ABSTRACT
Long-term protection against meningococcal disease relies on antibody persistence after vaccination. We report antibody persistence up to 5 y after vaccination in adolescents who received a single dose of either meningococcal serogroups A, C, W, Y tetanus toxoid conjugate vaccine (MenACWY-TT, Pfizer) or MenACWY polysaccharide vaccine (MenPS, GSK Vaccines) at the age of 11–17 y in the randomized controlled primary study NCT00464815. In this phase III, open, controlled, multi-center persistence follow-up study conducted in India and the Philippines (NCT00974363), antibody persistence was evaluated by a serum bactericidal antibody assay using rabbit complement (rSBA) yearly, up to year 5 after vaccination. Serious adverse events (SAEs) related to study participation were recorded. Five years after a single dose of MenACWY-TT, the percentage of participants (N = 236) with rSBA titers ≥1:8 was 97.5% for serogroup A, 88.6% for serogroup C, 86.0% for serogroup W and 96.6% for serogroup Y. The percentages in the MenPS group (N = 86) were 93.0%, 87.1%, 34.9% and 66.3%, respectively. Exploratory analysis indicated a higher percentage of subjects with rSBA titers ≥1:8 for serogroups W and Y, and higher rSBA geometric mean antibody titers for serogroups A, W and Y in the MenACWY-TT group than the MenPS group at each time point (years 3, 4 and 5). No differences between groups were observed for serogroup C. No SAEs related to study participation were reported. In conclusion, the results of this follow-up study indicate that antibodies persisted up to 5 y after a single dose of MenACWY-TT in adolescents.
Introduction
Neisseria meningitidis causes severe invasive disease, which typically presents as meningitis or septicemia.Citation1 The incidence of invasive meningococcal disease (IMD) is the highest in infants and young children, but a secondary peak occurs during adolescence.Citation2,3 Six serogroups (A, B, C, W, Y and X) are responsible for the majority of IMD worldwide, but their regional distribution varies and the predominant serogroup in any region can change over time.Citation4 Since 1982, 7 countries in Asia (India, Indonesia, Mongolia, Nepal, Pakistan, the Philippines and Vietnam) have experienced IMD epidemics due to serogroups A or C, most recently in 2005 in the Philippines and India.Citation5-7 Taiwan experienced a serogroup Y outbreak between 2001 and 2003, and serogroup W caused an outbreak among Hajj pilgrims and their contacts in Singapore in 2000–2001.Citation8,9 While little is known about the epidemiology of sporadic IMD in Asian countries, the available data suggest that the burden may be substantial, particularly in developing countries in the region, and that serogroups C, Y and W are potentially increasing in importance.Citation4,7
The burden of IMDs can be reduced through administration of effective meningococcal vaccines. Three quadrivalent meningococcal serogroups A, C, W and Y (MenACWY) conjugate vaccines are currently licensed for use. These vaccines differ in capsular polysaccharide content and carrier protein: Nimenrix™ (MenACWY-TT, Pfizer) contains 5 μg of each meningococcal capsular polysaccharide and is conjugated to tetanus toxoid; Menactra™ (MenACWY-DT, Sanofi Pasteur) contains 4 μg of each meningococcal capsular polysaccharide and is conjugated to diphtheria toxoid; and Menveo™ (MenACWY-CRM, GSK Vaccines) contains 10 μg of polysaccharide A and 5 μg each of the polysaccharides C, W and Y, and is conjugated to mutant diphtheria toxoid CRM197. For all 3 vaccines, immunization of adolescents is achieved using a single dose.
In the United States (US) where Menactra™ and Menveo™ are licensed for use, the Advisory Committee on Immunization Practices introduced a recommendation for administration of a booster dose of meningococcal conjugate vaccine 4–5 y after the initial vaccination during adolescence.Citation10 This decision was based on preliminary US data showing that overall vaccine effectiveness among adolescents was estimated to decrease from 95% within the first year after vaccination to 58% between 2 and 5 y after vaccination.Citation10 Available evidence suggests that immunity wanes over time regardless of the meningococcal conjugate vaccine used, with a decreasing percentage of adolescents who have seroprotective serum bactericidal antibody (SBA) titers up to 5 y after vaccination.Citation10-15
In the primary vaccination study conducted in India, the Philippines and Taiwan (Clinicaltrials.gov NCT00464815),Citation16 healthy adolescents 11–17 y of age who had not received any meningococcal vaccine within the previous 5 y were randomized (3:1) to receive a single dose of MenACWY-TT or a licensed quadrivalent meningococcal polysaccharide vaccine (MenPS; Mencevax™ACWY, Pfizer). One month post-vaccination, non-inferiority of MenACWY-TT compared to MenPS was demonstrated in terms of SBA titers using rabbit complement (rSBA). Vaccine response rates, and exploratory comparisons showed higher rSBA titers against all serogroups in MenACWY-TT recipients compared to MenPS recipients.
