ABSTRACT
In the 2015/16 influenza season, the Canadian National Advisory Committee on Immunization (NACI) recommended vaccination with quadrivalent inactivated influenza vaccine (QIV) for infants aged 6–23 months and trivalent inactivated influenza vaccines (TIVs) or QIVs in adults. The objective of this review (GSK study identifier: HO-13-14054) is to examine the epidemiology and disease burden of influenza in Canada and the economic benefits of vaccination. To inform this review, we performed a systematic literature search of relevant Canadian literature and National surveillance data. Influenza B viruses from phylogenetically-distinct lineages (B/Yamagata and B/Victoria) co-circulate in Canada, and are an important cause of influenza complications. Modeling studies, including those postdating the search suggest that switching from TIV to QIV in Canada reduces the burden of influenza and would likely be cost-effective. However, more robust real-world outcomes data is required to inform health policy decision makers on appropriate influenza vaccination strategies for Canada.
Introduction
Many countries have implemented influenza vaccination as targeted programs for people at high risk for influenza-related complications, while some countries such as Australia, Canada, and the United States (US) recommend universal vaccination against seasonal influenza.Citation1-3 The aim of influenza vaccination in Canada is to prevent serious outcomes such as hospitalization and death. As such, the National Advisory Committee on Immunization (NACI) stresses the need for vaccination, particularly for those people at high risk for influenza-related complications, including infants 6 to 59 months of age, adults 65 y and older, and people with medical co-morbidities.Citation4
Two phylogenetically-distinct influenza B lineages (B/Yamagata and B/Victoria) emerged globally in the early 1980s, and have co-circulated in the US since 2000.Citation5 Between 2000 and 2010, the influenza B strain in the seasonal trivalent influenza vaccine (TIV) did not match the prevalent B lineage in around half of the European and North American influenza seasons.Citation5 As a result, the World Health Organization (WHO) recommended the inclusion of an influenza strain from each of the influenza B lineages for the 2012/13 influenza season onwards.Citation6 Traditional TIVs include either B/Victoria or B/Yamagata whereas quadrivalent influenza vaccines (QIVs) include strains from both lineages. Thus the new QIVs are expected to reduce the burden of influenza B disease,Citation7,8 and this is supported by the available evidence. The first efficacy study of a QIV was a randomized, observer-blinded assessment of FluLaval™ TETRA in children from 3 to 8 y of age.Citation9 The study showed that the QIV versus control reduced real-time polymerase chain reaction (RT-PCR)-confirmed influenza A and B cases by 59.3% and reduced moderate-to-severe influenza cases by 74.2%.Citation9 A recent meta-analysis of randomized controlled trials of QIVs found that inactivated QIVs have equivalent immunogenicity and efficacy against the shared 3 strains included in TIVs, with superior immunogenicity against the B lineage not included in TIV.Citation10
In Canada, a number of influenza vaccines are currently licensed for use. These include 6 inactivated TIVs; split virus or subunit TIVs for intramuscular injection (Agriflu [Seqirus], Fluviral™ [GSK], Fluzone, Vaxigrip [Sanofi Pasteur], and Influvac [BGP Pharma ULC], and an MF59-adjuvanted TIV (Fluad [Seqirus]).Citation1 Three QIVs are also available; a live attenuated influenza vaccine (LAIV) for intranasal administration (FluMist Quadrivalent [MedImmune/AstraZeneca]) and 2 split-virion, inactivated QIVs (FluLaval™ TETRA [GSK] and Fluzone Quadrivalent [Sanofi Pasteur]).Citation1 TIVs and QIVs are recommended for use in healthy adults and those with chronic health problems from 18 y of age.Citation1 NACI preferentially recommends QIV for use in infants from 6 to 23 months of age and LAIV for children 2 to 6 y of age. In the 2015/16 season, NACI offered no preference between TIV, QIV, or MF59-adjuvanted TIV for those 65 y of age and older.Citation1
The principal objectives of this literature review are to examine the epidemiology and disease burden of influenza in Canada and the economic benefits of vaccination. To facilitate this, we performed a comprehensive literature review using a detailed search strategy conducted to identify original research of influenza in the general population in a Canadian setting (GSK study identifier: HO-13-14054).
Search strategy
Search strings were developed to identify potentially relevant sources based on incidence, prevalence, morbidity and mortality of influenza in Canada, along with costs and cost-effectiveness or cost-utility analyses. With these, searches were performed in PubMed, EMBASE and the Cochrane databases for the period from January 2002 to December 2013. The detailed search strategies for PubMed are described in Tables S1, S2 and S3. Search criteria used to identify studies of interest were broad to include epidemiological, clinical and cost-effectiveness studies reporting on influenza in the general population (not pregnant women, diabetics, or HIV+ [human immunodeficiency virus-positive] patients) in the Canadian setting. Identified literature was assessed independently by 2 reviewers for relevance, with any disagreements resolved by discussion and consensus. From these searches, we identified a total of 1,580 citations in the search of electronic databases. Following article appraisal, 64 articles were selected to inform this review. Of these, 27 studies contained relevant data on disease epidemiology, 42 studies on the burden of disease and costs of illness, and 7 studies on the economic evaluation of vaccines. An overview of the search results and selection is presented in Figure S1. We also searched ‘gray’ literature, including annual reports from Canada's influenza surveillance through the Canadian FluWatch surveillance system for the influenza seasons beginning 1999/00 up until 2009/10, including final weekly reports for 2010/11, 2011/12, and 2012/13, along with NACI statements describing vaccine match-mismatch data.
Canadian surveillance data
Using incidence of laboratory-confirmed cases of influenza taken from the Canadian FluWatch surveillance system for seasons from 1999/00 to 2012/13, the mildest season was 2002/03 (2,953 cases) and the most severe season was 2012/13 (31,737 cases).Citation11-22 Data for 2012/13 season were also obtained from NACI statement on seasonal influenza vaccine for 2013/14.Citation23 Influenza A was the dominant subtype circulating in all but 2 seasons (2000/01 and 2011/12). Influenza B subtype was reported in fewer than 20% of cases for most seasons, apart from 2000/01, 2002/03, 2005/06, 2007/08, and 2011/12 (68%, 45%, 39%, 44%, and 53% respectively) (). Excluding the pandemic seasons (2008/09 and 2009/10), influenza B has represented an average of 26% to 34% of all laboratory-confirmed influenza cases from 2000/01 to 2012/13, depending on whether one uses, respectively, data from Canada's Respiratory Virus Detection Surveillance System (RVDSS), or the National Microbiology Laboratory (NML) of the Public Health Agency of Canada (PHAC) [internal GSK analysis of publicly-available FluWatch data]. In 14 Canadian influenza seasons for which data was available, 3 seasons showed only one dominant B strain whereas 2 or 3 different strains were reported in the remaining seasons ().Citation11-22 Available FluWatch data show that in the seasons from 1999/00 to 2009/10, most cases were observed in children aged <5 years or adults aged ≥65 years ().Citation11-19 In all seasons, the proportion of seasonal influenza cases in children aged <5 years was >10%. In adults aged ≥65 years, the proportion of cases was particularly high in the 1999/00 season (42%), and in the 2004/05 season (51% and 22% for Influenza A and B, respectively). During three seasons, <10% of cases were elderly people, including the pandemic seasons (2008/09 and 2009/10).
