ABSTRACT
Background: We sought to estimate the national prevalence of HPV vaccine refusal and delay in a nationally-representative sample of parents of adolescents. We also compared parents who refused versus delayed HPV vaccine in terms of their vaccination beliefs and clinical communication preferences. Methods: In 2014 to 2015, we conducted an online survey of 1,484 US parents who reported on an 11- to 17-year-old child in their household. We used weighted multinomial logistic regression to assess correlates of HPV vaccine refusal and delay. Results: Overall, 28% of parents reported that they had ever “refused or decided not to get” HPV vaccine for their child, and an additional 8% of parents reported that they had “delayed or put off getting” HPV vaccine. Compared to no refusal/delay, refusal was associated with lower confidence in adolescent vaccination (relative risk ratio [RRR] = 0.66, 95% confidence interval [CI], 0.48–0.91), lower perceived HPV vaccine effectiveness (RRR = 0.68, 95% CI, 0.50–0.91), and higher perceived harms (RRR = 3.49, 95% CI, 2.65–4.60). In contrast, delay was associated with needing more information (RRR = 1.76, 95% CI, 1.08–2.85). Most parents rated physicians and information sheets as helpful for making decisions about HPV vaccination, although parents who reported refusal endorsed these resources less often. Conclusions: Our findings suggest that HPV vaccine refusal is common among parents of adolescents and may have increased relative to previous estimates. Because the vaccination beliefs and communication preferences of parents who refuse appear to differ from those who delay, targeted communication strategies may be needed to effectively address HPV vaccine hesitancy.
Human papillomavirus (HPV) vaccination coverage among US adolescents remains far below national goals with just 42% of girls and 28% of boys completing the 3-dose series by 2015.Citation1 In the extensive research literature that seeks to explain low HPV vaccination coverage, many studies have focused on parents of adolescents because they are most often responsible for making HPV vaccination decisions.Citation2 These studies, including our own, have typically examined the relationship between parents' vaccination beliefs, their intention to vaccinate their adolescents, and adolescents' ultimate vaccination status.Citation3-6 This research has been important for identifying potentially modifiable barriers to HPV vaccination, such as parents' need for more information and their perception that their children's risk of HPV infection is low.Citation3-6 However, despite the large volume of existing literature, basic gaps in our understanding of parents' decision making processes remain.
Most notably, we know surprisingly little about the epidemiology of HPV vaccine refusal and delay. These behaviors constitute important intermediary steps between parents' vaccination intentions and adolescents' vaccination status. Although the qualitative literature suggests that HPV vaccine refusal and delay are common,Citation7,8 only one study has estimated their national prevalence.Citation9 In 2010, a special addendum to the National Immunization Survey (NIS)-Teen assessed these behaviors, finding that 20% of parents of adolescent girls reported HPV vaccine refusal while an additional 11% reported delay.Citation9 Understanding parental refusal and delay of HPV vaccine is important for preventing these behaviors, but unfortunately, little is known about how the prevalence of HPV vaccine refusal and delay has changed since 2010, whether prevalence is different for boys vs. girls, or how parents who refuse differ from those who delay.
We sought to better understand HPV vaccine refusal and delay using data from a nationally-representative sample of parents of adolescents. The aims of this study were to: 1) estimate the national prevalence of HPV vaccine refusal and delay; 2) assess demographic and psychological correlates of refusal and delay; 3) compare parents' reasons for refusal versus delay; and 4) assess differences in parents' communication preference according to their refusal/delay status.
Results
Sample characteristics
About half (51%) of index children were male, and the mean age of children in the sample was 14 y (). Most were non-Hispanic white (58%), non-Hispanic black (12%), or Hispanic (21%). On indicators of socioeconomic status, over one-third of parents (39%) reported having a high school degree or less education and about one-fifth (22%) reported a household income of less than $35,000 per year.
Table 1. Sample characteristics (n = 1,484).
