ABSTRACT
Rotavirus gastroenteritis is a leading global cause of mortality and morbidity in young children due to diarrhea and dehydration. Over 85% of deaths occur in developing countries. In industrialised countries, 2 live oral rotavirus vaccines licensed in 2006 quickly demonstrated high effectiveness, dramatically reducing severe rotavirus gastroenteritis admissions in many settings by more than 90%. In contrast, the same vaccines reduced severe rotavirus gastroenteritis by only 30–60% in developing countries, but have been proven life-saving. Bridging this “efficacy gap” offers the possibility to save many more lives of children under the age of 5. The reduced efficacy of rotavirus vaccines in developing settings may be related to differences in transmission dynamics, as well as host luminal, mucosal and immune factors. This review will examine strategies currently under study to target the issue of reduced efficacy and effectiveness of oral rotavirus vaccines in developing settings.
Introduction
Rotavirus disease is a major cause of morbidity and mortality worldwide in children under 5 y of age.Citation1-3 In 2013, rotavirus was responsible for 37% of all deaths caused by diarrheal disease in children younger than 5 y of age, leading to 215,000 deaths globally.Citation3 While rotavirus is ubiquitous, the greatest disease burden and over 90% of related deaths occur in low-income and low-middle-income countries.Citation3
In 2006, the licensure of 2 rotavirus vaccines, RotaTeq® (RV5, Merck & Co., West Point, PA, USA) and Rotarix™ (RV1, GlaxoSmithKline Biologicals, Rixensart, Belgium) represented a significant advancement in the prevention of gastroenteritis-related morbidity and mortality, particularly in children under the age of 5.Citation4 The vaccines dramatically reduced the rate of severe rotavirus gastroenteritis in both industrialised and developing countries,Citation5 however the exhibited efficacy and subsequent effectiveness was lower in developing countries.Citation2 Similar findings have been reported in clinical trials using other live oral rotavirus vaccines, including the original rhesus-reassortant tetravalent (RRV-TV) and the monovalent Indian human-bovine G9P[11] strain vaccine 116E.Citation2,6,7
Hypotheses proposed to explain why rotavirus vaccines are less effective in the developing country setting are grouped broadly between increased exposure of children to rotavirus infection and reduced host immune responses to administered vaccines.Citation8,9
This review will examine a range of strategies that have been studied to target the issue of reduced efficacy and effectiveness of oral rotavirus vaccines in developing countries.
Background
Rotavirus structure and classification
Rotaviruses (genus Rotavirus) belong to the Reoviridae family,Citation2,10,11 and each rotavirus particle consists of 3 concentric capsid layers.Citation10-12 Rotaviruses are classified according to their 2 outer capsid proteins, with the current genotyping nomenclature referring to G-types (based on gene encoding the VP7 outer capsid protein) and P types (based on gene encoding the VP4 outer capsid protein). The most common rotavirus strains identified associated with human disease are G1P[8], G2P[4], G3P[8], G4P[8], G9P[8] and G12P[8] which constitute approximately 90% of rotavirus infections worldwide.Citation2,13
Rotavirus disease and immunity
Rotavirus disease can vary in severity, from asymptomatic disease, to mild diarrhea, profoundly severe diarrhea, and even death.Citation2,12 Primary rotavirus infection occurring in infancy is the episode most likely to manifest as severe or even fatal disease, especially in the period between 3 months and 2 y of age, while subsequent infections tend to be much milder in severity, or asymptomatic.Citation2,12 There is no “gold standard” immune correlate of protection against rotavirus disease, however, the current consensus is that serum rotavirus-specific IgA is the strongest indicator of rotavirus protection.Citation11,14,15 Serum IgG is less well correlated with protection from disease, however, may be transferred across the placenta to confer some passive immunity, while IgA is produced actively by the infant following infection or vaccination.Citation14,15
In developing settings, 80 percent of primary rotavirus infections occur prior to 1 y of age, with infants 6 to 9 months of age the most vulnerable to severe rotavirus disease.Citation2 Furthermore, unlike in many temperate, industrialised countries, the transmission of rotavirus infection is an all-year phenomenon, punctuated by periods of increased activity.Citation2,16 It is unsurprising that over 85% of deaths attributed to rotavirus occur in developing countries, especially in areas where limited access to prompt oral rehydration and healthcare services hinders the provision of appropriate and timely treatment.Citation2
For rotavirus vaccines to be most effective, they must be administered before the primary rotavirus infection, which is made difficult in developing countries by the earlier onset of both rotavirus infection and disease, and the scarcity of health resources to support population-wide immunisation programs, for timely and broad coverage.Citation2,17
Efficacy and effectiveness of rotavirus vaccines in developing countries
At present, 2 rotavirus vaccines are available internationally: RotaTeq® (RV5, Merck & Co., West Point, PA, USA) and Rotarix™ (RV1, GlaxoSmithKline Biologicals, Rixensart, Belgium). Both are live oral rotavirus vaccines licensed for human use in over 85 countries.Citation2 The first dose of both vaccines should be administered within 6 to 12 weeks of age in a bid to reduce the peak of rotavirus disease that occurs at 6 months of age.Citation2 RV5 is a pentavalent, human-bovine reassortant vaccine administered as 3 doses.Citation2 RV1 is a monovalent, attenuated human rotavirus vaccine administered as 2 doses.Citation2 In the clinical development of both vaccines, immune responses were found to be dependent upon sufficient titer of live vaccine viruses to be ingested at each dose, and presumably reach the lumen on the jejunum, where replication and induction of immune responses occur.Citation9