Figure 1. FluWatch surveillance system case-by-case data of laboratory confirmed influenza by season and virus subtype. Note: Data obtained from The Canadian FluWatch surveillance system unless otherwise indicated;Citation11-22 Data for 2008/09 and 2009/10 pandemic seasons have not been included for calculation of average influenza B incidence across seasons. †Data are based on aggregate cases for these years only. More sentinel laboratories report aggregate data then detailed data, therefore the case counts are expected to be higher. #Data obtained from FluWatch final cumulative weekly report in absence of a full annual report.Citation20-22
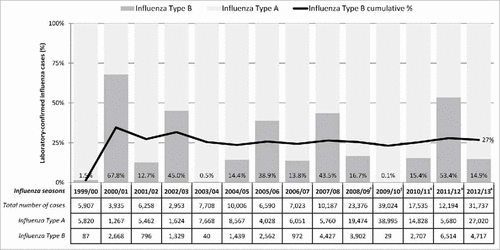
Table 1. FluWatch surveillance system reports of influenza B strains in Canada during influenza seasons from 1999 to 2012
Table 2. Influenza case distribution by age groups and viral subtype in Canada
Influenza attack rates
A number of studies report seasonal influenza attack rates from various settings, including 3 which report the incidence of A(H1N1)pdm09. Most studies were conducted in institutional settings rather than the community and contained limited data specific to influenza B (Table S4).Citation24-33 In one study of people dwelling in long-term care facilities, the incidence of influenza B was higher in the residents (6,400 per 100,000 population) than in the staff (3,100 per 100,000 population).Citation30 In another study of patients hospitalized with any influenza during the 2007/08 season, the incidence of influenza B was 23 per 1,000 patients.Citation31
Co-morbidities and mortality
The most common co-morbidities associated with seasonal influenza disease in Canadian children were pneumonia and asthma, and the most common co-morbidities in adults were identified as cardiac conditions and respiratory disorders such as pneumonia and chronic lung disease (Table S5).Citation24,26,27,29,32,34-37
Between 2008 and 2012, Statistics Canada reported an annual death toll between 5,106 and 5,826 due to influenza and pneumonia (ICD-10 codes J09 – J18).Citation38 Many studies have reported influenza-related mortality data in Canada, describing either the probability of death, or formal mortality rates, or both measures (Table S6).Citation24,26-29,34-36,39-61 In these, mortality varied widely (0–27%) depending on the population, strain type, and pandemic or non-pandemic seasons.
General practitioner (GP) and emergency room (ER) visits
GP visit data for influenza infection were reported in 3 studies.Citation28,33,37 In a study of an elderly population in a nursing home with seasonal influenza, 66% of those with influenza received visits from a physician,Citation28 and a telephone survey of 1,009 Canadian households reported a rate of 0.7 GP visits related to influenza-like illness (ILI) per household.Citation33 Information on ER visits was provided by one study which reported that the rate of influenza-attributable ER visits during a non-pandemic influenza season was 460 per 100,000 population.Citation62
Antibiotics and antivirals
From the available data, around 60% of patients with seasonal influenza are reported to have received antibiotics ().Citation24,27-29,34-36,39,40,60,61 In children, the probability of receiving antivirals during seasonal years ranged from 6.6%Citation61 to 13%,Citation63 while the probability in adults ranged from 29%Citation26 to 79.9%.Citation36 However, in contrast with seasonal influenza, antiviral use during pandemic years reached 84%Citation44 in children and 94.4% in adults.Citation49
Table 3. Burden of seasonal influenza in Canada expressed as probabilities of outcomes
Hospitalization and intensive care unit (ICU) admission
Numerous studies conducted in the Canadian setting report on the probability of influenza-related hospitalization (),Citation28,29,37,43,56,58,64 the rate of influenza-related hospitalization in the general or hospital population,Citation40,47,57,58,65-67 and the length of hospitalization stay (LOS).Citation24,34,36,37,41-43,49-54,58,60,61,63 The probability of hospitalization during non-pandemic influenza seasons was higher in children (35.0%)Citation43 than in elderly people (1.3–11.0%).Citation28,29 Hospital admission rates for individuals with non-pandemic A/H1N1 influenza ranged from 3.9 to 270-340 hospitalizations per 100,000 population.Citation40,65-67 Overall, for non-pandemic influenza seasons, LOS ranged from 2 to 10 d.Citation24,34,36,42,43,60,61
In general, the probability of admission to the ICU was higher in adults than children (). Probability of ICU admission for children ranged from 0.9%Citation43 to 26.1%Citation63 and probabilities in adults ranged from 4.9%Citation47 to 30.0%.Citation40 Rates of ICU admission ranged from 0.2 per 100,000 populationCitation39 to 4.4 per 100,000 population.Citation57 There was a wide range of probabilities reported for mechanical ventilation, ranging from 6.2%Citation60 to 66.0%Citation63 in children, and 9.4%Citation27 to 55.0% in adults.Citation39
Influenza and productivity loss
Few studies with data on loss of work, or absence from school, or quality-of-life associated with influenza illness were identified in the literature. In an ILI telephone survey, it was estimated that 2 d of work or school were lost per household.Citation33 In another study, it was estimated that during non-pandemic influenza seasons from 1997/98 to 2008/09, on average 14 work hours were lost per influenza patient, which translated to an aggregated 20 work days lost per 100 full-time employees. For pandemic strain, while absenteeism rates were similar to those seen in seasonal influenza, an average of 25 work hours were lost per patient.Citation68
Cost of influenza
Limited data exists for the average cost per case for influenza in Canada.Citation28 In one study reported in 2002, the authors compared a population of elderly patients who received rapid virus testing to those who did not during the 1998/99 influenza season. Including costs of drugs, laboratory tests, and hospitalizations, the group who received rapid viral testing had a cost of CAN$673.30, compared with CAN$313.85 in the control group.Citation28
Modeling the cost of influenza vaccination
Between 2002 and end of 2013 a number of health economic influenza vaccine studies from Canada were reported, evaluating cost-effectiveness/cost-utility studies of seasonal TIVs, cost of vaccination strategies in targeted groups, and estimated cost-utility analyses and willingness-to-pay ().Citation69-75
Table 4. Summary of economic studies of seasonal influenza vaccination in Canada
A study of MF59-TIV compared with TIV used an age-structured model in which the population was divided into 5 different compartments or disease states: susceptible, vaccinated, exposed, infectious, and recovered.Citation69 The probability of moving between compartments was derived from epidemiological data and calibrated to Canadian influenza data (1997 to 2004 seasons). The simulation allowed new individuals to enter through birth or leave through non-influenza deaths over a 10 y time horizon. In the base case, the cost of using MF59-TIV over 10 y was higher (CAN$837.0 million) than TIV (CAN$730.5 million). These costs were offset by reducing the healthcare cost of influenza from CAN$501.76 million with TIV to CAN$473.50 million with MF59-TIV. The incremental cost per quality-adjusted life year (QALY) gained of MF59-TIV relative to TIV was CAN$2,111 in older adults (≥65 years).Citation69 In another modeling study, a decision tree was used to assess the cost-effectiveness of trivalent LAIV vs injectable inactivated TIV in children aged 2–17 y with a 1-year time horizon. The study showed that overall, trivalent LAIV dominated TIV and provided an estimated cost savings per vaccinated child of CAN$4.20 from a healthcare perspective and CAN$35.34 from a societal perspective.Citation70