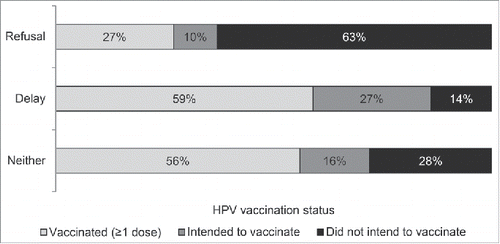
HPV vaccine refusal and delay, vaccination status, and intention to vaccinate
Full sample. Overall, 28% of parents reported having ever refused HPV vaccine, 8% reported delay, and 64% reported neither refusal nor delay. HPV vaccine initiation was less common among adolescents whose parents had ever refused (27%) vs. delayed (59%) or neither (56%) (p < 0.05) (). Among unvaccinated adolescents (n = 799), intention to get HPV vaccine was also lower among parents who reported refusal (46/321 or 14%) versus delay (30/48 or 66%) or neither (152/430 or 37%) (p < 0.05).
Figure 1. HPV vaccination status and intention to vaccinate for adolescents whose parents reported having ever refused HPV vaccine (n = 432), delayed HPV vaccine (n = 118), or neither (n = 934).
Girls, ages 13–17. For 13- to 17-year-old girls (n = 535), 169 parents (30%) reported having ever refused HPV vaccine, 61 parents (12%) reported delay, and 305 parents (58%) reported neither refusal nor delay. The proportion of girls who had initiated the HPV vaccine series was about one-third for those whose parents reported refusal (54/169 or 31%) vs. about two-thirds for those whose parents reported delay (42/61 or 64%) or neither (219/305 or 73%). Among unvaccinated girls (n = 220), intention to get HPV vaccine was lower among parents who reported refusal (15/115 or 13%) versus delay (15/19 or 81%) or neither (34/86 or 41%).
Correlates of HPV vaccine refusal and delay
Refusal. Compared to no refusal/delay, HPV vaccine refusal was more common among parents from households with high vs. low income (relative risk ratio [RRR] = 1.48, 95% confidence interval [CI], 1.02–2.15, ). Refusal was less common among parents who had high confidence in adolescent vaccination (RRR = 0.66, 95% CI, 0.48–0.91) or perceived high HPV vaccine effectiveness (RRR = 0.68, 95% CI, 0.50–0.91). Refusal was more common among parents who perceived high potential harm from HPV vaccination (RRR = 3.49, 95% CI, 2.65–4.60). Refusal was not associated with children's sex, age, or race/ethnicity, or with parents' educational attainment or uncertainty about HPV vaccine.
Table 2. Multivariable correlates of HPV vaccine refusal and delay (n = 1,484)
Delay. Compared to no refusal/delay, HPV vaccine delay was more common among parents reporting on female versus male children (RRR = 1.74, 95% CI, 1.14–2.65) and older children, ages 16–17, vs. younger children, ages 11–12 (RRR = 2.04, 95% CI, 1.16–3.59). Delay was also more common among parents who reported high uncertainty about HPV vaccine (RRR = 1.76, 95% CI, 1.08–2.85). Delay was not associated with children's race/ethnicity, parents' educational attainment, annual household income, or parents' perceptions of HPV vaccine effectiveness, harms, or vaccination confidence.
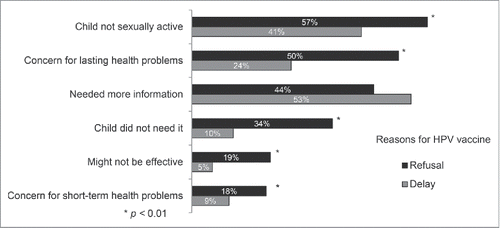
Reasons for HPV vaccine refusal and delay
Parents who refused versus delayed HPV vaccine more often reported their reason was believing their child was not sexually active (57% vs. 41%, ), concern about lasting health problems (50% versus 24%), or believing their child did not need HPV vaccine (34% vs. 10%, all p < 0.01). Similar proportions of parents who refused versus delayed indicated needing more information as their reason (44% vs. 53%). Parents who refused or delayed less often reported their reason was concern about HPV vaccine effectiveness (19% versus 5%) or concern about short-term health problems (18% vs. 9%).
