ABSTRACT
Bispecific antibody engineering, in which binding specificities toward 2 distinct epitopes are combined into a single molecule, can greatly enhance immunotherapeutic properties of monoclonal antibodies. While the bispecific antibody approach has been applied widely to targets for indications such as cancer and inflammation, the development of such agents for viral immunotherapy is only now emerging. Here, we review recent advances in the development of bispecific antibodies for viral immunotherapy, highlighting promising in vitro and in vivo results.
Introduction
Passive administration of monoclonal antibodies (mAbs) is a promising therapeutic platform for treatment of viral infections.Citation1 Currently, there is only one FDA-approved antiviral mAb, Synagis®, for prophylaxis of respiratory syncytial virus (RSV) in premature infants.Citation2 However, a number of mAbs are in preclinical or clinical development for chronic viruses (e.g., HIV-1) as well as acute infections (e.g., influenza, Ebola virus, and West nile virus).Citation3-6 In other indications, mAbs are advantageous relative to other therapeutic platforms because they are well tolerated (favorable safety profile), are highly specific for their target with few off target effects, and have long serum half-life due to the Fc region.Citation7 These advantages also extend to viral immunotherapy and, in cases where the antibody specificity is directed toward non-host (i.e. viral) epitopes, the safety profile may even be better than other mAbs that target human components.
The majority of antiviral mAb treatments consist of a single mAb that targets a single epitope on the virus surface (“monotherapy”); however, in several cases, a combination therapy consisting of 2 or more mAbs is being advanced. Most immunotherapeutic mAbs are targeted toward the glycoprotein of a particular virus, which is required for cell entry. In many viruses, the glycoproteins across species or strains within the same viral family contain a high degree of sequence variability, and thus a monotherapy may not be effective against all strains. Furthermore, monotherapies that target a single epitope are more susceptible to viral escape mutations and thus development of resistance. Cocktails of antibodies targeting multiple epitopes are capable of increasing potency as well as breadth, and potentially mitigate against escape mutations.Citation4,8-11 While there may be clear therapeutic benefits for advancement of a cocktail of mAbs rather than a monotherapy, the development of mAb cocktails also imposes additional regulatory and manufacturing hurdles.
Recent advancements in recombinant antibody engineering technologies have allowed the generation and clinical development of antibodies with enhanced function.Citation12,13 Bispecific antibodies (), which can bind 2 or more separate and distinct epitopes, have been broadly applied across a number of disease indications. They have been engineered to bind 2 epitopes on the same cell, 2 epitopes on different cells, or different epitopes of the same antigen. In viral immunotherapy, bispecific mAb design can be used to lower the complexity of mAb cocktails, allowing simultaneous targeting of 2 or more distinct epitopes in a single entity. This approach potentially combines the therapeutic advantage of targeting multiple epitopes on the glycoprotein with the simplicity of developing and manufacturing a single molecule, as opposed to a mixture of 2 or more conventional mAbs. Furthermore, bispecific antibody engineering provides the opportunity to tailor multifunctional molecules to match the proposed mechanism of action, for example targeting both viral and host components simultaneously.Citation13-15
Figure 1. Schematic representation of different antibody formats. The combination of the variable regions of 2 monoclonal antibodies (A) can be achieved via numerous bispecific antibody formats (B). Here exemplified is a single chain variable fragment fusion to an IgG (scFv-IgG), a dual-affinity retargeting immunoglobin fusion protein (DART-Ig), a dual-variable domain immunoglobin (DVD-Ig), as well as an asymmetric IgG in which each arm engages a different epitope (e.g., Duobody or CrossMab).
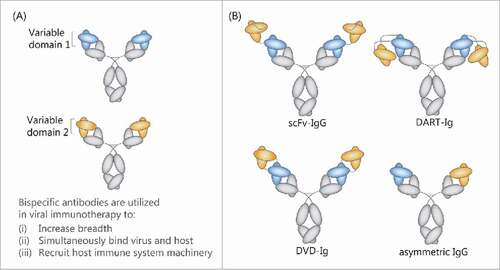
Here, we provide a brief overview of recent developments toward application of bispecific antibody design for viral immunotherapy. Much of the discussion focuses on formats that contain the Fc-region of immunoglobulin, which provides the potential for long pharmacokinetic half-life in vivo and for Fc-mediated antiviral mechanisms ().Citation7 Virus-targeting bispecific antibodies can be grouped into 3 major subclasses (i) molecules with increased species or strain breadth that target several viral epitopes; (ii) antibodies that bind both virus and host epitopes; and (iii) antibodies that recruit the host cells immune system machinery (). Currently, a variety of bispecific antibody formats are applied in the development of antiviral multivalent antibodies (). Methods that have been used for the multimerization of such antibodies include antibody conjugates, asymmetric IgG-like molecules, as well as single chain variable fragment (scFv) fusions to IgG molecules. These different formats and conjugation methods are each associated with particular advantages that may be tailored to the specific application.