Several studies discuss vaccinating different population sub-groups or regions. A cost-utility analysis of the A(H1N1)pdm09 mass immunization program in Ontario predicted a cost of CAN$118 million (CAN$1,645/QALY gained) to immunize 30% of the provincial population.Citation71 In a cost-utility analysis of Ontario's universal vaccination program, universal versus targeted group vaccination predicted a cost of CAN$10,797/QALY gained, which is below the threshold usually considered cost-effective in Canada (CAN$40,000–50,000/QALY gained).Citation72 In another study published in 2006, a decision analysis was used to assess the cost-effectiveness of universal vaccination of infants/toddlers aged 6–23 months compared with vaccinating high-risk children only.Citation73 Analyses were conducted for a cohort of 500,000 children assuming different vaccination coverage between influenza seasons (100% in year 1, 33% thereafter). In the first year, the incremental cost of vaccination from the healthcare system perspective was CAN$17 per day of illness averted, CAN$900,000/QALY gained, and CAN$6 million per death averted. In subsequent years, costs among those vaccinated would equal those unvaccinated (break-even) at a cost of CAN$6.81 per vaccine dose from a healthcare perspective, and CAN$11.90 per vaccine dose from a societal perspective.Citation73
Contingent valuation has been used to determine the willingness-to-pay for access to a pandemic influenza vaccine, and found that households were willing to pay CAN$417.35 for immediate A(H1N1)pdm09 vaccination.Citation74 In another analysis to determine key cost drivers in providing influenza vaccines within budget conducted in a health unit in Ontario, costs relating to labor (nursing and clerical), supplies, disposals, facilities, mileage, and program costs for influenza clinics (including labor costs) were estimated. This study reported that the most significant cost variables were labor costs and number of vaccines given per nurse per hour.Citation75
Discussion
Based on previous modeling of US surveillance data across 10 influenza seasons, the public health benefit of switching from TIV to QIV is likely to vary depending upon the epidemic intensity of influenza B during each season.Citation8 As such, many countries, including Canada, have established sentinel surveillance networks to help monitor influenza disease and to guide public health decision-making. Using data from the national surveillance system and published studies identified by a comprehensive literature search across 2002–2013, we have described the burden of seasonal influenza disease and the cost-effectiveness of vaccination in Canada, with the aim of developing a dataset that may be used to develop future analytical frameworks to model the effect of QIV in Canada.
According to Canadian national surveillance, influenza A was the dominant subtype circulating in all but 2 seasons (2000/01 and 2011/12), whereas influenza B was less prevalent, representing an average of 17% of all laboratory-confirmed cases during the 2001/02 to 2012/13 influenza seasons.Citation4 However, the surveillance data showed that influenza B strains from the Yamagata and Victoria lineages co-circulated in 7 of the 12 influenza seasons, resulting in mismatch with the TIV. Viral circulation and the occurrence of B lineage vaccine mismatch reported in Canada was generally consistent with that observed in Europe and the US.Citation5,76-78
Studies in the US and Europe suggest that A/H3N2 is the most common subtype associated with complications and death in older adults, followed by influenza B, and then A/H1N1.Citation79-81 In children and young adults, influenza B is suggested to pose the highest risk of severe outcomes.Citation5,7,82 As we describe above, laboratory confirmed influenza infection rates in Canada were generally highest among elderly populations, followed by children aged <5 years. These two groups, along with people with medical co-morbidities, are at increased risk for influenza complications and are recommended for annual seasonal vaccination in most industrialized countries.Citation4
The WHO reports that the global attack rate of influenza is about 10–20%,Citation83 while an analysis based on US hospital discharge records collected for 22 seasons (1979–2001), estimated that 230,000 influenza-related hospital admissions occurred annually.Citation79 In a more recent surveillance study of laboratory-confirmed influenza-related hospitalizations between 2005 and 2008 in the US, the annual age-adjusted rate of influenza-related hospitalizations was 12.2 per 100,000 persons.Citation84 The rate of hospitalization reported in Canada is consistent with that reported in the US. For example, in an evaluation of influenza-related hospitalization in Toronto in which the median age of patients was about 76–77 years, the rate of hospitalization ranged from 2.6 per 100,000 population in the 2005/06 season to 27.1 per 100,000 population in the 2010/11 season.Citation27
We are aware that a number of Canadian studies have been published since the 2013 search cut-off data used to inform this review, some of which we describe below. From an epidemiological perspective, the Serious Outcomes Surveillance Network of the Canadian Immunization Research Network reported an interim analysis for the 2014/15 influenza season. They found that the great majority (99%) of laboratory-confirmed influenza hospitalizations with a known influenza virus type reported in their sentinel hospitals was due to influenza A. Almost 70% of hospitalized laboratory-confirmed influenza cases were >75 years, and 7.9% of all hospitalized cases died in hospital.Citation85
From a health economic perspective, a recent systematic review provided a qualitative appraisal of all vaccine economic evaluations in Canada published between 1993 and 2013, including influenza cost-effectiveness studies.Citation86 While this review's aim was primarily to evaluate and report on study characteristics rather than outcomes per se, those identified for influenza were described either as cost-effective or dominant. Subsequent to the systematic search, 2 further economic evaluations of the benefits of switching from TIV to QIV have been published, one of which used a static model, and the second a dynamic model.Citation87,88 The first study used a probabilistic and static cost-utility model adapted for Ontario, and found that, in comparison to TIV, universal vaccination with QIV is estimated to realize reductions of an additional 2,516 influenza cases, 1,683 influenza-associated medical visits, 27 influenza-associated hospitalizations, and 5 influenza-associated deaths. Furthermore, QIV would generate 76 additional QALYs with a net budget impact of CAN$4,784,112 from a societal perspective, and with an incremental cost-effectiveness ratio (ICER) of CAN$63,773/QALY relative to TIV.Citation87 The second study used an age-structured compartmental dynamic transmission model applied to the overall Canadian population.Citation88 This study found that switching from TIV to QIV across Canada would prevent an estimated 328 (6.8%) deaths; 1,876 (5.7%) hospitalizations; 3,395 (5.7%) ER visits; 52,200 (4.9%) doctor's office visits; and 135,538 (4.6%) symptomatic cases of influenza in an average season. The incremental cost-utility ratio (ICUR) for QIV compared with TIV was CAN$7,961 per QALY gained.Citation88