RV5 and RV1 have proven highly efficacious and effective in industrialised countries, providing at least 90% protection against severe rotavirus gastroenteritis and at least 74% protection against rotavirus gastroenteritis of any severity.Citation18-24 In contrast, studies conducted in low-income countries in Africa and Asia revealed that the efficacy of the 2 vaccines ranged from 56% to 64% in protecting against severe rotavirus gastroenteritis.Citation25-30 In addition, in these settings vaccine efficacy waned between the first and second years after immunisation.Citation25-30 These findings are supported by a recent Cochrane systematic review which revealed that RV1 and RV5 demonstrated diminished efficacy against severe rotavirus gastroenteritis in WHO subregions classified as having “high” rates of childhood mortality and “high” or “very high” rates of adult mortality, compared with subregions with “very low” or “low” rates of child and adult mortality. This reduced vaccine efficacy persisted after 1 to 2 y of follow-up.Citation31
The largest RV5 Phase III study in the developing world included infants from Asia and Africa.Citation25-29 The efficacy of RV5 against severe rotavirus gastroenteritis in African infants in their first year of life was 64% (95% CI 40-79), with the vaccine also reducing rotavirus gastroenteritis of any severity by 31% (95% CI 17-42).Citation25 Results from the Asian infants were comparable, with RV5 reducing severe rotavirus gastroenteritis by 48% (95% CI 22– 66) over the complete study period.Citation27 Point estimates of vaccine efficacy reduced between the first and second year of life, from 51% (95% CI 13-73) to 46% (95% CI 1-71).Citation27 In a large multicentre randomized, controlled trial conducted in South Africa and Malawi, RV1 reduced severe rotavirus gastroenteritis by 61% (95% CI 44-73).Citation29 Despite the reduced performance of RV5 and RV1 in developing regions, the World Health Organization has recommended global rotavirus immunization for all infants based on the vaccines' ability to reduce the risk of rotavirus gastroenteritis in all regions of the world. In the context of the higher incidence of severe rotavirus disease in developing regions, rotavirus vaccines are still beneficial in reducing the toll of gastroenteritis morbidity and mortality.Citation2
Hypotheses for reduced efficacy of rotavirus vaccines in developing settings
Many explanations have been considered to explain the divergence in the efficacy of rotavirus vaccines between industrialized and developing countries. These factors include the higher early transmission rates of rotavirus infection in developing settings, and luminal, mucosal and immune factors of the host that may interfere with one or more stages of rotavirus vaccine take and resultant immune responses.Citation2,9,11
Host luminal factors, such as gastric acidity, variations in gut microbiota, and host mucosal factors, such as the presence of breast milk constituents at the time of immunisation, may directly alter or inhibit rotavirus vaccine particles reducing the titer of vaccine that reaches the small bowel.Citation9,11
The gut microbiome of infants in developing countries differs significantly from that of infants in industrialised countries, with higher rates of bacterial overgrowth, increased parasitic load and “tropical enteropathy.’Citation9,32-34 The interaction of gut microbes with rotavirus vaccine may reduce the titer of vaccine virus available to infect the target cells within the small intestine, where viral replication occurs to induce immune responses. Thus it is possible that the composition of the intestinal microbiota influences vaccine immune responses, and contributes to the decreased vaccine efficacy observed in Africa and Asia.Citation35 Microbes implicated in the reduced effectiveness of oral vaccines such as cholera, polio and rotavirus, are those associated with poor sanitation and increased faecal-oral transmission, such as Klebsiella, Escherichia coli and Bacteroides.Citation36 A study conducted in 2016 also revealed that Bangladeshi infants with detectable enterovirus infection at the time of their first dose of the Rotarix vaccine demonstrated lower rotavirus-specific serum IgA responses, lower rates of seroconversion and higher rates of rotavirus gastroenteritis.Citation37 These findings support the hypothesis that concurrent enteric infections may reduce the immunogenicity and efficacy of rotavirus vaccines.
The oral transmission of breast milk constituents, particularly rotavirus-specific antibodies, to infants around the time of rotavirus immunisation, was thought to be a primary factor for reducing titres of rotavirus vaccines delivered to the small bowel.Citation38,39 However, 2 studies demonstrated no impact of withholding breast feeding around the time of rotavirus vaccination on seroconversion rates in South African or Indian infants.Citation39,40 A more recent study in Mexican infants once again demonstrated that breastfeeding was associated with reduced immune responses to rotavirus vaccines.Citation41 These findings suggest that the inhibitory effect of breast milk constituents while less applicable than originally thought, at least upon humoral immune responses, remain a potential factor behind reduced immunogenicity and efficacy of rotavirus vaccines.