Bispecific antibodies targeting 2 distinct viral epitopes
Bispecific antibodies that engage 2 distinct viral epitopes have potential to provide enhanced breadth and potency. As such, Wagner et al. reported a format where 2 full-sized anti-influenza A IgG antibodies with individual subgroup specificity were fused via their C-termini applying sortase transpeptidation and click chemistry.Citation16 The C-C fusion of this covalently linked IgG antibody heterodimer does not require any mutations and it is therefore believed to not compromise antibody stability or Fc-receptor binding capacity. The antibody dimer retained the activity and stability of the 2 original antibodies against a wide range of influenza subtypes in a suitable mouse model. However, the C-C linkage could be immunogenic, a notion that requires further testing. Zanin et al. developed two neutralizing monoclonal antibodies against H5 influenza viruses of different clades, and combined them into a single bispecifc Fc-domain fusion protein (referred to as Fc-dual-affinity retargeting molecule, or FcDART).Citation17 The generated FcDART consist of 2 Fc-fusion protein chains that dimerize to form antibody derived binding sites. One polypeptide chain connects the light chain variable region (vL) of the first antibody with the heavy chain variable region (vH) of the second antibody via a Gly-Ser linker, and the second polypeptide contains the complementary variable regions. Assembly of a DART occurs via heterodimeriztion of the 2 chains and results in a single protein that is bispecific and tetravalent. The anti-influenza FcDART was shown to possess the broad-spectrum activity and protective efficacy of both of its parental monoclonal antibodies in mouse infection models. Additionally, the FcDART demonstrated 100% protection in infected ferrets, which represents a more faithful model for influenza infection. Therefore, a therapy consisting of FcDART alone might be as efficacious as a cocktail containing both monoclonal antibodies against a broad spectrum of antigenically diverse influenza viruses.
Similarly, enhanced activity was also demonstrated for bispecific antibodies targeting the antigenically diverse envelope protein (Env) of HIV-1. Asokan et al. constructed asymmetric bispecific IgGs in which each arm binds a different broadly susceptible epitope on Env monovalently, applying the CrossMAb format.Citation18 The generated bispecific IgGs not only retained functional binding and neutralization by both arms, but also neutralized 94–97% of antigenically diverse viruses in a panel of 206 HIV-1 strains. The bispecific molecule with the highest neutralization activity was infused intravenously to rhesus macaques and demonstrated similar pharmacokinetic properties as the combination of its parental antibodies. To test the idea that increased parent affinity might enhance neutralizing activity, Mouquet et al. engineered scFv-Fc IgG-like molecules, also called immunoadhesins, that can bind HIV-1 bivalently by engaging 2 distinct subunits of Env.Citation19 It was shown that heterotypic binding enhanced neutralization compared with the parental antibodies. By engineering an asymmetric bispecific IgG1 molecule that contains the hinge domain of an IgG3, Bounazos et al. recently reported additional success in the synergistic combination of different anti HIV-Env antibodies.Citation20 The length and flexibility of the IgG3 hinge domain allows for hetero-bivalent binding to 2 essential epitopes on 2 adjacent glycoproteins of the HIV-1 trimer spike, thereby increasing antibody avidity while facilitating spike inactivation. Compared to unmodified asymmetric bispecific molecules, the synergistic activity of molecules with modified hinge domains was shown to be superior in viral neutralization as well as in the protection of HIV-1 infected humanized mice.
