 2011; 29(46):8285-8292; PMID:21907255; http://dx.doi.org/10.1016/j.vaccine.2011.08.108
- Kanungo S, Desai SN, Nandy RK, Bhattacharya MK, Kim DR, Sinha A, Mahapatra T, Yang JS, Lopez AL, Manna B, et al. Flexibility of oral cholera vaccine dosing-a randomized controlled trial measuring immune responses following alternative vaccination schedules in a cholera hyper-endemic zone. PLoS Negl Trop Dis 2015; 9(3):e0003574; PMID:25764513; http://dx.doi.org/10.1371/journal.pntd.0003574
- WHO. Outbreak news. Oral cholera vaccine campaign among internally displaced persons in South Sudan. Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations 2014; 89(20):205-6.
- Desai SN, Cravioto A, Sur D, Kanungo S. Maximizing protection from use of oral cholera vaccines in developing country settings: an immunological review of oral cholera vaccines. Hum Vaccin Immunother 2014; 10(6):1457-65; PMID:24861554; http://dx.doi.org/10.4161/hv.29199
- Chen WH, Cohen MB, Kirkpatrick BD, Brady RC, Galloway D, Gurwith M, Hall RH, Kessler RA, Lock M, Haney D, et al. Single-dose Live Oral Cholera Vaccine CVD 103-HgR Protects Against Human Experimental Infection With Vibrio cholerae O1 El Tor. Clin Infect Dis 2016; 62(11):1329-35; PMID:27001804; http://dx.doi.org/10.1093/cid/ciw145