Chronic immunosuppressive disease and malnutrition, particularly deficiencies in micronutrients such as zinc, appear to impair the ability of the host to mount an effective immune response to administered vaccines.Citation42-44 Similarly, zinc supplementation has been shown to reduce the frequency and severity of diarrheal and respiratory illnesses in children.Citation42 The disproportionate burden of HIV, tuberculosis and malaria, in conjunction with a higher incidence of nutritional deficiencies, in developing and low-income regions may also contribute to the reduced immunogenicity and efficacy of rotavirus vaccines in these areas.Citation8,9
Strategies to improve rotavirus vaccine immunogenicity and efficacy in developing countries
Rotavirus vaccines, if priced at levels thought achievable for manufacturers to still make a profit once economies of manufacturing scale have been factored, have been evaluated as a cost-effective intervention in many developing settings.Citation45 Additionally, the improving coverage and timeliness of Expanded Program on Immunisation (EPI) vaccination schedules in most developing settings during early infancy offers promise for trial efficacy results to translate well into effectiveness at the population level, even in areas with poor access to healthcare resources.Citation46
Current approaches in rotavirus vaccine research center on developing new or modified vaccine formulations; studying potential candidates for use as rotavirus vaccine adjuvants; altered scheduling of existing rotavirus immunisations; and increasing the number of vaccine doses.
Development of new rotavirus vaccine candidates
At present, “new generation” rotavirus vaccine candidates utilizing different strains, formulations and routes of administration are under clinical development.Citation47
Vaccine development is still primarily directed toward live attenuated rotavirus vaccines, with multiple candidates currently under study around the world. This is based in part due to the natural history of rotavirus showing that primary infection protects against severe disease in subsequent infection, and the success of the current licensed rotavirus vaccines. Two vaccines derived from strains isolated from newborns have also demonstrated promising results.
One such vaccine, ROTAVAC® (Bharat Biotech, India), is a live-attenuated vaccine derived from a single G9P[11] human-bovine 116E ressortant strain isolated from an Indian child. The vaccine is administered as 3 infant doses commencing at 6-12 weeks of age and was licensed for use in India in early 2014.Citation6 In Phase III trials, ROTAVAC® reduced severe rotavirus gastroenteritis by 56.4% in the first year of life, a similar efficacy rate as that of RV5 and RV1 in developing countries.Citation6
Another neonatal rotavirus vaccine candidate in clinical development, RV3-BB, is based on an attenuated G3P[6] human strain identified in asymptomatic Australian newborns. Phase II safety and immunogenicity trials in New Zealand demonstrated high levels of vaccine take in a developed setting following the administration of 3 doses commencing either in the first week of life or at 6-12 weeks of age.Citation48 Vaccine take was demonstrated in 90% of neonatal participants, and 93% of infant participants.Citation48 Larger trials in Indonesia are examining immunogenicity and efficacy in a developing setting.
Other live attenuated vaccines, based on non-neonatal strains, are in various stages of development. Rotavin-M1™ (human G1P[8] human strain) and the Lanzhou (lamb-derived) monovalent vaccine G10P[12], have been locally licensed in Vietnam and China respectively.Citation47 In addition, Phase III trials are underway in India for the tetravalent bovine-human reassortant rotavirus vaccine (BRV-TV, Serum Institute of India) after Phase IIb trials demonstrated seroconversion rates of 60% following administration of 3 vaccine doses.Citation49-51
Several non-replicating parenteral formulations are also being evaluated in a range of animal models. The inactivated rotavirus particles, protein sub-units or “virus-like particles” (VLPs, structurally-similar to live virus) are being investigated as rotavirus vaccine candidates.Citation52 A candidate P2-VP8 subunit vaccine has been shown to be well-tolerated in human Phase 1 clinical studies.Citation53 It is hoped that these candidates will improve efficacy against severe rotavirus gastroenteritis and be associated with lower manufacturing costs.Citation47
While parenteral vaccine candidates are under clinical development, the mucosal route has additional potential benefits in the context of rotavirus immunization. Oral vaccines are able to induce both mucosal and systemic immune responses necessary for long-term rotavirus immunity.Citation54,55 Live oral vaccines are also associated with conferring herd immunity among unimmunized infants by potential faecal-oral transmission from immunised infants.Citation46,55 Furthermore, “needle-free” administration is preferable for mass rotavirus immunization in low-resource settings where HIV infection, viral hepatitis and other blood-borne communicable diseases remain a risk if needles are reused.Citation56-58
In general, oral vaccines have less demanding logistical requirements in terms of administration and disposal, and can be administered by staff with limited training.Citation46,55,56 In addition, oral vaccines may allow local production of rotavirus vaccines, potentially reducing vaccine cost and further improving access to rotavirus immunization in developing countries.Citation52,56 Conversely, the cold-chain requirements for parenteral vaccines may be less onerous than for oral rotavirus vaccines.