Guo and colleagues engineered a dual variable domain (DVD) bispecific antibody from 2 distinct monoclonal antibodies against hepatitis B surface antigen (HBsAg) that synergistically neutralized hepatitis B virus (HBV) infection.Citation21 In DVDs the vH and vL of one antibody are genetically fused to the N-terminus of the heavy and light chain of a second antibody via a short peptide linker, respectively, thereby retaining the Y-shape of an IgG molecule (). The resulting bispecific molecule was shown to have superior HBV-neutralizing activity in comparison to the combination of both parental monoclonal antibodies, possibly through steric hindrance or induction of HBsAg conformational changes. The same group recently described another DVD bispecific antibody, that neutralizes all 4 closely related but serologically distinct dengue viruses (DENV-1-4).Citation22 The fused variable domains of this molecule bind the domain of the DENV surface protein E, that is responsible for virus attachment (DIII), while the variable domains of the IgG scaffold bind to the E protein domain DII, which contains the fusion loop that inserts into the host cell during viral entry. The DVD molecule was confirmed to be more effective at protecting against DENV than each of its parental mAbs individually as well as the combination of the 2 in a suckling mouse model. Additionally, the mutation of 9 amino acids within the Fc domain eliminated antibody dependent enhancement (ADE) of infection in myeloid cells expressing Fc-γ receptors (FcγR). Earlier, Brien et al. already reported an IgG fusion DART protein (DART-Ig; ) that was also engineered to lack FcγR binding capacity and to bind 2 spatially distinct and cross-reactive epitopes on DII and DIII on the surface of the DENV virion.Citation23 The generated molecule possessed neutralizing activity in vitro and therapeutic activity in a mouse ADE disease model. An observed improved avidity of the tetravalent DART-Ig for DENV E protein did not translate into greater inhibitory activity in vitro and in vivo, possibly due to the quasi-icosahedral structure of the DENV virion, precluding binding geometries necessary for simultaneous recognition of adjacent epitopes. In 2015, a set of bispecific antibodies was developed in which scFvs of an Ebola virus (Zaire) specific antibody were fused to the N –and C-termini of a Sudan virus-specific antibody (scFv-Ig; ). These two species are the most divergent within the ebolavirus family but have collectively accounted for 95% of Ebola-related deaths from 1976–2014. The bispecific molecules exhibited potent neutralization, and present the first example of antibodies to confer a high degree (in one case 100%) post-exposure protection of mice from 2 distinct Ebola virus species.Citation24
Bispecific antibodies that interact with host and viral epitopes
The approach of constructing bispecific antiviral antibodies that simultaneously bind to viral and host epitopes has been mainly utilized in HIV studies to target CD4 receptor presenting T-cells. By binding to CD4, these bispecifics are able to increase its local concentration at the point of viral entry and prevent HIV from infecting CD4+ T cells. Pace et al., developed a bispecific antibody where scFvs of broadly neutralizing anti-Env antibodies were fused to the N-terminus of a humanized anti-CD4 antibody via a Gly-Ser linker.Citation14 The same research group engineered another bispecific antibody where 2 copies of a single domain antibody that binds to the CD4-induced site on Env is fused to the C-terminus of the heavy chain of the same anti-CD4+ antibody by a Gly-Ser linker.Citation25 Both bispecific molecules showed improved antiviral activity and were able to neutralize viruses that exhibit resistance to parental antibodies in vitro. Aiming to engineer synergistic bispecific antibodies that preserve the architecture of a normal IgG, Ho and colleagues developed asymmetric IgGs () with the Fab from either an anti-CD4 or anti-CCR5 antibody applying the CrossMab technology.Citation26 Two of these molecules showed increased neutralization in a panel of HIV-1 isolates, and one antibody significantly reduced the viral load in HIV-infected humanized mice.
Unlike HIV, which binds to the surface exposed CD4 receptor to facilitate host-cell attachment and entry, ebolaviruses engage the intracellular late endosome residing receptor Niemann-Pick C1 (NPC1) during host cell entry and infection. This engagement is absolutely required for host cell infection and the receptor-binding site (RBS) on the filovirus glycoprotein is shielded prior to physical sequestration in late endosomes. To target this interaction, recently a novel “Trojan Horse” bispecifc antibody strategy was described, in which the viable domains of NPC-1 or GP-RBS specific antibodies are fused to an antibody that binds to a conserved surface exposed GP-epitope, applying the DVD technology.Citation27 It was shown that the bispecific molecules, but not their parental monoclonal antibodies, neutralized all known ebolaviruses by utilizing the viral particle themselves for endosomal delivery, and conferred post exposure protection against multiple filoviruses in vivo.
Bispecific antibodies recruiting the host cell machinery
Bispecific molecules can also be utilized to recruit host cell immune system machinery. Taylor et al., developed heteropolymers (HPs) to clear various prototype pathogens by covalently crosslinking a monoclonal antibody specific for primate E complement receptor 1 with another monoclonal antibody specific to a target antigen with a nonreducible thioether bond.Citation28,29 Potential for viral immunotherapy was shown in HPs that bound to the Dengue glycoprotein and inactivated Marburg virus.Citation30,31 The HPs demonstrated rapid and specific binding of their respective pathogens to monkey and human erythrocytes in vitro. Furthermore, the dengue-specific HP demonstrated clearance of dengue passive viremia when administered to previously challenged cynomolgus macques.