Since vaccine-specific antibody persistence data, along with estimates of long-term effectiveness, are needed to inform and guide the development of booster recommendations, all participants who completed the primary vaccination study in India and the Philippines were invited to return 2 y after vaccination for assessment of antibody persistence, and yearly thereafter until year 5 (NCT00974363). Persistence data at year 2 are presented elsewhere.Citation17 Here, we report antibody persistence in this cohort of adolescents until 5 y after vaccination with either MenACWY-TT or MenPS.
Results
Of 790 eligible participants contacted from the primary study, 643 returned at year 3, 541 at year 4 and 478 at year 5 (). No participants were excluded because they had previously developed meningococcal disease. The median time since the primary vaccination was 61 months at year 5 (). Demographic characteristics of participants in the MenACWY-TT and MenPS groups were similar at year 3 and year 4 (). At year 5, 50.8% of participants in the MenACWY-TT group and 40.7% in the MenPS group were female.
Figure 1. Flow of participants through the study. ATP = according-to-protocol cohort for persistence; TVC = total vaccinated cohort; N = number of participants in each group.
Table 1. Summary of demographic characteristics at follow-up time points (according-to-protocol cohorts for persistence at year 3, year 4 and year 5).
Five years after primary vaccination, the percentage of participants (N = 236) in the MenACWY-TT group with rSBA antibody titers ≥1:8 was 97.5% for serogroup A, 88.6% for serogroup C, 86.0% for serogroup W and 96.6% for serogroup Y (). In the group that received MenPS (N = 86), the percentages were 93.0%, 87.1%, 34.9% and 66.3%, respectively. At year 5, the exploratory analyses did not suggest any difference between groups in terms of the percentage of participants reaching these rSBA cut-offs for serogroup C, while for serogroup A, a higher percentage of participants reached the 1:128 cut-off in the MenACWY-TT group (). For serogroups W and Y, exploratory analysis suggested that a higher percentage of participants reached the rSBA cut-offs of 1:8 and 1:128 in the MenACWY-TT group than the MenPS group at each time point.
Table 2. Percentage of participants with rSBA titers ≥1:8 and ≥1:128 up to 5 y after vaccination with MenACWY-TT or MenPS at 11-–17 y of age (according-to-protocol cohorts for persistence at year 3, year 4 and year 5).
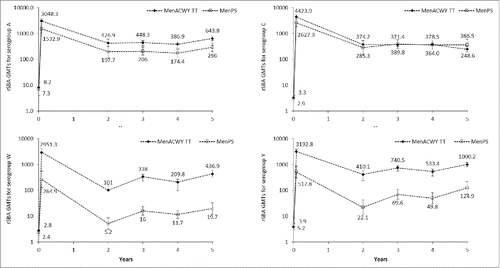
At each time point, exploratory analyses indicated that the rSBA geometric mean antibody titers (GMTs) for serogroups A, W and Y were higher in the MenACWY-TT group than in the MenPS group ().
Table 3. rSBA antibody titers 3, 4 and 5 y after vaccination with MenACWY-TT or MenPS at 11-17 y of age (according-to-protocol cohorts for persistence at year 3, year 4 and year 5).
An increase in GMT was observed between year 4 and year 5 for serogroups A, W and Y. At year 5, the rSBA GMT for serogroup C remained similar, or lower, than that observed at year 4 ().
From year 3, the rSBA assay used to test the persistence of antibodies was performed at a different laboratory (Public Health England [PHE], Manchester, United Kingdom) than the rSBA assay used prior to year 3 (GSK laboratories). To allow a direct longitudinal comparison of antibody persistence in the 2 vaccine groups, a subset of samples (N = 756) collected prior to year 3 were re-tested at the PHE laboratory. The post-hoc analysis showed a sharper decline both in the percentage of participants with rSBA titers ≥1:8 () and GMT values (), in the MenPS group compared to the MenACWY-TT group for serogroups W and Y. For each meningococcal serogroup, an increasing trend was observed in the percentage of subjects with rSBA titers ≥1:8 from the MenACWY-TT group, at each time point, starting from year 2. For both vaccines, rSBA GMTs for serogroups A and C persisted at similar levels between year 2 and year 5, with a small increase between year 4 and year 5 for serogroup A, while an increasing trend in rSBA GMTs was observed for serogroups W and Y (). No serious adverse events (SAEs) related to study participation were reported from the last visit of the primary vaccination study up to year 5.