Rotavirus vaccine adjuvants
The use of adjuvants offers a strategy to improve the efficacy and effectiveness of rotavirus vaccines in developing and low-income regions where vaccine take and immunogenicity is reduced. Vaccine adjuvants are compounds, such as salts, oil emulsions and bacterial components, co-administered with a vaccine to stimulate or amplify the host's immune responses to enhance the immunogenicity of the vaccine antigen.Citation17,54,59-61 Vaccine adjuvants such as aluminium salts and oil emulsions were heavily studied for use with the parenteral inactivated polio vaccine (IPV), to attempt to reduce vaccine dose and, hence, improve early schedule completion and decrease vaccine costs.Citation62
However, few adjuvant candidates have been tested for use with live, oral rotavirus vaccines.
Limited studies explored the effect of probiotic adjuvant co-administration with live, oral rotavirus vaccines.Citation35,63 Only one small RRV-TV study in Finnish infants randomized to lactobacillus GG has been able to demonstrate an improvement in IgA seroconversion.Citation35 No other study has demonstrated improved correlates of rotavirus immunity associated with adjuvant use. As such, an effective adjuvant for oral rotavirus vaccines is yet to be developed. However, there is discussion surrounding the possibility of using novel drugs as vaccine adjuvants.
One potential adjuvant that we recently evaluated in a murine model is Nitazoxanide (Alinia®). Nitazoxanide is a broad-spectrum oral antimicrobial that belongs to a novel class of drugs called thiazolides, currently licensed by the US Food and Drug Administration for the treatment of Cryptosporidium and Giardia-related diarrheal diseases.Citation64,65 Nitazoxanide has since been shown to be an effective antiviral against rotavirus and a variety of other viruses in reducing viral load and illness duration.Citation64 Nitazoxanide has been associated with significantly-reduced symptom duration and viral shedding in adults and adolescents with acute uncomplicated influenza in a multi-centered, double-blinded randomized, controlled trial.Citation64,66 Two randomized, placebo-controlled trials also demonstrated that nitazoxanide significantly reduced illness durationCitation67,68 and length of hospitalisationCitation68 in children hospitalised for rotavirus gastroenteritis. At present, a multicentre, double-blind randomized controlled trial is underway to examine oral nitazoxanide in the treatment of acute gastroenteritis in indigenous children in the Northern Territory of Australia.Citation69
Recent studies have supported an immunomodulatory role for nitazoxanide, suggesting its antiviral properties stem from both its capacity to enhance host interferon production as well as direct antiviral action.Citation64,70 Nitazoxanide reduced symptom duration and viral shedding in patients with acute uncomplicated influenza in a recent multi-centered, double-blinded randomized, controlled trial.Citation66,71 Promisingly, serologic immune responses to influenza in patients treated with nitazoxanide were increased, despite apparent reduced viral exposure.
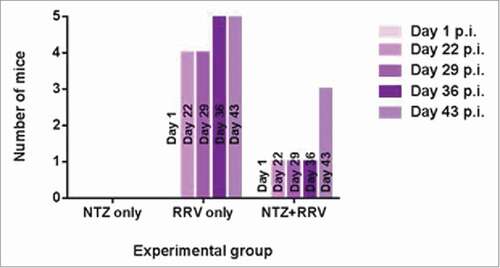
We have previously examined in a murine challenge model whether nitazoxanide affected the immunogenicity of live oral rotavirus doses (unpublished data, Appendix). In an adult mouse model, nitazoxanide therapy administered close to the time of rotavirus immunisation resulted in significantly lower median geometric mean titres of rotavirus-specific serum IgA compared with rotavirus administered alone, and fewer mice developed detectable rotavirus-specific IgA (). These results suggest that unlike with influenza, nitazoxanide does not appear to augment humoral immune responses in a murine model. Further evidence would be needed to support a role for nitazoxanide as a rotavirus vaccine adjuvant.