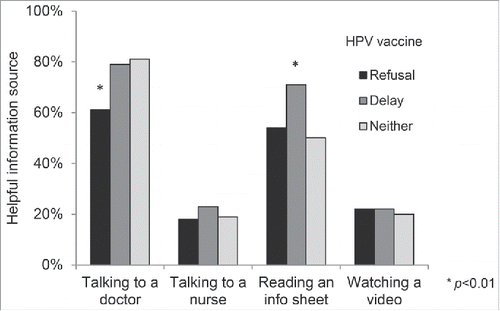
Clinical communication preferences
Helpful sources of information. Overall, a majority of parents (75%) said talking to a doctor would be helpful when deciding about HPV vaccination, although parents reporting HPV vaccine refusal (61%) endorsed this response less often than those reporting delay (79%) or neither refusal nor delay (81%) (, ). About half of parents (53%) perceived reading an information sheet as being helpful, with parents who reported delay (71%) more often endorsing this option than those reporting refusal (54%) or neither (50%). Parents less often perceived talking to a nurse (19%) or watching an informational video (21%) as being helpful.
Table 3. Parents’ preferences for clinical communication about HPV vaccination (n = 1,484).
Preferred age for provider recommendation. Overall, few parents (16%) believed providers should never routinely recommend HPV vaccine, although this perception was more common among parents reporting refusal (30%) versus delay (4%) or neither (12%). Most parents believed that providers should begin recommending HPV vaccine to adolescents on time by age 12 (39%) or behind schedule at age 13 or later (45%). Parents reporting delay (60%) more often indicated a preference for behind schedule recommendations compared to those reporting refusal (44%) or neither (44%).
Decisional timeframe. Overall, most parents preferred to make a same-day decision about HPV vaccination with little or no discussion (42%) or to make the decision at a later visit (42%). Parents who reported refusal (60%) or delay (51%) more often indicated a preference for deciding at a later visit compared to those reporting neither (33%). Few parents (16%) preferred a long discussion with their child's provider.
Discussion
We found that HPV vaccine refusal and delay were prevalent in our nationally-representative sample of parents of adolescents. Overall, 28% of parents reported having ever refused HPV vaccine, and consistent with prior research,Citation9,17 this behavior was more common among parents from high- vs. low-income households. In terms of change over time, our estimate for the prevalence of HPV vaccine refusal for 13- to 17-year-old girls was substantially higher than what the NIS-Teen reported, using the same measures, for 2010 (30% versus 20%, respectively).Citation9 This finding suggests that refusal may have become more common in recent years, which could be, in part, because providers are recommending HPV vaccination more often, thereby giving parents more opportunities to refuse.Citation5,18 Alternatively, the change in refusal prevalence could reflect an increase in parents' concerns or a mode effect, whereby parents were more comfortable reporting refusal in our online survey vs. during the telephone interviews conducted for NIS-Teen.Citation18 Whatever the case, this finding is troubling given that vaccine refusal is associated with under-immunization and constitutes a considerable burden to the healthcare system in terms of provider time and frustration.Citation9,19 Ongoing surveillance is needed to provide a better understanding of HPV vaccine refusal over time.
In addition to refusal, 8% of parents reported HPV vaccine delay. In terms of demographic variation, delay was more common among parents of girls versus boys and older vs. younger adolescents. For 13- to 17-year-old girls, our estimate of the proportion of parents who delayed HPV vaccine was very similar to that reported by NIS-Teen in 2010 (12% versus 11%).Citation9 This finding suggests that the prevalence of delay has remained stable over time.
Parents who refused vs. delayed HPV vaccine differed in terms of their vaccination behavior and intentions. Among parents with a history of refusal, over one-third (37%) went on to initiate HPV vaccination or intended to do so in the next year. Among parents with a history of delay, well over three-quarters (86%) initiated HPV vaccination or intended to do so. In this way, parents who delayed were similar to those who had neither refused nor delayed. Taken together, these findings suggest that providers should not give up when they encounter HPV vaccine hesitancy because many parents eventually change their minds and accept HPV vaccine. By continuing to offer counseling and recommendations, providers may have considerable success in raising HPV vaccination coverage among their adolescent patient populations.