In 2004, the NACI recommended annual influenza vaccination in healthy infants/toddlers aged from 6 to 23 months in addition to the groups already recommended for vaccination and most Canadian provinces adopted the recommendation within their publically-funded immunization programs.Citation89 In the 2014/15 influenza season, universal influenza vaccination of healthy individuals from 6 months of age was recommended by NACI in Canada.Citation4
The incremental reduction in the burden of influenza B disease associated with switching from TIV to QIV was initially modeled by the US Centers for Disease Control and Prevention (CDC).Citation8 It was estimated that this switch would result in fewer influenza cases, hospitalizations, and deaths, dependant on the proportion of cases due to influenza B included in the analysis. As we describe above, modeling studies performed for the Canadian population show similar benefits.Citation87,88
We should acknowledge that the present review has some limitations. While we performed a comprehensive systematic search to identify and select studies to inform this review, no formal systematic appraisal of these studies was performed. As such, bias, both in the study selection and in the original research (including reporting and selection bias) may exist, and this may impact upon our summary of the data. It may be noted however, that while reporting bias may exist, some consider these to have a particular impact when discussing vaccine effectiveness (VE) data. In our review, we specifically do not discuss VE data, in part as data is limited for the Canadian population, although we accept that this omission may also be a limitation of this review.
Another limitation was in the search cut-off date. While we have sought to mitigate the latter by making some mention of relevant more recent literature, it remains that other relevant recent literature has not been included in this review. In addition, reporting differences exist between epidemiological data reported in journals and those reported in surveillance reports (‘gray’ literature). The latter, inclusion of which we consider a strength of the review, often report preliminary, aggregated data, with less detail than formal reports and should be considered as complementary to those data reported in formal publications.
In summary, Canadian national surveillance data show that over 11 seasons, influenza A was the predominant influenza virus, and excluding the pandemic seasons, about 26–34% of cases were attributed to influenza B viruses. Published studies suggest that influenza-related complications are an important cause of hospitalization, antibiotic use, and mortality. Economic models indicate that universal vaccination with TIVs is cost-saving in Canada, with more recent models suggesting that switching from TIV to QIV may further reduce the burden of influenza B disease and be cost-effective. Nevertheless, it remains that outcomes data from the Canadian setting is limited and further real-world data is required to help inform health policy decision makers on appropriate influenza vaccination strategies for Canada.
Trademark disclosure
Agriflu and Fluad are trademarks of Seqirus.FluLaval and Fluviral are trademarks of the GSK group of companies.FluMist is a trademark of MedImmune, LLC/the AstraZeneca group of companies.Fluzone and Vaxigrip are trademarks of Sanofi Pasteur.Influvac is a trademark of Abbott Biologicals B.V.
Abbreviations
| CDC | = | Centers for Disease Control and Prevention |
| ER | = | Emergency Room |
| GP | = | General practitioner |
| HIV+ | = | human immunodeficiency virus-positive |
| ICU | = | Intensive Care Unit |
| ICER | = | incremental cost-effectiveness ratio |
| ICUR | = | incremental cost-utility ratio |
| ILI | = | influenza-like illness |
| LAIV | = | live attenuated influenza vaccine |
| LOS | = | length of hospitalization stay |
| NACI | = | National Advisory Committee on Immunization |
| NML | = | National Microbiology Laboratory |
| RT-PCR | = | real-time polymerase chain reaction |
| PHAC | = | Public Health Agency of Canada |
| QALY | = | quality-adjusted life year |
| QIV | = | quadrivalent influenza vaccine |
| RVDSS | = | Respiratory Virus Detection Surveillance System |
| TIV | = | trivalent influenza vaccine |
| US | = | United States |
| VE | = | vaccine effectiveness |
| WHO | = | World Health Organization |
Disclosure of potential conflicts of interest
EWT was employed by the GSK group of companies at the time of the conduct of the literature review and during the development of the manuscript and is currently employed by Takeda. MKr & MKo are employees of Optum, which was contracted by the GSK group of companies to conduct this literature review. RS & SGN are employees of the GSK group of companies. RS holds shares in the GSK group of companies as part of her employee remuneration.
Authors' contributions
EWT, MKr, Mko & RS contributed to the design of the literature review, MKr supervised article selection, acted as the arbitrator of any dispute, and supervised extraction of the data from the selected articles, EWT, MKr, & Mko drafted the report of the literature review, EWT, MKr, Mko, RS & SGN interpreted the data. All authors participated in the development of this manuscript. All authors had full access to the data and gave final approval before submission. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The work described was carried out in accordance with ICMJE recommendations for conduct, reporting, editing and publications of scholarly work in medical journals. The corresponding author had final responsibility to submit for publication.
Supplementary Figure and Tables
Download Zip (415.9 KB)Acknowledgments
The authors thank Eva Razumienko (GSK) for her contribution to the literature review (writing of a chapter of the report). The authors would like to acknowledge Graeme Ball (OptumInsight) for screening the articles, Jatin Gupta and Robin Jha (OptumInsight) for extracting data from the selected articles into structured tables and Qing Gu (OptumInsight) for both articles screening and data extraction. The authors thank Grégory Leroux (Business & Decision Life Sciences on behalf of GSK) for editorial support and publication development management, Véronique Gochet (Business & Decision Life Sciences on behalf of GSK) for editorial support, and Amrita Ostawal and Annick Moon (freelance medical writers, on behalf of GSK) for medical writing.
Funding
GlaxoSmithKline Biologicals SA funded all costs associated with the literature review (GSK study identifier: HO-13-14054) and with the development and publication of this manuscript.