Figure 2. Percentage of participants with rSBA titers ≥1:8 over time. Footnote: Post-hoc analysis of a subset of samples from the according-to-protocol (ATP) cohort for immunogenicity (primary study) and the ATP cohort for persistence at year 2, all participants in the ATP cohorts for persistence at years 3, 4 and 5. Error bars show 95% confidence intervals. Pre = pre-vaccination; Month 1 = 1 month after vaccination; Year 2–5 = 2 to 5 y after vaccination.
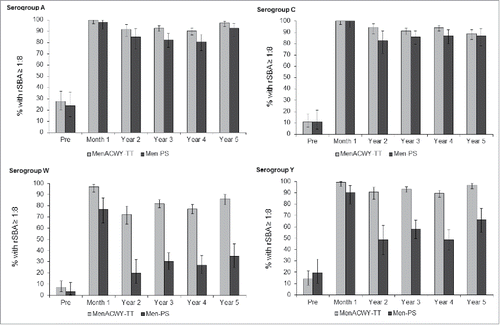
Figure 3. rSBA geometric mean titers (GMTs) over time. Footnote: Post-hoc analysis of a subset of samples from the according-to-protocol cohort (ATP) for immunogenicity (primary study) and the ATP cohort for persistence at year 2, all participants in the ATP cohorts for persistence at years 3, 4 and 5. Error bars show 95% confidence intervals. Time points shown are pre-vaccination, 1 month post-vaccination and 2 to 5 y after vaccination.
Discussion
This study evaluated antibody persistence in a large cohort of adolescents vaccinated up to 5 y previously with a single dose of quadrivalent MenACWY-TT. Antibody persistence appeared sustained, with at least 77.2% of vaccinees maintaining rSBA titers ≥1:8 for each serogroup at year 4, and at least 86.0% at year 5. In the ACWY-TT group, GMTs values observed for serogroup A, appeared higher compared to the MenPS group, while for serogroups W and Y, both GMTs and percentage of participants with rSBA titers ≥1:8 showed an ascending trend. At year 5, differences were maintained for serogroups W and Y in terms of percentage of subjects with rSBA titers ≥1:8 (86% and 96.6%) and A, W and Y in terms of GMTs (643.8 for serogroup A, 436.9 for serogroup W and 1000.2 for serogroup Y).
A small increase in rSBA GMTs between year 4 and year 5 was observed in both study groups for serogroups A, W and Y. Increases in rSBA over time for some serogroups after vaccination have been observed in other studies of MenACWY-TT conducted in other countries (Finland, Germany, US), and in different age groups.Citation15,18,19 This may be suggestive of the development of natural immunity either through exposure to meningococci (nasopharyngeal carriage) or to bacteria that induce a cross-reactive response in the rSBA assay. However, meningococcal nasopharyngeal carriage is low in India,Citation20 and serogroups W and Y are not commonly isolated from patients with IMD in India and South East Asia,Citation21 suggesting that boosting by circulating meningococci may not have had a major impact on the observed rSBA titers in this study. The observed year-by-year oscillations in rSBA titers may also be explained by the assay variability and by the fact that subsequent visits were not always performed by the same participants.
Our results are consistent with previous studies of antibody persistence following primary vaccination with MenACWY-TT in adolescents and adults, in which persisting antibody levels after MenACWY-TT were similar or higher than after vaccination with MenPS.Citation18,19,22 In industrialized countries, adolescence is associated with increased exposure to meningococci due to environmental and social behaviors favoring close contacts, such as dormitory living or night club attendance.Citation23,24 IMD often occurs soon after nasopharyngeal acquisition of a new strain,Citation25,26 although longer periods of asymptomatic carriage of meningococci have been reported.Citation27 The incubation period of IMD is short, indicating that immune memory responses, which usually take at least 5 and up to 7 d to develop,Citation28,29 may not occur soon enough to prevent fulminant invasive disease. In view of demonstrated vaccine failure in participants subsequently shown to have robust immune memory responses,Citation30 maintenance of circulating antibodies is considered critical in providing ongoing protection against IMD.