In addition to vaccination behavior, concerns appeared to differ for parents who refused versus delayed HPV vaccine. Correlates of vaccine refusal included lower overall confidence in adolescent vaccination generally as well as lower perceived effectiveness and higher perceived harms of HPV vaccination specifically. When asked their reasons for refusal, parents most often reported believing their child was not sexually active and concern about lasting health problems. By contrast, the only psychological correlate of vaccine delay was higher uncertainty, with parents' most common reason for delay being the need for more information. These findings suggest that HPV vaccine refusal may be motivated by specific and sometimes serious concerns that correspond to the Health Belief Model's constructs of perceived benefits and threats,Citation6,20 with delay stemming from a more generalized feeling of ambivalence. If so, persuading the “fence sitters” who delay may be a fairly straightforward matter of providing information, while the “worried” who refuse may require more extensive and targeted counseling [Citation21, p. 84–85].
In terms of how to provide such counseling, parents reported differences in their clinical communication preferences according to their history of HPV vaccine refusal or delay. Consistent with prior research,Citation22,23 most respondents indicated that talking to a physician or reading an information sheet would be helpful for making a decision about HPV vaccination, although parents who reported refusal less often perceived these resources to be helpful. We were encouraged to note that, across respondent groups, relatively few parents believed that providers should never routinely recommend HPV vaccine. Similarly, few parents in any group perceived the need for long discussions about HPV vaccination. We did find, however, that over half of parents who reported delay indicated a preference for receiving recommendations behind schedule, and many parents in both groups preferred to make a decision about HPV vaccination at a visit subsequent to receiving a recommendation. These preferences likely pose a barrier to the timely delivery of HPV vaccine and suggest the need for providers to emphasize the advantages of “on time” HPV vaccination, which include convenience and enhanced protection against HPV.Citation5
The findings of our study point to several areas for future research. First, given the differences we observed among parents who refused vs. delayed HPV vaccine, researchers should seek to evaluate targeted strategies for providing information to vaccine hesitant parents. By identifying and addressing parents' specific concerns, providers may be able to counsel parents more effectively and efficiently, which is an important goal given providers' perception that discussing HPV vaccine takes far longer than other adolescent vaccines.Citation24 Second, because many parents in our sample indicated a preference for deciding about HPV vaccination after receiving a recommendation, future research should explore the impact of providing anticipatory guidance about adolescent vaccination. By giving parents prior notice that their children will be due to receive HPV vaccine, providers may be able to give parents more time to prepare.Citation9,25 Finally, given the dearth of data on HPV vaccine refusal and delay, additional research is needed to explore topics such as why parents who initially refuse or delay HPV vaccine later go on to get the vaccine. Understanding how parental refusal and delay interact with other key determinants of under-immunization (e.g., absent or low-quality provider recommendations) is also important.
Strengths of this study include a large, nationally-representative sample and a good response rate. To our knowledge, this study is the first to assess the national prevalence of HPV refusal and delay since the 2010 NIS-Teen. Surveillance of these behaviors is important given that refusal and delay are specific to parents' role in vaccine delivery, unlike vaccination status which reflects a combination of parent-, provider-, and health systems-related factors. Limitations of this study include its cross-sectional design which prevents us from assessing the temporal relationship between HPV vaccine refusal and delay and parents' vaccination behavior and concerns. That our measures rely on parents' self-report is also a limitation. Future research can build on the present study by prospectively assessing the relationship between parents' concerns and their decisions to refuse, delay, or accept HPV vaccine and by validating parental report of refusal and delay with provider-reported measures.