References
- Public Health Agency of Canada. An advisory committee statement (ACS) - National Advisory Committee on Immunization (NACI). Statement on Seasonal Influenza Vaccine for 2015–2016; Available from: http://www.phac-aspc.gc.ca/naci-ccni/flu-2015-grippe-eng.php [Accessed 18 February 2016]
- Australian Government Department of Health. Clinical advice for immunisation providers regarding the administration of 2014 seasonal influenza vaccines. March 2014; Available from: http://www.immunise.health.gov.au/internet/immunise/Publishing.nsf/content/ATAGI-advice-TIV [Accessed 18 February 2016]
- United States Centers for Disease Control and Prevention. CDC's advisory committee on immunization practices (ACIP) recommends universal annual influenza vaccination. 2010; Available from: http://www.cdc.gov/media/pressrel/2010/r100224.htm [Accessed 18 February 2016]
- Public Health Agency of Canada. An Advisory Committee Statement (ACS) - National Advisory Committee on Immunization (NACI). Statement on Seasonal Influenza Vaccine for 2014–2015. http://www.phac-aspc.gc.ca/naci-ccni/flu-grippe-eng.php [Accessed 18 February 2016]
- Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother 2012; 8:81-8; PMID:22252006; https://doi.org/http://dx.doi.org/10.4161/hv.8.1.17623
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2012–2013 northern hemisphere influenza season. 2012; Available from: http://www.who.int/influenza/vaccines/virus/recommendations/201202_recommendation.pdf [Accessed 18 February 2016]
- Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine 2010; 28(Suppl 4):D45-53; PMID:20713260; https://doi.org/http://dx.doi.org/10.1016/j.vaccine.2010.08.028
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993-8; PMID:22226861; https://doi.org/http://dx.doi.org/10.1016/j.vaccine.2011.12.098
- Jain VK, Rivera L, Zaman K, Espos RA, Jr., Sirivichayakul C, Quiambao BP, Rivera-Medina DM, Kerdpanich P, Ceyhan M, Dinleyici EC, et al. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med 2013; 369:2481-91; PMID:24328444; https://doi.org/http://dx.doi.org/10.1056/NEJMoa1215817
- Moa AM, Chughtai AA, Muscatello DJ, Turner RM, MacIntyre CR. Immunogenicity and safety of inactivated quadrivalent influenza vaccine in adults: A systematic review and meta-analysis of randomised controlled trials. Vaccine 2016; 34(35):4092-102; PMID:27381642; https://doi.org/http://dx.doi.org/10.1016/j.vaccine.2016.06.064
- Squires SG, Pelletier L, Zabchuck P, Winchester B, Tam T. Influenza in Canada: 1999–2000 season. Can Commun Dis Rep 2001; 27(1):1-9; PMID:11195964
- Macey JF, Zabchuck P, Winchester B, Tam TWS. Influenza in Canada: 2000–2001 season. Can Commun Dis Rep 2002; 28(3):17-28; PMID:11852481
- Macey JF, Tam TWS, Li Y, Winchester B, Zabchuck P. Influenza in Canada: 2001–2002 season. Can Commun Dis Rep 2003; 29(6):45-59; PMID:12666540
- Aziz S, Tam TWS, Macey JF, Li Y, Jain S, Boulos D, Zheng H, Winchester B, Zabchuck P. Influenza in Canada: 2003–2004 season. Can Commun Dis Rep 2005; 31(1):1-19
- Xie L, Squires SG, Macey JF, Aziz S, Winchester B, Zheng H, Tam TWS. Influenza in Canada: 2004–2005 season. Can Commun Dis Rep 2006; 32(6):57-74; PMID:16649289
- Reyes F, Macey JF, Aziz S, Li Y, Watkins K, Winchester B, Zabchuck P, Zheng H, Huston P, Tam TWS, et al. Influenza in Canada: 2005–2006 season. Can Commun Dis Rep 2007; 33(3):21-41; PMID:17323533
- Reyes F, Aziz S, Li Y, Macey JF, Winchester B, Garner M, Huston P, King A. Influenza in Canada: 2006–2007 season. Can Commun Dis Rep 2008; 34(3):1-25
- Domingo F, Aziz S, Winchester B, Li Y, Bettinger J, Rahimi Khameneh S, Pelletier L. Influenza in Canada: 2007–2008 season. Can Commun Dis Rep 2012; 37(S1)
- Domingo F, Winchester B, Sumner M, Li Y, Bastien N, Bettinger J, Bryson M, Do MT, Akwar H. An epidemiologic and virologic summary of seasonal and pandemic influenza in Canada, 2008–2009 to 2009–2010 seasons. Public Health Agency of Canada 2013
- FluWatch Weekly Report, August 14 to 27, 2011 (Weeks 33 and 34). In: FluWatch [Internet]. Canada: Public Health Agency of Canada. 2011. Available from: http://publications.gc.ca/collections/collection_2014/aspc-phac/HP58-1-2011-34-eng.pdf. [Accessed 13 April 2016]
- FluWatch Weekly Report, August 12 to August 25, 2012 (Weeks 33 and 34). In: FluWatch [Internet]. Canada: Public Health Agency of Canada. 2012. Available from: http://publications.gc.ca/collections/collection_2013/aspc-phac/HP58-1-2012-34-eng.pdf. [Accessed 13 April 2016]
- FluWatch Weekly Report, August 11 to 24, 2013 (Weeks 33 and 34). In: FluWatch [Internet]. Canada: Public Health Agency of Canada. 2013. Available from: http://publications.gc.ca/collections/collection_2013/aspc-phac/HP58-1-2013-34-eng.pdf. [Accessed 13 April 2016]
- Public Health Agency of Canada. An Advisory Committee Statement (ACS) - National Advisory Committee on Immunization (NACI). Statement on seasonal influenza vaccine for 2013–2014. Can Commun Dis Rep 2013; 39(ACS-4)
- McGeer A, Green KA, Plevneshi A, Shigayeva A, Siddiqi N, Raboud J, Low DE. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis 2007; 45:1568-75; PMID:18190317; https://doi.org/http://dx.doi.org/10.1086/523584
- Schull MJ, Mamdani MM, Fang J. Influenza and emergency department utilization by elders. Acad Emerg Med 2005; 12:338-44; PMID:15805325; https://doi.org/http://dx.doi.org/10.1111/j.1553-2712.2005.tb01953.x
- McGeer A, Gravel D, Taylor G, Weir C, Frenette C, Vayalumkal J, Wong A, Moore D, Michaud S, Amihod B. Influenza in adults admitted to Canadian hospitals: data from two seasons. Clin Microbiol Infect 2009; 15(Suppl s4):S26
- McGeer A, Devlin R, Downey S, Drews K, Green J, Gubbay K, Hassan K, Katz T, Mazzulli M, Muller A, et al. Did the 2009 influenza pandemic change influenza prevention, diagnosis and management? Evidence from surveillance for influenza-associated hospitalisation in Canada. Clin Microbiol Infect 2012; 18(Suppl s3):129-30
- Church DL, Davies HD, Mitton C, Semeniuk H, Logue M, Maxwell C, Donaldson C. Clinical and economic evaluation of rapid influenza a virus testing in nursing homes in Calgary, Canada. Clin Infect Dis 2002; 34:790-5; PMID:11830797; https://doi.org/http://dx.doi.org/10.1086/338960
- Bowles SK, Lee W, Simor AE, Vearncombe M, Loeb M, Tamblyn S, Fearon M, Li Y, McGeer A. Use of oseltamivir during influenza outbreaks in Ontario nursing homes, 1999–2000. J Am Geriatr Soc 2002; 50:608-16; PMID:11982659; https://doi.org/http://dx.doi.org/10.1046/j.1532-5415.2002.50153.x