Changes to rotavirus immunisation schedules
Changes to the immunisation schedule of live oral non-rotavirus vaccines, such as the administration of the oral polio vaccine closer to the time of birth, have been associated with increased rates of seroconversion in some populations.Citation72 However, changes to the schedule for rotavirus immunisation are complicated by the need to establish protection before the period of heightened risk of rotavirus gastroenteritis in early infancy and the increased risk of intussusception observed when older candidate vaccines were commenced after the age of 3 months.Citation2
Early rotavirus vaccine administration close to birth is an attractive potential strategy, and has been trialled in New Zealand, Indonesia and Ghana, with initial results suggesting similar vaccine take to that achieved by infant dosing.Citation7,48 The major potential benefit of vaccines administered at birth is that immune protection may be conferred prior to the early onset of rotavirus infection and the associated risk of severe disease in infants in developing regions, without an increased risk of intussusception.Citation48 However, the immaturity of the neonatal immune system may hinder a full response to vaccines administered at this time. In addition, neonatal dosing regimens are more likely to be successfully delivered to target infants in low-income regions where the timeliness and coverage of EPI vaccinations decreases with each successive scheduled timepoint.Citation17,48,73,74
Increasing the number of rotavirus vaccine doses
Waning vaccine efficacy from one year post-immunisation may be addressed through the administration of additional vaccine doses.Citation75 Lopman et al proposed that extra vaccine doses later in the EPI schedule may target the severe symptomatic nature of higher-order infections in low-income countries, estimating a 9% improvement in RV1 vaccine efficacy with a 3-dose course at 6, 10 and 14 weeks.Citation75 This is supported by a previous study that demonstrated that the same 3-dose RV1 course was efficacious against severe rotavirus gastroenteritis, without an increased risk of intussusception following the third dose.Citation29 The administration of an additional dose at a later age, such as concomitantly with measles-containing vaccines at 9 months of age, may pose a potential solution to the observed decreased efficacy in the 2nd year of life. The development of neonatal vaccine candidates may also present another potential time point for increasing the number of vaccine doses.Citation6,48
Conclusion
While already life-saving in developing settings, improving the immunogenicity and effectiveness of rotavirus vaccines in these settings remains an urgent priority. Why vaccine effectiveness remains low in developing countries remains unknown but may be due to the interplay between the luminal factors, mucosal factors and immune characteristics discussed in this article. A variety of avenues are being pursued to improve our understanding, ranging from the development of novel rotavirus vaccine formulations, exploration of potential vaccine adjuvants, changes to the number of doses or the timing of vaccine doses administered. It is hoped that one or a combination of these approaches will provide insights into disease pathogenesis and provide a key platform that will assist in reducing the toll of rotavirus upon the world's most vulnerable children.
Figure 1. Frequency of mice positive for rotavirus-specific serum IgA per experimental group over complete study duration. Four-week-old, female C57BL/6 mice were administered one of the following: nitazoxanide (NTZ only); Rhesus rotavirus (RRV only); or both the nitazoxanide regime and Rhesus rotavirus (NTZ+RRV). Serum was collected at various timepoints and analyzed for the presence of rotavirus-specific serum IgA. Colored bars correspond to the number of mice positive for rotavirus-specific serum IgA within each experimental group on days 1, 22, 29, 36 or 43 post-immunisation (p.i.). (Refer to Appendix for full description of the Methods employed.)
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Department of Pediatrics, Monash University.
References
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar U. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136-41; PMID:22030330; https://doi.org/http://dx.doi.org/10.1016/S1473-3099(11)70253-5
- World Health Organization. Rotavirus vaccines. WHO position paper- January 2013. Wkly Epidemiol Rec 2013; 88:49-64; PMID:23424730
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. Global, regional, and national estimates of rotavirus mortality in children. Clin Infect Dis 2016; 62:S96-105; PMID:27059362; https://doi.org/http://dx.doi.org/10.1093/cid/civ1013