Long-term antibody persistence has also been shown following vaccination with MenACWY-DT,Citation11,12 and MenACWY-CRM.Citation11,13,31 Since these studies were conducted in different laboratories and using different sources of complement, their results should be compared with caution, as only relative trends can be observed. In a head-to-head study assessing antibody persistence following primary vaccination with MenACWY-TT or MenACWY-DT vaccines,Citation31 exploratory analyses suggested that the percentage of participants with SBA using human complement (hSBA) titers ≥1:8 at year 5 was higher for serogroup C in the MenACWY-TT-primed group than in the MenACWY-DT-primed group.Citation15 Together, a growing body of data suggests that MenACWY-TT induces long-lasting immunity after a single dose in adolescents, that is at least as good, or better, than that induced by the MenACWY-DT vaccine.
Limitations of this study include the change in rSBA assay that occurred at year 3, which did not allow us to make direct comparisons with results obtained at earlier time points. However, we attempted to account for this by performing a post-hoc analysis on a subset of samples representative of the distribution of observed titers at each time point. This analysis supported the original conclusions of the primary vaccination study which was done using a rSBA assay at a different laboratory. This study was also limited by the fact that rSBA titers may vary over time during persistence studies. This observation may be due to the variability in the study population and to the fact that there was not sufficient serum to test a given persistence year time point with all previous time points. The variability in rSBA titers may also be due to boosting through asymptomatic carriage. Limitations of this study included also the long study duration that could have led to bias in results due to loss to follow-up and differences in relative retention between groups. However, the impact of this potential bias was probably limited since our modeling analysis, which included rSBA results for both groups, showed results in line with the observed rSBA antibody titers. Another limitation of this study is the fact that immune responses were only evaluated by an rSBA assay, while both rSBA and hSBA titers have been considered as correlates of efficacy for the licensure of meningococcal conjugate vaccines, and a correlation between rSBA and hSBA assays against serogroup C has been observed, it was not observed for the other serogroups.Citation32 Higher titers are usually obtained with the rSBA assay because N. meningitidis are more easily lysed by rabbit complement.Citation33 Finally, we were unable to compare immune responses with a control group that received another MenACWY conjugate vaccine because no such vaccine was licensed for use in the participating countries at the time of the study.
In conclusion, the 5-year persistence data indicate good long-term antibody persistence induced by a single dose of MenACWY-TT in adolescents.
Methods
Study details
The follow-up study from year 3 to year 5 took place between 28 August 2010 and 08 April 2013 (ClinicalTrials.gov NCT00974363). The primary study was conducted in the Philippines, India and Taiwan.Citation16 Based on the enrolment for the primary study, participation from the Indian and Philippines sites was considered sufficient in terms of the sample size to be followed for antibody persistence. The two study centers in Taiwan were therefore not included in the follow-up study. There were 4 participating centers in India and one center in the Philippines at year 3, 3 participating centers in India and the Philippines site at year 4, and 2 centers in India and one in the Philippines at year 5. Participants were able to take part in any antibody persistence study visit 1, 2, 3, 4, or 5 independently of the other study visits, at their own or at their parent or legal guardian's discretion. They were not allowed to participate if they had developed meningococcal disease or received any meningococcal vaccination since the primary vaccination study.
The study protocols and associated documents were reviewed and approved by ethics committees in each participating country. The study was to be conducted in accordance with Good Clinical Practice (GCP), all applicable regulatory requirements and the Declaration of Helsinki. Each subject was to complete an assent or consent form, and their parent(s)/guardian(s) were to provide written informed consent if the subject was <18 years of age at the time of each visit.
During the conduct of the study, GSK Vaccines became aware of deviations from GCP at the study sites in India and the Philippines. These deviations and the actions taken by GSK are reported in Quiambao et al., 2016.Citation17 At the year 3 and year 4 persistence time points the identified issues included improper consent and/or assent for 3 participants, and documentation lacking at one site in India confirming the appropriate storage of serum samples. From a subject rights perspective, the immunogenicity data of participants without proper informed consent and/or assent should not be used. Therefore, GSK performed a re-analysis of the data excluding all participants with improper consent. In addition, 27 participants at the year 3 and 15 participants at the year 4 time points were eliminated from the according-to-protocol (ATP) persistence cohort since proper storage of the samples could not be confirmed at this site, which could have affected immunogenicity results. The composition of the ATP cohorts at each time point is provided in .