- Mahmud SM, Thompson LH, Nowicki DL, Plourde PJ. Outbreaks of influenza-like illness in long-term care facilities in Winnipeg, Canada. Influenza Other Respir Viruses 2013; 7:1055-61; PMID:23145997; https://doi.org/http://dx.doi.org/10.1111/irv.12052
- Kuster SP, Drews S, Green K, Blair J, Davis I, Downey J, Fowler R, Katz K, Lapinsky S, McRitchie D, et al. Epidemiology of influenza-associated hospitalization in adults, Toronto, 2007/8. Eur J Clin Microbiol Infect Dis 2010; 29:835-43; PMID:20428910; https://doi.org/http://dx.doi.org/10.1007/s10096-010-0935-x
- Janjua NZ, Skowronski DM, Hottes TS, Osei W, Adams E, Petric M, Lem M, Tang P, De Serres G, Patrick DM, et al. Transmission dynamics and risk factors for pandemic H1N1-related illness: outbreak investigation in a rural community of British Columbia, Canada. Influenza Other Respir Viruses 2012; 6:e54–62; PMID:22385647; https://doi.org/http://dx.doi.org/10.1111/j.1750-2659.2012.00344.x
- Ammons B, Barnes S, Xiong W, Linse T, Loeb M, Smieja M. Influenza-like illness amongst Canadians in Winter 2008: a nationally representative, telephone-based survey. Int J Antimicrob Agents 2009; 34(Suppl 2):S110; ; https://doi.org/http://dx.doi.org/10.1016/S0924-8579(09)70487-X
- Tran D, Vaudry W, Moore DL, Bettinger JA, Halperin SA, Scheifele DW, Aziz S. Comparison of children hospitalized with seasonal versus pandemic influenza A, 2004–2009. Pediatrics 2012; 130:397-406; PMID:22931901; https://doi.org/http://dx.doi.org/10.1542/peds.2011-3216
- McGeer A, Green K, Drews S, Davis I, Downey J, Katz K, Rose D, Sarabia A, Simor A, Pataki R, et al. Epidemiology of influenza illness requiring intensive care unit admission in Toronto, Canada. Clin Microbiol Infect 2009; 15(Suppl s4):S26
- Mitchell R, Taylor G, McGeer A, Frenette C, Suh KN, Wong A, Katz K, Wilkinson K, Amihod B, Gravel D. Understanding the burden of influenza infection among adults in Canadian hospitals: a comparison of the 2009–2010 pandemic season with the prepandemic and postpandemic seasons. Am J Infect Control 2013; 41:1032-7; PMID:24176768; https://doi.org/http://dx.doi.org/10.1016/j.ajic.2013.06.008
- Pollock SL, Sagan M, Oakley L, Fontaine J, Poffenroth L. Investigation of a pandemic H1N1 influenza outbreak in a remote First Nations community in northern Manitoba, 2009. Can J Public Health 2012; 103:90-3; PMID:22530528
- Statistics Canada. Deaths, by cause, Chapter X: Diseases of the respiratory system (J00 to J99), age group and sex, Canada. Statistics Canada CANSIM table 102–0530. 2014; Available from: http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=1020530 [Accessed 18 February 2016]
- Hassan K, Devlin R, Downey J, Drews S, Green K, Gubbay J, Katz K, Low D, Ma C, Mazzulli T, et al. Epidemiology and clinical features of severe influenza in adults: 7 years of surveillance in Toronto, Canada. Abstract presented at the 22nd European Congress of Clinical Microbiology and Infectious Diseases. March 31-April 3, 2012; London, United Kingdom.
- Wilkinson KD, Taylor G, Gravel D, Frenette C, McGeer A, Moore D, Suh K, Vayalumkal J, Wong A, Tong A, et al. Laboratory Confirmed Seasonal Influenza in Adults in a Network of Canadian Hospitals 2006–2009. Abstract [n 892] presented at the International Conference on Healthcare-Associated Infections. March 18–22, 2010; Atlanta, Georgia, United States.
- Jouvet P, Hutchison J, Pinto R, Menon K, Rodin R, Choong K, Kesselman M, Veroukis S, Andre Dugas M, Santschi M, et al. Critical illness in children with influenza A/pH1N1 2009 infection in Canada. Pediatr Crit Care Med 2010; 11:603-9; PMID:20308929; https://doi.org/http://dx.doi.org/10.1097/PCC.0b013e3181d9c80b
- O'Riordan S, Barton M, Yau Y, Read SE, Allen U, Tran D. Risk factors and outcomes among children admitted to hospital with pandemic H1N1 influenza. CMAJ 2010; 182:39-44; PMID:19926677; https://doi.org/http://dx.doi.org/10.1503/cmaj.091724
- Aguirre E, Papenburg J, Ouakki M, Fontela PS, Guimont C, De Serres G, Boivin G. Comparison of pandemic and seasonal influenza in the pediatric emergency department. Pediatr Infect Dis J 2011; 30:633-9; PMID:21289529; https://doi.org/http://dx.doi.org/10.1097/INF.0b013e3182103d54
- Flechelles O, Fowler R, Jouvet P. H1N1 pandemic: clinical and epidemiologic characteristics of the Canadian pediatric outbreak. Expert Rev Anti Infect Ther 2013; 11:555-63; PMID:23750727; https://doi.org/http://dx.doi.org/10.1586/eri.13.40
- Allard R, Leclerc P, Tremblay C, Tannenbaum TN. Diabetes and the severity of pandemic influenza A (H1N1) infection. Diabetes Care 2010; 33:1491-3; PMID:20587722; https://doi.org/http://dx.doi.org/10.2337/dc09-2215
- Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009; 302:1872-9; PMID:19822627; https://doi.org/http://dx.doi.org/10.1001/jama.2009.1496
- Campbell A, Rodin R, Kropp R, Mao Y, Hong Z, Vachon J, Spika J, Pelletier L. Risk of severe outcomes among patients admitted to hospital with pandemic (H1N1) influenza. CMAJ 2010; 182:349-55; PMID:20159893; https://doi.org/http://dx.doi.org/10.1503/cmaj.091823
- Sood MM, Rigatto C, Zarychanski R, Komenda P, Sood AR, Bueti J, Reslerova M, Roberts D, Mojica J, Kumar A. Acute kidney injury in critically ill patients infected with 2009 pandemic influenza A(H1N1): report from a Canadian province. Am J Kidney Dis 2010; 55:848-55; PMID:20303633; https://doi.org/http://dx.doi.org/10.1053/j.ajkd.2010.01.011
- Jung J, Fowler R, Long J, Zarychanski R, Rodin R, Cook DJ, Jouvet P, Marshall J, Kumar A. On behalf of the ICU-flu investigators. 2009–2010 H1N1-related critical illness among aboriginal canadians and non-aboriginal canadians. Am J Respir Crit Care Med 2011; 183:A3140
- Gu S, Long J, Menon K, Cook D, McGeer A, Kumar A, Jouvet P, Marshall J, Hutchison J, Fowler R. Comparison of the first and second waves Of H1N1-related critical illness in Canada. Am J Respir Crit Care Med 2011; 183:A3141
- McNeil SA, Johnstone J, Ambrose A, Loeb M, Russell M, Trottier S, Boivin G, McCarthy A, Henderson E, Stiver G, et al. Sentinel Surveillance for Influenza in Canadian Hospitals: Experience of the Public Health Agency of Canada (PHAC)/Canadian Institutes of Health Research (CIHR) Influenza Research Network (PCIRN) Adult Serious Outcomes Surveillance Network. Can J Infect Dis Med Microbiol 2010; 21(4):214.