- World Health Organization. Rotavirus vaccines. Wkly Epidemiol Rec 2007; 82:285-95; PMID:17691162
- World Health Organization. Rotavirus vaccines: an update. Wkly Epidemiol Rec 2009; 84:533-37; PMID:20034143
- Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, Goyal N, Kawade A, Kang G, Rathore SS, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet 2014; 383:2136-43; https://doi.org/http://dx.doi.org/10.1016/S0140-6736(13)62630-6
- Armah GE, Kapikian AZ, Vesikari T, Cunliffe N, Jacobson RM, Burlington DB, Ruiz LP. Efficacy, immunogenicity, and safety of two doses of a tetravalent rotavirus vaccine RRV-TV in Ghana with the first dose administered during the neonatal period. J Infect Dis 2013; 208:423-31; PMID:23599316; https://doi.org/http://dx.doi.org/10.1093/infdis/jit174
- Qadri F, Bhuiyan TR, Sack DA, Svennerholm A. Immune responses and protection in children in developing countries induced by oral vaccines. Vaccine 2013; 31:452-60; PMID:23153448; https://doi.org/http://dx.doi.org/10.1016/j.vaccine.2012.11.012
- Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral rotavirus vaccines: how well will they work where they are needed most? J Infect Dis 2009; 200:S39-48; https://doi.org/http://dx.doi.org/10.1086/605035
- Desselberger U, Huppertz HI. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J Infect Dis 2011; 203:188-95; PMID:21288818; https://doi.org/http://dx.doi.org/10.1093/infdis/jiq031
- Clarke E, Desselberger U. Correlates of protection against human rotavirus disease and the factors influencing protection in low-income settings. Mucosal Immunol 2015; 8:1-17; PMID:25465100; https://doi.org/http://dx.doi.org/10.1038/mi.2014.114
- Greenberg HB, Estes MK. Rotaviruses: from pathogenesis to vaccination. . Gastroenterology 2009; 136:1939-51; PMID:19457420; https://doi.org/http://dx.doi.org/10.1053/j.gastro.2009.02.076
- Banyai K, Laszlo B, Duque J, Steele AD, Nelson EA, Gentsch JR, Parashar UD. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: insights for understanding the impact of rotavirus vaccination programs. Vaccine 2012; 30:A122-30; PMID:22520121; https://doi.org/http://dx.doi.org/10.1016/j.vaccine.2011.09.111
- Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine 2006; 24:2718-31; PMID:16446014; https://doi.org/http://dx.doi.org/10.1016/j.vaccine.2005.12.048
- Patel M, Glass RI, Jiang B, Santosham M, Lopman B, Parashar U. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Dis 2013; 208:284-94; PMID:23596320; https://doi.org/http://dx.doi.org/10.1093/infdis/jit166
- Paul A, Gladstone BP, Mukhopadhya I, Kang G. Rotavirus infections in a community based cohort in Vellore, India. Vaccine 2014; 32S:A49-54; https://doi.org/http://dx.doi.org/10.1016/j.vaccine.2014.03.039
- Cherian T, Wang S, Mantel C. Rotavirus vaccines in developing countries: the potential impact, implementation challenges, and remaining questions. Vaccine 2012; 30S:A3-A6; https://doi.org/http://dx.doi.org/10.1016/j.vaccine.2011.10.007
- Vesikari T, Matson DO, Dennehy P, van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Eng J Med 2006; 354:23-33; PMID:16394299; https://doi.org/http://dx.doi.org/10.1056/NEJMoa052664
- Block SL, Vesikari T, Goveia MG, Rivers SB, Adeyi BA, Dallas MJ, Bauder J, Boslego JW, Heaton PM. Efficacy, immunogenicity, and safety of a pentavalent human-bovine (WC3) reassortant Rotavirus vaccine at the end of shelf life. Pediatrics 2007; 119:11-8; PMID:17200266; https://doi.org/http://dx.doi.org/10.1542/peds.2006-2058
- Goveia MG, DiNubile MJ, Dallas MJ, Heaton PM, Kuter BJ, Team RS. Efficacy of pentavalent human-bovine (WC3) reassortant rotavirus vaccine based on breastfeeding frequency. Pediatr Infect Dis J 2008; 27:656-8; PMID:18520448; https://doi.org/http://dx.doi.org/10.1097/INF.0b013e318168d29e
- Ruiz-Palacios GM, Perez-Schael I, Velázquez FR, Abate H, Breuer T, Costa Clemens S, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Eng J Med 2006; 354:11-22; PMID:16394298; https://doi.org/http://dx.doi.org/10.1056/NEJMoa052434
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370:1757-63; PMID:18037080; https://doi.org/http://dx.doi.org/10.1016/S0140-6736(07)61744-9
- Clark HF, Burke CJ, Volkin DB, Offit P, Ward RL, Bresee JS, Dennehy P, Gooch WM, Malacaman E, Matson D. Safety, immunogenicity, and efficacy in healthy infants of G1 and G2 human reassortant rotavirus vaccine in a new stabilizer/buffer liquid formulation. Pediatr Infect Dis J 2003; 22:914-20; PMID:14551493; https://doi.org/http://dx.doi.org/10.1097/01.inf.0000091887.48999.77
- Clark HF, Bernstein DI, Dennehy PH, Offit P, Pichichero M, Treanor J, Ward RL, Krah DL, Shaw A, Dallas MJ, et al. Safety, efficacy and immunogenicity of a live, quadrivalent human-bovine reassortant rotavirus vaccine in healthy infants. J Pediatr 2004; 144:184-90; PMID:14760258; https://doi.org/http://dx.doi.org/10.1016/j.jpeds.2003.10.054