There has been much recent interest in engineering bispecific molecules that are able to activate and direct cytotoxic T-cells. For example, bispecific T-cell engagers (BiTEs) contain both an antigen-specific arm and an anti-CD3 arm, which activates and redirects cytotoxic CD8+ T-cells to antigen-specific cells.Citation32 Given the success of T-cell engaging molecules in cancer immunotherapy, there is much potential for adoption of similar strategies to eliminate the viral particles themselves, or infected cells. For example, an anti-cytomegalovirus (CMV) bispecific molecule possessing an anti-CD3 arm has been reported.Citation33 To achieve bispecificity, an anti-CD3 monoclonal antibody was chemically heteroconjugated with a CMV specific human IgG via a nonreducable thioether bond. The bispecific antibody was able to redirect specific cytotoxicity to CMV-infected cells and induce cell lysis of target cells at an effector to target ratio as low as 1:1. Similar approaches have been applied in HIV-1, where the CD3-binding moiety serves a 2-fold purpose in directing cytotoxic CD8+ T-cells and activating latently infected cells. In what has been called a “kick and kill” strategy, the anti-CD3 arm promotes viral production by binding to latently infected CD4+ T-cells and stimulating Env gene expression. The antibody then crosslinks CD3 on CD8+ T-cells to bring the cytotoxic cell in close proximity to recently activated CD4+ T-cells with Env on the surface to induce cell lysis.Citation34 Interest in the “kick and kill” strategy for HIV-1 stems from the persistence of latently infected cells in HIV-1 infected individuals treated with antiretroviral therapy (ART). While ART can significantly reduce the viral load in an infected individual, it is unable to fully eradicate HIV-1 from the system due to the virus establishing a stable latent infection in resting CD4+ T-cells with minimal expression of HIV genes.Citation35 Fortunately, 2 bispecific anti-HIV antibodies have shown promise in the lysis of these reservoirs and potential use in ART-treated patients. Additionally, an immunomodulatory protein consisting of the scFv of an anti-human CD3 monoclonal antibody that is linked to the light chain of a HIV-1 broadly neutralizing antibody by a Gly-Ser linker showed similar results in CD8+ T-cell activation and in vivo safety in ART-treated SHIV-infected macaques.Citation36
Multispecific antibody derived formats lacking an Fc-region
Success has also been found in multifunctional anti-viral antibody-mimetics. Genetic fusions of recombinant single-domain antibody fragments derived from camelid heavy chain antibodies (VHHs) have gained attention due to their high stability and easy production through prokaryotic expression. For example, 2 VHHs that bind to different epitopes on the rotavirus capsid were displayed on the surface of Lactobacillus paracasei as linear bivalent proteins that are fused without linkers.Citation37 These were shown to be superior at reducing the rate of diarrhea when used for prophylactic and therapeutic intervention in a mouse model of rotavirus infection. Additionally, Hultberg et al. reported multimeric constructs developed by fusing different llama heavy chain antibody fragments with Gly-Ser linkers of varying length.Citation38 Multivalent molecules were constructed with VHHs against the trimeric envelope proteins of RSV, rabies virus and H5N1 influenza. Despite functional differences in the protein among the viruses, multimerization of the VHHs exhibited increased potency and cross-protection capacity against different viral strains for all 3 viruses. Recently, a DART engineered with the arm of a CD3-specific monoclonal antibody and the arm of one of 2 anti-Env HIV antibodies showed increased CD8+ T-cell activation and lysis with lymphocytes derived from patients on ART.Citation39 Additionally, a bispecific antibody-based molecule has been reported in which 2 Fabs of broadly neutralizing anti-HIV antibodies were conjugated to various lengths of double-stranded DNA. The architecture of the Env spike trimer prevents bivalent binding by the Fabs of IgG and reduces neutralization potency. The flexibility and length in the DNA linkers overcome this barrier and allow for bivalent binding of the Fabs to the Env spike trimer via intra-spike crosslinking. When the molecules were engineered with the Fabs of 2 different broadly neutralizing anti-HIV antibodies, the neutralization potency increased 100-fold.