Immunogenicity assessment
Blood samples were collected from all participants who returned at each follow-up time point. Samples from year 3 onward were tested using a rSBA assay performed at the meningococcal surveillance laboratory of Dr. Ray Borrow at PHE in the (UK) and based on the Centers for Disease Control's protocol.Citation34 The cut-off of the assay was a 1:8 dilution. An antibody titer ≥1:8 is considered indicative of seroprotection for meningococcal serogroup C,Citation35 and was also applied to the other serogroups.Citation36 A more conservative cut-off of 1:128 was also reported.
Statistical analyses
The primary study objective at each time point was to evaluate the persistence of bactericidal antibodies up to 5 y after vaccination in terms of the percentage of participants in each group with rSBA titers ≥1:8 for each vaccine meningococcal serogroup. Secondary objectives included assessment of participants with persisting rSBA titers ≥1:128 and rSBA GMTs. SAEs considered by the investigator to be related to study participation or a GSK medication were captured retrospectively at each follow-up visit.
The primary analysis of persistence was done on the ATP persistence cohorts which included participants who had complied with all protocol-defined procedures and who had data available for at least one immunogenicity endpoint. Participants who were immunocompromised or who had received immunosuppressants or blood products including immunoglobulin were excluded from the ATP analyses.
A subset of samples obtained in the primary vaccination study (NCT00464815) and in the follow-up study at year 2 (NCT00955682) were re-tested at PHE. The samples were selected using a Gibbs sampling from the ATP immunogenicity and persistence cohorts, allowing a representative sample of the distribution of titers for each meningococcal antigen. One month after vaccination, participants from the ATP cohort for immunogenicity were ranked according to their results with the rSBA assay and classified in 25 consecutive classes, each representing 4% of the results. The participants were sampled per study group, per assay, and per time point. For each timepoint, the first 6 participants of the MenACWY-TT group and the first 3 participants of the MenPS group who had a sufficient quantity of serum were selected to be tested at PHE. The same subject was not necessarily sampled at each time point, given limitations in the volume of serum per subject for each time point.
For each vaccine serogroup, an exploratory evaluation of the differences in the immune response at each time point was performed between the MenACWY-TT and MenPS groups. In terms of differences in percentages of participants with rSBA antibody titers ≥1:8 and ≥1:128, 2 vaccine groups were considered potentially different if the standardized asymptotic 95% confidence intervals (CIs) for the differences in rates between the 2 vaccine groups did not contain the value “0.” In terms of GMTs, 2 vaccine groups were considered potentially different if the 95% CIs for the GMTs ratios between the 2 vaccine groups, which were computed using an Analysis of Variance (ANOVA) model on the log10-transformed titers with the vaccine group and country as fixed effects in the model (pooled variance), did not contain the value “1.” Note that potential differences should be interpreted with caution considering that there was no adjustment for multiplicity for these comparisons, and that significant findings may have occurred by chance alone.
Statistical analyses were performed using SAS software (SAS Institute Inc., Cary, NC, US) version 9.22.
Trademarks
MENVEO is a registered trademark of the GSK group of companies. MENACTRA is a registered trademark of Sanofi Pasteur. MENCEVAX is a registered trademark of Pfizer. NIMENRIX is a registered trademark of the GSK group of companies, licensed to Pfizer.
Abbreviations
| ATP | = | According–to-protocol |
| CI | = | Confidence interval |
| CRM | = | cross-reactive material |
| DT | = | Diphtheria toxoid |
| GMT | = | Geometric mean antibody titer |
| IMD | = | Invasive meningococcal disease |
| rSBA | = | Serum bactericidal antibody assay using rabbit complement |
| MenACWY-TT | = | Pfizer's quadrivalent meningococcal serogroups A, C, W and Y vaccine conjugated to tetanus toxoid |
| MenPS | = | Quadrivalent meningococcal serogroups A, C, W and Y polysaccharide vaccine |
| PHE | = | Public Health England |
| SD | = | Standard deviation |
| TT | = | Tetanus toxoid |
| UK | = | United Kingdom |
| US | = | United States |
Disclosure of potential conflicts of interest
BPQ received research grants and consulting fee to conduct the present study and support for meetings, travel or accommodation expenses in the past 5 y from the GSK group of companies. BPQ also received payment for service on speakers' bureaus from Sanofi Pasteur. The institute of BPQ received fee for the present study from the GSK group of companies. The institute of AB received grants to conduct the present study and other clinical trials and for attending investigator meetings from the GSK group of companies. Dr AB also received support from the GSK group of companies for attending vaccinology meetings. The institution of APD received money from the GSK group of companies for Ethics Committee fees and for each subject enrolled (Payment to MAMC Society for Promotion of Medical Research). APD also received support from the GSK group of companies for travel to Investigator meeting. JH declares that he has no competing interests.