Conclusion
Parents who refused HPV vaccine differed from those who delayed in terms of their vaccination behavior, concerns, and communication preferences. While parents who delayed were distinguished only by their need for more information, parents who refused had distinct concerns and less often initiated or intended to initiate HPV vaccination. Relatively few parents in our sample were opposed to provider recommendations for HPV vaccination, but many who delayed expressed a preference for receiving recommendations behind schedule. Furthermore, only about half of parents who refused or delayed preferred same-day vaccination. Since many parents who refused or delayed went on to get HPV vaccine, our findings suggest that providers who encounter hesitancy should persist in offering recommendations, while understanding that parents' concerns and communication preferences vary. By developing targeted strategies, future studies may provide a way to better meet parents' communication needs, thereby increasing the acceptance of HPV vaccination and, in turn, adolescents' protection against future HPV-associated cancers.
Methods
Participants
We surveyed parents of adolescents in November 2014 to January 2015. Parents were members of a nationally-representative, online panel of US adults maintained by a survey research company.Citation10 The survey company constructed the panel using a dual-frame sampling approach consisting of random digit dialing supplemented by address based sampling; this approach provided coverage for households with and without landline telephones. Panel members were eligible to participate if they were the parent of an 11- to 17-year-old child. To facilitate the ongoing participation of low-resource households, the survey company provided internet access and an electronic device for panel members who lacked these resources. Other members received points redeemable for small cash payments.
The survey company invited 2,845 parents to participate, and 1,504 parents were eligible and completed the survey. Our response rate, calculated using American Association for Public Opinion Research Response Rate 5, was 61%Citation11 For this study, we excluded respondents who did not provide data on whether they had ever refused or delayed HPV vaccine (n = 20). Our final analytic sample consisted of 1,484 parents. The University of North Carolina Institutional Review Board approved this study.
Measures
Our survey assessed HPV vaccine refusal and delay using 2 separate items from the 2010 NIS-TeenCitation12: “Has there ever been a time when you [refused or decided not to get/delayed or put off getting] the HPV vaccine for [NAME]?” Separately for each item, parents who responded “yes” next indicated their reason(s) for refusal or delay, using a list of 10 response options. We categorized parents as having refused HPV vaccine (with or without delay), delayed only, or neither delayed nor refused. We combined parents who had refused only with those who had both refused and delayed based on prior research that suggests similarities between these groups in terms of HPV vaccine-related knowledge, attitudes, and behavior.Citation9
Our survey assessed HPV vaccination status with one item: “How many shots of the HPV vaccine has [NAME] had?”Citation6 We defined HPV vaccine initiation as responses of one or more shots. For adolescents who had not completed the HPV vaccine series, the survey also assessed parents' intention to get HPV vaccine in the next year.
Our survey assessed 4 constructs related to parents' vaccine-related perceptions. One item assessed the perceived effectiveness of HPV vaccine (“effectiveness”), 2 items assessed perceived harms of HPV vaccine (“harms”), one item assessed the perceived need for more information about HPV vaccine (“uncertainty”), and 4 items assessed confidence about adolescent vaccination more generally (“vaccination confidence”). We adapted these items from 2 validated measures of vaccination beliefs.Citation13-15 All items used response scales that ranged from 1 (“strongly disagree”) to 5 (“strongly agree”). For constructs with multiple items, we averaged responses across items. For each construct, we then categorized responses as “low” (≤3) or “high” (>3).
Our survey assessed preferences for clinical communication about HPV vaccination with 4 items. Parents rated information sources, such as talking to a doctor, in terms of whether they would be helpful for making a decision about HPV vaccination. Parents also indicated the age at which they believed providers should start recommending HPV vaccine. Based on guidelines for HPV vaccine administration,Citation16 we categorized responses as “on time” (by age 12), “late” (age 13 or older), or “never.” Finally, parents reported their preferred timeframe for decision making: same-day decision after little or no discussion with a provider; same-day decision after a long discussion; or decision at a later visit.
Our survey assessed the demographic characteristics of children on sex, age, and race/ethnicity. The survey company provided data on parents' sex, educational attainment, annual household income, and state of residence.
Statistical analysis
We calculated the prevalence of HPV vaccine refusal and delay for the overall sample as well as for girls, ages 13–17. We performed the latter subgroup analysis to facilitate comparison with 2010 NIS-Teen findings. We used chi-squared tests to assess differences in survey responses by refusal/delay category. We used multinomial logistic regression to simultaneously identify bivariate correlates of HPV vaccine refusal and delay, as compared to neither refusal nor delay. We then entered statistically significant correlates (p < 0.05) into a multivariable model.