- Muscedere J. Prevalent and incident bacterial infections among Canadian critically Ill patients with influenza A (H1N1) 2009 preliminary data. Am J Respir Crit Care Med 2011; 183:A4915.
- Muscedere J, Ofner M, Kumar A, Long J, Lamontagne F, Cook D, McGeer A, Chant C, Marshall J, Jouvet P, et al. The occurrence and impact of bacterial organisms complicating critical care illness associated with 2009 influenza A(H1N1) infection. Chest 2013; 144:39-47; PMID:23392627; https://doi.org/http://dx.doi.org/10.1378/chest.12-1861
- Bagshaw SM, Sood MM, Long J, Fowler RA, Adhikari NK. Acute kidney injury among critically ill patients with pandemic H1N1 influenza A in Canada: cohort study. BMC Nephrol 2013; 14:123; PMID:23763900; https://doi.org/http://dx.doi.org/10.1186/1471-2369-14-123
- Taylor G, Wilkinson K, Gravel D, Amihod B, Frenette C, Moore D, McGeer A, Suh K, Wong A, Mitchell R. Laboratory-confirmed pandemic h1n1 influenza in hospitalized adults: findings from the Canadian Nosocomial Infections Surveillance Program (CNISP), 2009–2010. BMC Proc 2011 2011; 5(6):P81; https://doi.org/https://doi.org/10.1186/1753-6561-5-S6-P81
- Cutler J, Schleihauf E, Hatchette TF, Billard B, Watson-Creed G, Davidson R, Li Y, Bastien N, Sarwal S. Investigation of the first cases of human-to-human infection with the new swine-origin influenza A (H1N1) virus in Canada. CMAJ 2009; 181:159-63; PMID:19620268; https://doi.org/http://dx.doi.org/10.1503/cmaj.090859
- Helferty M, Vachon J, Tarasuk J, Rodin R, Spika J, Pelletier L. Incidence of hospital admissions and severe outcomes during the first and second waves of pandemic (H1N1) 2009. CMAJ 2010; 182:1981-7; PMID:21059773; https://doi.org/http://dx.doi.org/10.1503/cmaj.100746
- World Health Organization. Epidemiological summary of pandemic influenza A (H1N1) 2009 virus - Ontario, Canada. 2009. Wkly Epidemiol Rec 2009; 84:485-91; Available from: http://www.who.int/wer/2009/wer8447.pdf [Accessed 18 February 2016]; PMID:19928301
- Wilkinson KD, Taylor G, Gravel D, Amihod B, Frenette C, McGeer A, Moore D, Suh K, Tong A, Vayalumkal J, et al. Laboratory-confirmed influenza in hospitalized adults: findings from the Canadian nosocomial infections surveillance program, June 1 to December 31, 2009. Abstract [n LB 3] presented at the International Conference on Healthcare-Associated Infections. March 18–22, 2010; Atlanta, Georgia, United States.
- Moore DL, Vaudry W, Scheifele DW, Halperin SA, Dery P, Ford-Jones E, Arishi HM, Law BJ, Lebel M, Le Saux N, et al. Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003–2004. Pediatrics 2006; 118:e610–9; PMID:16950953; https://doi.org/http://dx.doi.org/10.1542/peds.2005-2744
- Public Health Agency of Canada. The epidemiology of influenza in children hospitalized in Canada, 2004–2005, in immunization monitoring program active (IMPACT) centres. Can Commun Dis Rep 2006; 32:77-86; PMID:16649286
- Schanzer DL, Schwartz B. Impact of seasonal and pandemic influenza on emergency department visits, 2003–2010, Ontario, Canada. Acad Emerg Med 2013; 20:388-97; PMID:23701347; https://doi.org/http://dx.doi.org/10.1111/acem.12111
- Fanella ST, Pinto MA, Bridger NA, Bullard JM, Coombs JM, Crockett ME, Olekson KL, Poliquin PG, Van Caeseele PG, Embree JE. Pandemic (H1N1) 2009 influenza in hospitalized children in Manitoba: nosocomial transmission and lessons learned from the first wave. Infect Control Hosp Epidemiol 2011; 32:435-43; PMID:21515973; https://doi.org/http://dx.doi.org/10.1086/659401
- Mostaço-Guidolin LC, Towers SM, Buckeridge DL, Moghadas SM. Age distribution of infection and hospitalization among Canadian First Nations populations during the 2009 H1N1 pandemic. Am J Public Health 2013; 103:e39–44; https://doi.org/http://dx.doi.org/10.2105/AJPH.2012.300820
- Schanzer DL, Langley JM, Tam TW. Hospitalization attributable to influenza and other viral respiratory illnesses in Canadian children. Pediatr Infect Dis J 2006; 25:795-800; PMID:16940836; https://doi.org/http://dx.doi.org/10.1097/01.inf.0000232632.86800.8c
- Schanzer DL, Langley JM, Tam TW. Role of influenza and other respiratory viruses in admissions of adults to Canadian hospitals. Influenza Other Respir Viruses 2008; 2:1-8; PMID:19453488; https://doi.org/http://dx.doi.org/10.1111/j.1750-2659.2008.00035.x
- Schanzer DL, McGeer A, Morris K. Statistical estimates of respiratory admissions attributable to seasonal and pandemic influenza for Canada. Influenza Other Respir Viruses 2013; 7:799-808; PMID:23122189; https://doi.org/http://dx.doi.org/10.1111/irv.12011
- Schanzer DL, Zheng H, Gilmore J. Statistical estimates of absenteeism attributable to seasonal and pandemic influenza from the Canadian Labour Force Survey. BMC Infect Dis 2011; 11:90; PMID:21486453; https://doi.org/http://dx.doi.org/10.1186/1471-2334-11-90
- Fisman DN, Tuite AR. Estimation of the health impact and cost-effectiveness of influenza vaccination with enhanced effectiveness in Canada. PLoS One 2011; 6:e27420; PMID:22110645; https://doi.org/http://dx.doi.org/10.1371/journal.pone.0027420