VB, DK, MVDW and JMM are employees of GSK Vaccines. MVDW declares stock ownership in the GSK group of companies. JM declares restricted share in the GSK group of companies.
Authors' contributions
BPQ, APD, AB and HJ were investigators involved in the supervision of the study, administrative, logistic and technical supports, the recruitment and the medical evaluation of participants, the evaluation of any reported adverse events / serious adverse events for severity and causality, the collection and interpretation of the data, and the drafting and approval of the manuscript. JMM, MVDW (clinical development scientists), VB (biostatistician) and DK (biostatistician) are employed by GSK Vaccines and were involved in all stages of the study (study design, data analyses and interpretations, drafting and approval of the manuscript).
Acknowledgments
The authors are indebted to the study participants, clinicians, nurses, and laboratory technicians at the study site, as well as to clinical investigators for their contribution to this study. In particular we thank Dr Sonia T. Carlos. We also thank the following employees of GSK Vaccines for their valuable contributions: Gulhan Denizer for assistance in preparation of, or contribution to, protocols and study reports; Shailesh Mehta and Salvacion Gatchalian for their assistance in coordination of the study; Emmanuel Aris, Laurence Fissette and Lisa Allamassey for their input into the statistical analysis; and Pascal R. Lestrate for conducting laboratory assays. Medical writing services were provided by Dr Joanne Wolter (independent, on behalf of GSK Vaccines). Dr Regis Azizieh (XPE Pharma & Science, on behalf of GSK Vaccines) provided coordination and editorial assistance.
Funding
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of the present manuscript. The corresponding author had full access to the data and was responsible for submission of the publication.
References
- Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med 2001; 344:1378-88; PMID:11333996; http://dx.doi.org/10.1056/NEJM200105033441807
- Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, Pondo T, Arnold KE, Baumbach J, Bennett N, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis 2010; 50:184-91; PMID:20001736; http://dx.doi.org/10.1086/649209
- Trotter CL, Chandra M, Cano R, Larrauri A, Ramsay ME, Brehony C, Jolley KA, Maiden MC, Heuberger S, Frosch M. A surveillance network for meningococcal disease in Europe. FEMS Microbiol Rev 2007; 31:27-36; http://dx.doi.org/10.1111/j.1574-6976.2006.00060.x
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27 Suppl 2:B51-63; PMID:19477562; http://dx.doi.org/10.1016/j.vaccine.2009.04.063
- Nair D, Dawar R, Deb M, Capoor MR, Singal S, Upadhayay DJ, Aggarwal P, Das B, Samantaray JC. Outbreak of meningococcal disease in and around New Delhi, India, 2005–2006: a report from a tertiary care hospital. Epidemiol Infect 2009; 137:570-6; http://dx.doi.org/10.1017/S0950268808001398
- World Health Organization. Meningococcal disease in the Philippines - update 2. Emergencies preparedness, response programme [Internet]; 2005 Jan 28 [cited 2016 Aug 29]. Available from: http://www.who.int/csr/don/2005_01_28a/en/
- Soriano-Gabarro M, Wolter J, Ng T. Historical and emerging meningococcal disease in Asia. Abstract presented at: 5th World Congress of the World Society for Pediatric Infectious Diseases; 2007 Nov 15-18; Bangkok, Thailand
- Chiou CS, Liao JC, Liao TL, Li CC, Chou CY, Chang HL, Yao SM, Lee YS. Molecular epidemiology and emergence of worldwide epidemic clones of Neisseria meningitidis in Taiwan. BMC Infect Dis 2006; 6:25; PMID:16412228; http://dx.doi.org/10.1186/1471-2334-6-25