Our analyses used survey weights to generate nationally-representative estimates. We conducted analyses data using Stata Version 12.0 (College Station, TX). Statistical tests were 2-tailed with a critical alpha of 0.05.
Abbreviations
| CDC | = | Centers for Disease Control and Prevention |
| CI | = | confidence interval |
| HPV | = | human papillomavirus |
| NIS | = | National Immunization Survey |
| RRR | = | relative risk ratio |
Disclosure of potential conflicts of interest
NB has received HPV vaccine-related grants from or been on paid advisory boards for Merck, GlaxoSmithKline, and Pfizer; he served on the National Vaccine Advisory Committee Working Group on HPV Vaccine and is chair of the National HPV Vaccination Roundtable. The remaining authors (MG, WC, MM) have no conflicts of interest to report.
Funding
This study was funded via an unrestricted educational grant to NB from Merck, Sharp and Dohme (Grant #8970803). Authors' time was supported by grants from the National Cancer Institute (K22 CA186979 for MG; R25 CA116339 for WC). Funders played no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
References
- Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD, Curtis CR, MacNeil J, Markowitz LE, Singleton JA. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 Years - United States, 2015. MMWR Morb Mortal Wkly Rep 2016; 65:850-8; PMID:27561081; http://dx.doi.org/10.15585/mmwr.mm6533a4
- McRee AL, Reiter PL, Brewer NT. Vaccinating adolescent girls against human papillomavirus-Who decides? Prev Med 2010; 50(4):213-4; http://dx.doi.org/10.1016/j.ypmed.2010.02.001
- Brewer NT, Gottlieb SL, Reiter PL, McRee AL, Liddon N, Markowitz L, Smith JS. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis 2011; 38:197-204; PMID:20838362; http://dx.doi.org/10.1097/OLQ.0b013e3181f12dbf
- Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr 2014; 168:76-82; PMID:24276343; http://dx.doi.org/10.1001/jamapediatrics.2013.2752
- Stokley S, Jeyarajah J, Yankey D, Cano M, Gee J, Roark J, Curtis RC, Markowitz L. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014–United States. MMWR Morb Mortal Wkly Rep 2014; 63:620-4; PMID:25055185
- Reiter PL, Brewer NT, Gottlieb SL, McRee AL, Smith JS. Parents' health beliefs and HPV vaccination of their adolescent daughters. Soc Sci Med 2009; 69(3):475-80; PMID:19540642; http://dx.doi.org/10.1016/j.socscimed.2009.05.024
- Perkins RB, Clark JA, Apte G, Vercruysse JL, Sumner JJ, Wall-Haas CL, Rosenquist AW, Pierre-Joseph N. Missed opportunities for HPV vaccination in adolescent girls: a qualitative study. Pediatrics 2014; 134(3):e666-74; PMID:25136036; http://dx.doi.org/10.1542/peds.2014-0442
- Hughes CC, Jones AL, Feemster KA, Fiks AG. HPV vaccine decision making in pediatric primary care: a semi-structured interview study. BMC Pediatr 2011; 11:74; PMID:21878128
- Dorell C, Yankey D, Jeyarajah J, Stokley S, Fisher A, Markowitz L, Smith PJ. Delay and refusal of human papillomavirus vaccine for girls, National Immunization Survey-Teen, 2010. Clin Pediatr (Phila) 2014; 53:261-9; PMID:24463951; http://dx.doi.org/10.1177/0009922813520070
- GfK. KnowledgePanel design summary. Available at: http://www.gfk.com/Documents/GfK-KnowledgePanel-Design-Summary.pdf. Accessed October 1, 2015
- The American Association for Public Opinion Research. Standard definitions: final dispositions of case codes and outcome rates for surveys. 5th ed. Lenexa, Kansas: AAPOR; 2008