- Tarride JE, Burke N, Von Keyserlingk C, O'Reilly D, Xie F, Goeree R. Cost-effectiveness analysis of intranasal live attenuated vaccine (LAIV) versus injectable inactivated influenza vaccine (TIV) for Canadian children and adolescents. Clinicoecon Outcomes Res 2012; 4:287-98; PMID:23055756; https://doi.org/http://dx.doi.org/10.2147/CEOR.S33444
- Sander B, Bauch C, Fisman DN, Fowler R, Kwong JC, McGeer A, Zivkovic Gojovic M, Krahn M. Is a mass immunization program for pandemic (H1N1) 2009 good value for money? Early evidence from the Canadian experience. PLoS Curr 2009; 1:RRN1137; PMID:20043032; https://doi.org/http://dx.doi.org/10.1371/currents.RRN1137
- Sander B, Kwong JC, Bauch CT, Maetzel A, McGeer A, Raboud JM, Krahn M. Economic appraisal of Ontario's Universal Influenza Immunization Program: a cost-utility analysis. PLoS Med 2010; 7:e1000256; PMID:20386727; https://doi.org/http://dx.doi.org/10.1371/journal.pmed.1000256
- Skowronski DM, Woolcott JC, Tweed SA, Brunham RC, Marra F. Potential cost-effectiveness of annual influenza immunization for infants and toddlers: experience from Canada. Vaccine 2006; 24:4222-32; PMID:16423432; https://doi.org/http://dx.doi.org/10.1016/j.vaccine.2005.12.036
- Asgary A. Assessing households' willingness to pay for an immediate pandemic influenza vaccination programme. Scand J Public Health 2012; 40:412-7; PMID:22798286; https://doi.org/http://dx.doi.org/10.1177/1403494812453884
- Mercer NJ. Cost analysis of public health influenza vaccine clinics in Ontario. Can J Public Health 2009; 100:340-3; PMID:19994733
- Eiros-Bouza JM, Perez-Rubio A. [Burden of influenza virus type B and mismatch with the flu vaccine in Spain]. Rev Esp Quimioter 2015; 28:39-46; PMID:25690144
- Heikkinen T, Ikonen N, Ziegler T. Impact of influenza B lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999–2012. Clin Infect Dis 2014; 59:1519-24; PMID:25139969; https://doi.org/http://dx.doi.org/10.1093/cid/ciu664
- Karve S, Meier G, Davis KL, Misurski DA, Wang CC. Influenza-related health care utilization and productivity losses during seasons with and without a match between the seasonal and vaccine virus B lineage. Vaccine 2013; 31:3370-88; PMID:23707697; https://doi.org/http://dx.doi.org/10.1016/j.vaccine.2013.04.081
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333-40; PMID:15367555; https://doi.org/http://dx.doi.org/10.1001/jama.292.11.1333
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179-86; PMID:12517228; https://doi.org/http://dx.doi.org/10.1001/jama.289.2.179
- Zambon MC, Stockton JD, Clewley JP, Fleming DM. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet 2001; 358:1410-6; PMID:11705487; https://doi.org/http://dx.doi.org/10.1016/S0140-6736(01)06528-X
- Cottrell SL, Moore C, Dexter L, Thomas DR, Salmon RL. Unusually high impact of influenza B during the early 2012–2013 influenza season in Wales–epidemiology and clinical analysis of the first 100 cases. Influenza Other Respir Viruses 2013; 7:1013-6; PMID:24034594; https://doi.org/http://dx.doi.org/10.1111/irv.12151
- World Health Organization. Immunization, vaccines and biologicals. Influenza. 2008; Available from: http://www.who.int/immunization/topics/influenza/en/ [Accessed 18 February 2016]
- Dao CN, Kamimoto L, Nowell M, Reingold A, Gershman K, Meek J, Arnold KE, Farley M, Ryan P, Lynfield R, et al. Adult hospitalizations for laboratory-positive influenza during the 2005–2006 through 2007–2008 seasons in the United States. J Infect Dis 2010; 202:881-8; PMID:20677944; https://doi.org/http://dx.doi.org/10.1086/655904
- McNeil SA, Andrew MK, Ye L, Haguinet F, Hatchette TF, ElSherif M, LeBlanc J, Ambrose A, McGeer A, McElhaney JE, et al. Interim estimates of 2014/15 influenza vaccine effectiveness in preventing laboratory-confirmed influenza-related hospitalisation from the Serious Outcomes Surveillance Network of the Canadian Immunization Research Network, January 2015. Euro Surveill 2015; 20(5):21024; PMID:25677052; https://doi.org/http://dx.doi.org/10.2807/1560-7917.ES2015.20.5.21024
- Chit A, Lee JK, Shim M, Nguyen VH, Grootendorst P, Wu J, Van Exan R, Langley JM. Economic evaluation of vaccines in Canada: a systematic review. Hum Vaccin Immunother 2016; 12(5):1257-64; PMID:26890128; https://doi.org/http://dx.doi.org/10.1080/21645515.2015.1137405
- Chit A, Roiz J, Aballea S. An assessment of the expected cost-effectiveness of quadrivalent influenza vaccines in Ontario, Canada using a static model. PLoS One 2015; 10(7):e0133606; PMID:26222538; https://doi.org/http://dx.doi.org/10.1371/journal.pone.0133606
- Thommes EW, Ismaila A, Chit A, Meier G, Bauch CT. Cost-effectiveness evaluation of quadrivalent influenza vaccines for seasonal influenza prevention: a dynamic modeling study of Canada and the United Kingdom. BMC Infect Dis 2015; 15:465; PMID:26503131; https://doi.org/http://dx.doi.org/10.1186/s12879-015-1193-4
- Orr P, An Advisory Committee Statement (ACS). National Advisory Committee on Immunization (NACI). Statement on influenza vaccination for the 2004–2005 season. Can Commun Dis Rep 2004; 30:1-32; PMID:15239483