Potential drawbacks of bispecific antibodies
From a developmental and regulatory perspective, combining several antibodies into a single therapeutic makes development less complex and more cost-effective, since production and quality control as well as preclinical and clinical testing will be reduced to a single molecule. However, bispecific antibodies are also associated with some disadvantages relative to canonical mAbs, and these limitations should be considered depending on the desired outcome. Asymmetric IgGs (e.g. DuoBodies, CrossMab) bind monovalently to their targets, which might have consequences for activity, especialy in the context of viruses that have high glycoprotein spike density per virion and thus may be more potently neutralized by mAbs that engage epitopes bivalently to cross-link subunits or spikes.Citation40,41 The production of asymmetric IgGs also requires either a reduction step to convert homodimers into heterodimers, or coexpression of 2 antibody fragments. This additional requirement might hamper high scale production of these molecules. Some bispecific antibody formats might possess reduced physiochemical stability or be prone to aggregation, for example, when lacking a Fc domain, if a constant domain mutation is required to enable IgG heterodimerization, or if long flexible polypeptide linker regions are included in the design. This can comprise stability and may affect binding to Fc receptors.Citation42,43 So far, little attention has been given to the immunogenicity and pharmacokinetics of multispecifc antibodies within the specific context of viral immunotherapy. However, recent studies on bispecific antibodies generated for different purposes, indicate that the orientation as well as the nature of the fusion partner can affect the pharmacokinetics of immunoconjugates of IgGs, as it may result in conformational change or misfolding in certain combinations.44,45 However, the in vivo half-life for any particular antibody (engineered or natural) is unpredictable and requires empirical case-by case assessments. In general, enhanced pharmacokinetics of molecules containing an IgG scaffold can be attributed to 2 major factors; (i) their lager size (>150 kDa) precludes renal clearance, which is responsible for the rapid elimination of smaller constructs (60 kDa), such as scFvs; and (ii) their dynamic binding to the neonatal Fc receptor extends serum half-lives.45 An intravenously administered bispecifc antibody targeting different epitopes on the HIV-1 envelope was shown to have a similar serum half-life (ca. 10 days) to the parental IgGs in rhesus marcaques and persisted in circulation for 4 weeks while retaining neutralization activity in the serum.Citation18 Like the parental IgGs, this antibody contained a mutated CH3 domain to improve antibody persistence. Development for clinical application might require further engineering of existing bispecific formats. As such, hinge stabilized or aglycosylated IgG4 scaffolds can be applied to decrease heterogenicity.46 Furthermore, anti- and pro-inflammatory properties can be regulated through the alteration of glycosylation, and the serum half-life can be increased by Fc engineering.46 Other aspects that remain to be carefully addressed when preparing multispecific antibodies for clinical development are formulation and dosing, especially if different amounts of individual components are required in the respective canonical antibody cocktails.
Conclusion
To date, a vast repertoire of bispecific antibody formats is available, which has been primarily applied in the therapy of malignancies and inflammatory conditions. The studies we outlined here highlight that bispecific antibodies offer numerous functionalities and targeting mechanisms that can be exploited for viral immunotherapy. Despite potential limitations, bispecific antibody engineering can be utilized to enhance the effectiveness of antiviral immunotherapies. As individual epitopes may not provide sufficient therapeutic benefit when targeted alone, significant therapeutic benefits of these targets can be gained from additive and/or synergy effects of bispecific molecules. Moreover, bispecifc antibody engineering may open up new therapeutic venues when utilizing one of the 2 specificities for the delivery of the second specificity to its target site. Thus, there is much opportunity for the application of such methods to other viruses and to develop next-generation therapeutics.
Table 1. Fc-region containing bispecific antibody formats that have been generated for viral immunotherapy
Disclosure of potential conflicts of interest
JRL and EKN are named coinventors on patents encompassing bispecific antibodies described in references 24 and 27.
Acknowledgment
We thank Anna Z. Wec for assistance with figure preparation and helpful discussions.
Funding
We gratefully acknowledge NIH grant U19-AI109762 (Centers of Excellence for Translational Research) and an Irma T. Hirschl/Monique Weill-Caulier Career Scientist Award (to J.R.L.). E.K.N. was supported by a DAAD (Deutscher Akademischer Austausch Dienst, German Academic Exchange Service) fellowship.