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606-14; PMID:20692030; https://doi.org/http://dx.doi.org/10.1016/S0140-6736(10)60889-6
- Armah GE, Breiman RF, Tapia MD, Dallas MJ, Neuzil KM, Binka FN, Sow SO, Ojwando J, Ciarlet M, Steele AD. Immunogenicity of the pentavalent rotavirus vaccine in African infants. Vaccine 2012; 30:A86-93; PMID:22520142; https://doi.org/http://dx.doi.org/10.1016/j.vaccine.2011.10.006
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TP, Luby SP, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615-23; PMID:20692031; https://doi.org/http://dx.doi.org/10.1016/S0140-6736(10)60755-6
- Shin S, Anh DD, Zaman K, Yunus M, Mai LTP, Thiem VD, Azim T, Victor JC, Dallas MJ, Steele AD, et al. Immunogenicity of the pentavalent rotavirus vaccine among infants in two developing countries in Asia, Bangladesh and Vietnam. Vaccine 2012; 30:A106-13; PMID:22520119; https://doi.org/http://dx.doi.org/10.1016/j.vaccine.2011.11.091
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Eng J Med 2010; 362:289-98; PMID:20107214; https://doi.org/http://dx.doi.org/10.1056/NEJMoa0904797
- Neuzil KM, Zaman K, Victor JC. A proposed framework for evaluating and comparing efficacy estimates in clinical trials of new rotavirus vaccines. Vaccine 2014; 32:A179-84; PMID:25091673; https://doi.org/http://dx.doi.org/10.1016/j.vaccine.2014.04.074
- Soares-Weiser K, Maclehose H, Bergman H, Ben-Aharon I, Nagpal S, Goldberg E, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev 2012; 11:CD008521
- Khin-Maung U, Bolin TD, Duncombe VM, Myo-Khin Nyunt-Nyunt-Wai, Pereira SP, Linklater JM. Epidemiology of small bowel bacterial overgrowth and rice carbohydrate malabsorption in Burmese (Myanmar) village children. Am J Trop Med Hyg 1992; 47:298-304; PMID:1388002
- dos Reis JC, de Morais MB, Oliva CA, Fagundes-Neto U. Breath hydrogen test in the diagnosis of environmental enteropathy in children living in an urban slum. Dig Dis Sci 2007; 52:1253-8; PMID:17372830
- Salazar-Lindo E, Allen S, Brewster DR, Elliott EJ, Fasano A, Phillips AD, Sanderson IR, Tarr PI. Intestinal infections and environmental enteropathy: Working Group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2004; 39:S662-9; PMID:15184767
- Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine 1995; 13:310-2; PMID:7631519
- Valdez Y, Brown EM, Finlay BB. Influence of the microbiota on vaccine effectiveness. Trends Immunol 2014; 35:526-37; PMID:25113637
- Taniuchi M, Platts-Mills JA, Begum S, Uddin MJ, Sobuz SU, Liu J, Kirkpatrick BD, Colgate ER, Carmolli MP, Dickson DM, et al. Impact of enterovirus and other enteric pathogens on oral polioand rotavirus vaccine performance in Bangladeshi infants. Vaccine 2016; 34:3068-75; PMID:27154394
- Moon SS, Tate JE, Ray P, Dennehy P, Archary D, Coutsoudis A, Bland R, Newell ML, Glass RI, Parashar U, et al. Differential profiles and inhibitory effect on rotavirus vaccines of nonantibody components in breast milk from mothers in developing and developed countries. Pediatr Infect Dis J 2013; 32:863-70; PMID:23584581
- Groome MJ, Moon SS, Velasquez D, Jones S, Koen A, van Niekerk N, Jiang B, Parashar UD, Madhi SA. Effect of breastfeeding on immunogenicity of oral live-attenuated human rotavirus vaccine: a randomized trial in HIV-uninfected infants in Soweto, South Africa. Bull World Health Organ 2014; 92:238-45; PMID:24700991
- Rongsen-Chandola T, Strand TA, Goyal N, Flem E, Rathore SS, Arya A, Winje BA, Lazarus R, Shanmugasundaram E, Babji S, et al. Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine 2014; 32:A134-9; PMID:25091668
- Bautista-Marquez A, Velasquez DE, Esparza-Aguilar M, Luna-Cruz M, Ruiz-Moran T, Sugata K, Jiang B, Parashar UD, Patel M, Richardson V. Breastfeeding linked to the reduction of both rotavirus shedding and IgA levels after Rotarix immunization in Mexican infants. Vaccine 2016; 34:5284-89; PMID:27663670
- Aggarwal R, Sentz J, Miller MA. Role of zinc administration in prevention of childhood diarrhea and respiratory illnesses: a meta-analysis. Pediatrics 2007; 119:1120-30; PMID:17545379
- Sazawal S, Black RE, Menon VP, Dinghra P, Caulfield LE, Dhingra U, Bagati A. Zinc supplementation in infants born small for gestational age reduces mortality: a prospective, randomized, controlled trial. Pediatrics 2001; 108:1280-6; PMID:11731649
- Zhao N, Wang X, Zhang Y, Gu Q, Huang F, Zheng W, Li Z. Gestational zinc deficiency impairs humoral and cellular immune responses to hepatitis B vaccination in offspring mice. PloS One 2013; 8:e73461; PMID:24069198
- Rose J, Hawthorn RL, Watts B, Singer ME. Public health impact and cost effectiveness of mass vaccination with live attenuated human rotavirus vaccine (RIX4414) in India: model based analysis. BMJ 2009; 339:1-12