- Wilder-Smith A, Goh KT, Barkham T, Paton NI. Hajj-associated outbreak strain of Neisseria meningitidis serogroup W135: estimates of the attack rate in a defined population and the risk of invasive disease developing in carriers. Clin Infect Dis 2003; 36:679-83; PMID:12627350; http://dx.doi.org/10.1086/367858
- Committee on Infectious Diseases. Meningococcal conjugate vaccines policy update: booster dose recommendations. Pediatrics 2011; 128:1213-8; http://dx.doi.org/10.1542/peds.2011-2380
- Gill CJ, Baxter R, Anemona A, Ciavarro G, Dull P. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents. Human Vaccines 2010; 6:881-7; http://dx.doi.org/10.4161/hv.6.11.12849
- Vu DM, Welsch JA, Zuno-Mitchell P, Dela Cruz JV, Granoff DM. Antibody persistence 3 years after immunization of adolescents with quadrivalent meningococcal conjugate vaccine. J Infect Dis 2006; 193:821-8; PMID:16479517; http://dx.doi.org/10.1086/500512
- Jacobson RM, Jackson LA, Reisinger K, Izu A, Odrljin T, Dull PM. Antibody persistence and response to a booster dose of a quadrivalent conjugate vaccine for meningococcal disease in adolescents. Pediatr Infect Dis J 2013; 32:e170-7; PMID:23114372; http://dx.doi.org/10.1097/INF.0b013e318279ac38
- Borja-Tabora C, Montalban C, Memish ZA, Van der Wielen M, Bianco V, Boutriau D, Miller J. Immune response, antibody persistence, and safety of a single dose of the quadrivalent meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine in adolescents and adults: results of an open, randomised, controlled study. BMC Infect Dis 2013; 13:116; PMID:23320781; http://dx.doi.org/10.1186/1471-2334-13-116
- Baxter R, Baine Y, Kolhe D, Baccarini C, Miller J, Van der Wielen M. Five-year antibody persistence and booster response to a single dose of meningococcal A, C, W and Y tetanus toxoid conjugate vaccine in adolescents and young adults: an open, randomized trial. Pediatr Infect Dis J 2015; 34:1236-43; PMID:26237742; http://dx.doi.org/10.1097/INF.0000000000000866
- Bermal N, Huang LM, Dubey AP, Jain H, Bavdekar A, Lin TY, Bianco V, Baine Y, Miller JM. Safety and immunogenicity of a tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine in adolescents and adults. Hum Vaccin 2011; 7:239-47; PMID:21343698; http://dx.doi.org/10.4161/hv.7.2.14068
- Quiambao BP, Jain H, Bavdekar A, Dubey AP, Kolhe D, Bianco V, Van der Wielen M, Miller JM. Persistence of the immune response two years after vaccination with quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine (MenACWY-TT) in Asian adolescents. Hum Vaccin Immunother 2016; 12:2162-8; PMID:27152501; http://dx.doi.org/10.1080/21645515.2016.1163455
- Knuf M, Baine Y, Bianco V, Boutriau D, Miller JM. Antibody persistence and immune memory 15 months after priming with an investigational tetravalent meningococcal tetanus toxoid conjugate vaccine (MenACWY-TT) in toddlers and young children. Hum Vaccin Immunother 2012; 8:866-72; PMID:22485049; http://dx.doi.org/10.4161/hv.20229
- Vesikari T, Forstén A, Van der Wielen M, Bianco V, Miller J. Persistent antibody responses up to 5 years following vaccination with MenACWY-TT in toddlers and children aged 1-10 years. Oral presentation at: 20 years EMGM (The European Meningococcal Disease Society); 2013 Sep 17–19; Bad Loipersdorf, Austria. Available at: http://emgm.eu/meetings/emgm2013/Abstract_Book_EMGM_2013.pdf
- Sinclair D, Preziosi MP, Jacob John T, Greenwood B. The epidemiology of meningococcal disease in India. Trop Med Int Health 2010; 15:1421-35; http://dx.doi.org/10.1111/j.1365-3156.2010.02660.x
- Vyse A, Wolter JM, Chen J, Ng T, Soriano-Gabarro M. Meningococcal disease in Asia: an under-recognized public health burden. Epidemiol Infect 2011; 139:967-85; PMID:21492496
- Ostergaard L, Van der Wielen M, Bianco V, Miller JM. Persistence of antibodies for 42 months following vaccination of adolescents with a meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine (MenACWY-TT). Int J Infect Dis 2013; 17:e173-6; PMID:23246368; http://dx.doi.org/10.1016/j.ijid.2012.10.001