References
- Graham BS, Ambrosino DM. History of passive antibody administration for prevention and treatment of infectious diseases. Curr Opin HIV AIDS 2015; 10:129-34; PMID:25760933; https://doi.org/http://dx.doi.org/10.1097/COH.0000000000000154
- Pollack P, Groothuis JR, Barbarotto G. Development and use of palivizumab (Synagis): a passive immunoprophylactic agent for RSV. J Infect Chemother 2002; 8:201-6; PMID:12373481; https://doi.org/http://dx.doi.org/10.1007/s10156-002-0178-6
- Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr, Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 2015; PMID:25855300
- Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, Fausther-Bovendo H, Wei H, Aviles J, Hiatt E, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514:47-53; PMID:25171469
- Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 2011; 333:843-50; PMID:21737702; https://doi.org/http://dx.doi.org/10.1126/science.1204839
- Vogt MR, Moesker B, Goudsmit J, Jongeneelen M, Austin SK, Oliphant T, Nelson S, Pierson TC, Wilschut J, Throsby M, et al. Human monoclonal antibodies against West Nile virus induced by natural infection neutralize at a postattachment step. J Virol 2009; 83:6494-507; PMID:19386704; https://doi.org/http://dx.doi.org/10.1128/JVI.00286-09
- Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7:715-25; PMID:17703228; https://doi.org/http://dx.doi.org/10.1038/nri2155
- Pal P, Dowd KA, Brien JD, Edeling MA, Gorlatov S, Johnson S, Lee I, Akahata W, Nabel GJ, Richter MK, et al. Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus. PLoS Pathog 2013; 9:e1003312; PMID:23637602; https://doi.org/http://dx.doi.org/10.1371/journal.ppat.1003312
- Howell KA, Qiu X, Brannan JM, Bryan C, Davidson E, Holtsberg FW, Wec AZ, Shulenin S, Biggins JE, Douglas R, et al. Antibody treatment of ebola and sudan virus infection via a uniquely exposed epitope within the glycoprotein receptor-binding site. Cell Rep 2016; 15:1514-26; PMID:27160900; https://doi.org/http://dx.doi.org/10.1016/j.celrep.2016.04.026
- Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 2013; 503:224-8; PMID:24172905
- Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M Jr, Lifson JD, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 2013; 503:277-80; PMID:24172896
- Winnall WR, Beasley MD, Center RJ, Parsons MS, Kiefel BR, Kent SJ. The maturation of antibody technology for the HIV epidemic. Immunol Cell Biol 2014; 92:570-7; PMID:24797582
- Lu D, Zhu Z. Construction and production of an IgG-Like tetravalent bispecific antibody, IgG-single-chain Fv fusion. Hum Monoclonal Antibodies: Springer 2014:185-213; PMID:24037843; https://doi.org/http://dx.doi.org/10.1007/978-1-62703-586-6_11
- Pace CS, Song R, Ochsenbauer C, Andrews CD, Franco D, Yu J, Oren DA, Seaman MS, Ho DD. Bispecific antibodies directed to CD4 domain 2 and HIV envelope exhibit exceptional breadth and picomolar potency against HIV-1. Proc Natl Acad Sci 2013; 110:13540-5; PMID:23878231; https://doi.org/http://dx.doi.org/10.1073/pnas.1304985110
- Fischer N, Léger O. Bispecific antibodies: molecules that enable novel therapeutic strategies. Pathobiology 2007; 74:3-14; PMID:17496428; https://doi.org/http://dx.doi.org/10.1159/000101046
- Wagner K, Kwakkenbos MJ, Claassen YB, Maijoor K, Böhne M, van der Sluijs KF, Witte MD, van Zoelen DJ, Cornelissen LA, Beaumont T, et al. Bispecific antibody generated with sortase and click chemistry has broad antiinfluenza virus activity. Proc Natl Acad Sci 2014; 111:16820-5; PMID:25385586; https://doi.org/http://dx.doi.org/10.1073/pnas.1408605111
- Zanin M, Keck Z-Y, Rainey GJ, Lam C-YK, Boon AC, Rubrum A, Darnell D, Wong SS, Griffin Y, Xia J, et al. An anti-H5N1 influenza virus FcDART antibody is a highly efficacious therapeutic agent and prophylactic against H5N1 influenza virus infection. J Virol 2015; 89:4549-61; PMID:25673719; https://doi.org/http://dx.doi.org/10.1128/JVI.00078-15