- Centers for Disease Control and Prevention. 2010 National Immunization Survey. Available at: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NIS/NISTEENPUF10_HHQUEX.pdf. Accessed October 1, 2015
- McRee AL, Brewer NT, Reiter PL, Gottlieb SL, Smith JS. The Carolina HPV Immunization Attitudes and Beliefs Scale (CHIAS): scale development and associations with intentions to vaccinate. Sex Transm Dis 2010; 37:234-9; PMID:19940807
- Gilkey MB, Magnus BE, Reiter PL, McRee AL, Dempsey AF, Brewer NT. The Vaccination Confidence Scale: a brief measure of parents' vaccination beliefs. Vaccine 2014; 32:6259-65; PMID:25258098; http://dx.doi.org/10.1016/j.vaccine.2014.09.007
- Gilkey MB, Reiter PL, Magnus BE, McRee AL, Dempsey AF, Brewer NT. Validation of the Vaccination Confidence Scale: a brief measure to identify parents at risk for refusing adolescent vaccines. Acad Pediatr 2016; 16(1):42-9; PMID:26300368; http://dx.doi.org/10.1016/j.acap.2015.06.007
- Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, Bocchini JA Jr, Unger ER. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63:1-30; PMID:25167164
- Smith PJ, Humiston SG, Marcuse EK, Zhao Z, Dorell CG, Howes C, Hibbs B. Parental delay or refusal of vaccine doses, childhood vaccination coverage at 24 months of age, and the Health Belief Model. Public Health Rep 2011; 126 Suppl 2:135-46; PMID:21812176
- Darden PM, Thompson DM, Roberts JR, Hale JJ, Pope C, Naifeh M, Jacobson RM. Reasons for not vaccinating adolescents: National Immunization Survey of Teens, 2008–2010. Pediatrics 2013; 131:645-51; PMID:23509163; http://dx.doi.org/10.1542/peds.2012-2384
- Kempe A, Daley MF, McCauley MM, Crane LA, Suh CA, Kennedy AM, Basket MM, Stokley SK, Dong F, Babbel CI, et al. Prevalence of parental concerns about childhood vaccines: the experience of primary care physicians. Am J Prev Med 2011; 40: 548-55; PMID:21496754; http://dx.doi.org/10.1016/j.amepre.2010.12.025
- Rosenstock IM. Historical origins of the Health Belief model. Health Educ Behav 1974; 2:328-35; http://dx.doi.org/10.1177/109019817400200403
- Gust D, Brown C, Sheedy K, Hibbs B, Weaver D, Nowak G. Immunization attitudes and beliefs among parents: beyond a dichotomous perspective. Am J Health Behav 2005; 29:81-92; PMID:15604052; http://dx.doi.org/10.5993/AJHB.29.1.7
- Reiter PL, McRee AL, Pepper JK, Chantala K, Brewer NT. Improving human papillomavirus vaccine delivery: a national study of parents and their adolescent sons. J Adolesc Health 2012; 51:32-7; PMID:22727074; http://dx.doi.org/10.1016/j.jadohealth.2012.01.006
- Vannice KS, Salmon DA, Shui I, Omer SB, Kissner J, Edwards KM, Sparks R, Dekker CL, Klein NP, Gust DA. Attitudes and beliefs of parents concerned about vaccines: impact of timing of immunization information. Pediatrics 2011; 127 Suppl 1:S120-6; PMID:21502250; http://dx.doi.org/10.1542/peds.2010-1722R
- Gilkey MB, Moss JL, Coyne-Beasley T, Hall ME, Shah PD, Brewer NT. Physician communication about adolescent vaccination: how is human papillomavirus vaccine different? Prev Med 2015; 77:181-5; PMID:26051197; http://dx.doi.org/10.1016/j.ypmed.2015.05.024
- Griffioen AM, Glynn S, Mullins TK, Zimet GD, Rosenthal SL, Fortenberry JD, Kahn JA. Perspectives on decision making about human papillomavirus vaccination among 11- to 12-year-old girls and their mothers. Clin Pediatr (Phila) 2012; 51:560-8; PMID:22589477