- Harrison L, Kreiner C, Shutt K, Messonnier N, O'Leary M, Stefonek K, Lin H, Lynfield R, Barrett NL, Arnold KE, et al. Risk factors for meningococcal disease in students in grades 9–12. Pediatr Infect Dis J 2008; 27:193-9; PMID:18277925; http://dx.doi.org/10.1097/INF.0b013e31815c1b3a
- MacLennan J, Kafatos G, Neal K, Andrews N, Cameron JC, Roberts R, Evans MR, Cann K, Baxter DN, Maiden MCJ, et al. Social behavior and meningococcal carriage in British teenagers. Emerg Infect Dis 2006; 12:950-7; http://dx.doi.org/10.3201/eid1206.051297
- Andersen J, Berthelsen L, Jensen BB, Lind I. Surveillance of cases of meningococcal disease associated with military recruits studied for meningococcal carriage. Scand J Infect Dis 2000; 32:527-31; PMID:11055659; http://dx.doi.org/10.1080/003655400458820
- Edwards EA, Devine LF, Sengbusch CH, Ward HW. Immunological investigations of meningococcal disease: III. Brevity of group C acquisition prior to disease occurrence. Scand J Infect Dis 1977; 9:105-10; PMID:408918; http://dx.doi.org/10.3109/inf.1977.9.issue-2.09
- Neal KR, Nguyen-van-Tam JS, Slack RCB, Kaczmarski EB, White A, Ala'Aldeen DAA. Seven-week interval between acquisition of a meningococcus and the onset of invasive disease. A case report. Epidemiol Infect 1999; 123:507-9; PMID:10694164; http://dx.doi.org/10.1017/S0950268899003192
- Snape MD, Kelly DF, Salt P, Green S, Snowden C, Diggle L, Borkowski A, Yu L-m, Moxon ER, Pollard AJ. Serogroup C meningococcal glycoconjugate vaccine in adolescents: persistence of bactericidal antibodies and kinetics of the immune response to a booster vaccine more than 3 years after immunization. Clin Infect Dis 2006; 43:1387-94; PMID:17083009; http://dx.doi.org/10.1086/508776
- Wing JB, Smart L, Borrow R, Findlow J, Findlow H, Heath AW, Read RC. Kinetics of immune responses to nasal challenge with meningococcal polysaccharide one year after serogroup-C glycoconjugate vaccination. Clin Infect Dis 2011; 52:1317-23; PMID:21596673; http://dx.doi.org/10.1093/cid/cir198
- Auckland C, Gray S, Borrow R, Andrews N, Goldblatt D, Ramsay M, Miller E. Clinical and immunologic risk factors for meningococcal C conjugate vaccine failure in the United Kingdom. J Infect Dis 2006; 194:1745-52; PMID:17109348; http://dx.doi.org/10.1086/509619
- Baxter R, Baine Y, Ensor K, Bianco V, Friedland LR, Miller JM. Immunogenicity and safety of an investigational quadrivalent meningococcal ACWY tetanus toxoid conjugate vaccine in healthy adolescents and young adults 10 to 25 years of age. Pediatr Infect Dis J 2011; 30:e41-e8; PMID:21200360; http://dx.doi.org/10.1097/INF.0b013e3182054ab9
- Borrow R, Andrews N, Goldblatt D, Miller E. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun 2001; 69:1568-73; PMID:11179328; http://dx.doi.org/10.1128/IAI.69.3.1568-1573.2001
- McIntosh ED, Broker M, Wassil J, Welsch JA, Borrow R. Serum bactericidal antibody assays - The role of complement in infection and immunity. Vaccine 2015; 33:4414-21; PMID:26187262; http://dx.doi.org/10.1016/j.vaccine.2015.07.019
- Maslanka SE, Gheesling LL, Libutti DE, Donaldson KBJ, Harakeh HS, Dykes JK, Arhin FF, Devi SJN, Frasch CE, Huang JC, et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin Diagn Lab Immunol 1997; 4:156-67; PMID:9067649
- Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection–serum bactericidal antibody activity. Vaccine 2005; 23:2222-7; PMID:15755600; http://dx.doi.org/10.1016/j.vaccine.2005.01.051
- Centers for Disease Control and Prevention (CDC). Inadvertent misadministration of meningococcal conjugate vaccine-United States, June-August 2005. MMWR Morb Mortal Wkly Rep 2006; 55:1016-7; PMID:16988640