- Asokan M, Rudicell R, Louder M, McKee K, O'l S, Stewart-Jones G, Wang K, Xu L, Chen X, Choe M, et al. Bispecific antibodies targeting different epitopes on the HIV-1 envelope exhibit broad and potent neutralization. J Virol 2015; 89:12501-12; PMID:26446600; https://doi.org/http://dx.doi.org/10.1128/JVI.02097-15
- Mouquet H, Warncke M, Scheid JF, Seaman MS, Nussenzweig MC. Enhanced HIV-1 neutralization by antibody heteroligation. Proc Natl Acad Sci 2012; 109:875-80; PMID:22219363; https://doi.org/http://dx.doi.org/10.1073/pnas.1120059109
- Bournazos S, Gazumyan A, Seaman MS, Nussenzweig MC, Ravetch JV. Bispecific Anti-HIV-1 Antibodies with Enhanced Breadth and Potency. Cell 2016; 165:1609-20; PMID:27315478; https://doi.org/http://dx.doi.org/10.1016/j.cell.2016.04.050
- Tan W, Meng Y, Li H, Chen Y, Han S, Zeng J, Huang A, Li B, Zhang Y, Guo Y. A bispecific antibody against two different epitopes on hepatitis B surface antigen has potent hepatitis B virus neutralizing activity. MAbs: Taylor & Francis 2013:946-55; PMID:24492346; https://doi.org/http://dx.doi.org/10.4161/mabs.26390
- Shi X, Deng Y, Wang H, Ji G, Tan W, Jiang T, Li X, Zhao H, Xia T, Meng Y, et al. A bispecific antibody effectively neutralizes all four serotypes of dengue virus by simultaneous blocking virus attachment and fusion. mAbs: Taylor & Francis 2016:574-84; PMID:26905804; https://doi.org/http://dx.doi.org/10.1080/19420862.2016.1148850
- Brien JD, Sukupolvi-Petty S, Williams KL, Lam C-YK, Schmid MA, Johnson S, Harris E, Diamond MS. Protection by immunoglobulin dual-affinity retargeting antibodies against dengue virus. J Virol 2013; 87:7747-53; PMID:23658441; https://doi.org/http://dx.doi.org/10.1128/JVI.00327-13
- Frei JC, Nyakatura EK, Zak SE, Bakken RR, Chandran K, Dye JM, Lai JR. Bispecific antibody affords complete post-exposure protection of mice from both Ebola (Zaire) and Sudan Viruses. Sci Rep 2016; 6:19193; PMID:26758505; https://doi.org/http://dx.doi.org/10.1038/srep19193
- Sun M, Pace CS, Yao X, Yu F, Padte NN, Huang Y, Seaman MS, Li Q, Ho DD. Rational design and characterization of the novel, broad and potent bispecific HIV-1 neutralizing antibody iMabm36. J Acquired Immune Defic Syndr (1999) 2014; 66:473-83; PMID:24853313; https://doi.org/http://dx.doi.org/10.1097/QAI.0000000000000218
- Huang Y, Yu J, Lanzi A, Yao X, Andrews CD, Tsai L, Gajjar MR, Sun M, Seaman MS, Padte NN, et al. Engineered bispecific antibodies with exquisite HIV-1-neutralizing activity. Cell 2016; 165:1621-31; PMID:27315479; https://doi.org/http://dx.doi.org/10.1016/j.cell.2016.05.024
- Wec AZ, Nyakatura EK, Herbert AS, Howell KA, Holtsberg FW, Bakken RR, Mittler E, Christin JR, Shulenin S, Jangra RK, et al. A “Trojan horse” bispecific antibody strategy for broad protection against ebolaviruses. Science 2016; 354:350-354; PMID:27608667
- Taylor RP, Martin EN, Reinagel ML, Nardin A, Craig M, Choice Q, Schlimgen R, Greenbaum S, Incardona NL, Ochs HD. Bispecific monoclonal antibody complexes facilitate erythrocyte binding and liver clearance of a prototype particulate pathogen in a monkey model. J Immunol 1997; 159:4035-44; PMID:9378993
- Taylor RP, Sutherland WM, Martin EN, Ferguson PJ, Reinagel ML, Gilbert E, Lopez K, Incardona NL, Ochs HD. Bispecific monoclonal antibody complexes bound to primate erythrocyte complement receptor 1 facilitate virus clearance in a monkey model. J Immunol 1997; 158:842-50; PMID:8993002
- Hahn CS, French OG, Foley P, Martin EN, Taylor RP. Bispecific monoclonal antibodies mediate binding of dengue virus to erythrocytes in a Monkey model of passive viremia. J Immunol 2001; 166:1057-65; PMID:11145685; https://doi.org/http://dx.doi.org/10.4049/jimmunol.166.2.1057
- Nardin A, Sutherland WM, Hevey M, Schmaljohn A, Taylor RP. Quantitative studies of heteropolymer-mediated binding of inactivated Marburg virus to the complement receptor on primate erythrocytes. J Immunol Methods 1998; 211:21-31; PMID:9617828; https://doi.org/http://dx.doi.org/10.1016/S0022-1759(97)00168-3
- Wolf E, Hofmeister R, Kufer P, Schlereth B, Baeuerle PA. BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Disc Today 2005; 10:1237-44; PMID:16213416; https://doi.org/http://dx.doi.org/10.1016/S1359-6446(05)03554-3
- Lum LG, Ramesh M, Thakur A, Mitra S, Deol A, Uberti JP, Pellett PE. Targeting cytomegalovirus-infected cells using T cells armed with anti-CD3 × anti-CMV bispecific antibody. Biol Blood Marrow Transplantation 2012; 18:1012-22; PMID:22313635; https://doi.org/http://dx.doi.org/10.1016/j.bbmt.2012.01.022
- Hua CK, Ackerman ME. Engineering broadly neutralizing antibodies for HIV prevention and therapy. Adv Drug Del Rev 2016; 103:157-73; PMID:26827912
- Shan L, Siliciano RF. From reactivation of latent HIV‐1 to elimination of the latent reservoir: The presence of multiple barriers to viral eradication. Bioessays 2013; 35:544-52; PMID:23613347; https://doi.org/http://dx.doi.org/10.1002/bies.201200170
- Sung JA, Pickeral J, Liu L, Stanfield-Oakley SA, Lam C-YK, Garrido C, Pollara J, LaBranche C, Bonsignori M, Moody MA, et al. Dual-Affinity Re-Targeting proteins direct T cell–mediated cytolysis of latently HIV-infected cells. J Clin Investigat 2015; 125:4077-90; PMID:26413868; https://doi.org/http://dx.doi.org/10.1172/JCI82314
- Pegu A, Asokan M, Wu L, Wang K, Hataye J, Casazza JP, Guo X, Shi W, Georgiev I, Zhou T, et al. Activation and lysis of human CD4 cells latently infected with HIV-1. Nat Commun 2015; 6:8447; PMID:26485194
- Pant N, Marcotte H, Hermans P, Bezemer S, Frenken L, Johansen K, Hammarström L. Lactobacilli producing bispecific llama-derived anti-rotavirus proteins in vivo for rotavirus-induced diarrhea. Fut Microbiol 2011; 6:583-93; PMID:21585264; https://doi.org/http://dx.doi.org/10.2217/fmb.11.32
- Hultberg A, Temperton NJ, Rosseels V, Koenders M, Gonzalez-Pajuelo M, Schepens B, Ibañez LI, Vanlandschoot P, Schillemans J, Saunders M, et al. Llama-derived single domain antibodies to build multivalent, superpotent and broadened neutralizing anti-viral molecules. PloS One 2011; 6:e17665; PMID:21483777; https://doi.org/http://dx.doi.org/10.1371/journal.pone.0017665
- Labrijn AF, Meesters JI, de Goeij BE, van den Bremer ET, Neijssen J, van Kampen MD, Strumane K, Verploegen S, Kundu A, Gramer MJ, et al. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci 2013; 110:5145-50; PMID:23479652; https://doi.org/http://dx.doi.org/10.1073/pnas.1220145110
- Strop P, Ho W-H, Boustany LM, Abdiche YN, Lindquist KC, Farias SE, Rickert M, Appah CT, Pascua E, Radcliffe T, et al. Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair. J Mol Biol 2012; 420:204-19; PMID:22543237; https://doi.org/http://dx.doi.org/10.1016/j.jmb.2012.04.020
- Von Kreudenstein TS, Escobar-Carbrera E, Lario PI, D'Angelo I, Brault K, Kelly JF, Durocher Y, Baardsnes J, Woods RJ, Xie MH, et al. Improving biophysical properties of a bispecific antibody scaffold to aid developability: quality by molecular design. MAbs: Taylor & Francis 2013:646-54; PMID:23924797; https://doi.org/http://dx.doi.org/10.4161/mabs.25632
- Miller BR, Demarest SJ, Lugovskoy A, Huang F, Wu X, Snyder WB, Croner LJ, Wang N, Amatucci A, Michaelson JS, et al. Stability engineering of scFvs for the development of bispecific and multivalent antibodies. Protein Eng Des Sel 2010; 23:549-57; PMID:20457695; https://doi.org/http://dx.doi.org/10.1093/protein/gzq028