- Levine MM. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol 2010; 8:129; PMID:20920375
- Status of vaccine research and development of next-generation rotavirus vaccines. WHO Product Development for Vaccines Advisory Committee Meeting. Geneva, Switzerland, 2014
- Bines JE, Danchin M, Jackson P, Handley A, Watts E, Lee KJ, West A, Cowley D, Chen MY, Barnes GL, et al. Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2015; 15:1389-97; PMID:26318715
- Dhingra MS, Kundu R, Gupta M, Kanungo S, Ganguly N, Singh MP, Bhattacharya MK, Ghosh R, Kumar R, Sur D, et al. Evaluation of safety and immunogenicity of a live attenuated tetravalent (G1–G4) Bovine-Human Reassortant Rotavirus vaccine (BRV-TV) in healthy Indian adults and infants. Vaccine 2014; 32S:A117-23
- Bernal A. Shantha's Investigational Rotavirus Vaccine Enters Phase III Clinical Trials in India. Sanofi Pasteur 2014
- Zade JK, Kulkarni PS, Desai SA, Sabale RN, Naik SP, Dhere RM. Bovine rotavirus pentavalent vaccine development in India. Vaccine 2014; 32:A124-8; PMID:25091665
- Estes M, Khan G, Neuzil K, Parashar U, Steele AD. Developing next generation rotavirus vaccines. In: Organization WH, ed. Global Vaccine and Immunization Research Forum. Bethesda, MD 2014
- Fix AD, Harro C, McNeal M, Dally L, Flores J, Robertson G, Boslego JW, Cryz S. Safety and immunogenicity of a parenterally administered rotavirus VP8 subunit vaccine in healthy adults. Vaccine 2015; 33:3766-72; PMID:26065919
- Kim S, Jang Y. Antigen targeting to M cells for enhancing the efficacy of mucosal vaccines. Exp Mol Med 2014; 46:e85; PMID:24626171
- Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med 2005; 11:S45-53; PMID:15812489
- Holmgren J, Czerkinsky C, Eriksson K, Mharandi A. Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine 2003; 21:89-95
- Simonsen L, Kane A, Lloyd J, Zaffran M, Kane M. Unsafe injections in the developing world and transmission of bloodborne pathogens: a review. Bull World Health Organ 1999; 77:789-800; PMID:10593026
- Levine MM. Can needle-free administration of vaccines become the norm in global immunization? Nat Med 2003; 9:99-103; PMID:12514720
- Aguilar JC, Rodriguez EG. Vaccine adjuvants revisited. Vaccine 2007; 25:3752-62; PMID:17336431
- Schijns VE. Mechanisms of vaccine adjuvant activity: initiation and regulation of immune responses by vaccine adjuvants. Vaccine 2003; 21:829-31; PMID:12547589
- Schijns VE. Immunological concepts of vaccine adjuvant activity. Curr Opin Immunol 2000; 12:456-63; PMID:10899018
- Hawken J, Troy SB. Adjuvants and Inactivated Polio Vaccine: A Systematic Review. Vaccine 2012; 30:6971-79; PMID:23041122
- Zhang W, Azevado M, Wen K, Gonzalez A, Saif LJ, Li G, Yousef AE, Yuan L. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine 2008; 26:3655-61; PMID:18524434
- Rossignol JF. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res 2014; 110:94-103; PMID:25108173
- Fox LM, Saravolatz LD. Nitazoxanide: a new thiazolide antiparasitic agent. Clin Infect Dis 2005; 40:1173-80; PMID:15791519
- Haffizulla J, Hartman A, Hoppers M, Resnick H, Samudrala S, Ginocchio C, Bardin M, Rossignol JF. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis 2014; 14:609-18; PMID:24852376
- Rossignol JF, Abu-Zekry M, Hussein A, Santoro MG. Effect of nitazoxanide for treatment of severe rotavirus gastroenteritis diarrhoea: randomised double-blind placebo-controlled trial. Lancet 2006; 368:124-9; PMID:16829296
- Teran CG, Teran-Escalera CN, Villarroel P. Nitazoxanide vs. probiotics for the treatment of acute rotavirus diarrhea in children: a randomized, single-blind, controlled trial in Bolivian children. Int J Infect Dis 2009; 13:518-23; PMID:19070525
- A randomised, placebo-controlled trial of oral nitazoxanide for the empiric treatment of acute gastroenteritis among Australian Indigenous children. In: Council AGNHaMR, ed.: Commonwealth of Australia, 2016
- La Frazia S, Ciucci A, Arnoldi F, Coira M, Gianferretti P, Angelini M, Belardo G, Burrone OR, Rossignol JF, Santoro MG. Thiazolides, a new class of antiviral agents effective against rotavirus infection, target viral morphogenesis, inhibiting viroplasm formation. J Virol 2013; 87:11096-106; PMID:23926336
- Clerici M, Trabattoni D, Pacei M, Biasin M, Rossignol JF. The antiinfective nitazoxanide shows strong immuno-modulating effects [abstract]. J Immunol 2011; 186:21
- World Health Organization. Polio vaccines: WHO position paper, January 2014. Wkly Epidemiol Rec 2014; 89:73-92; PMID:24707513
- Danchin MH, Kirkwood CD, Lee KJ, Bishop RF, Watts E, Justice FA, Clifford V, Cowley D, Buttery JP, Bines JE. Phase I trial of RV3-BB rotavirus vaccine: a human neonatal rotavirus vaccine. Vaccine 2013; 31:2610-6; PMID:23597719
- Clark A, Sanderson C. Timing of children's vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet 2009; 373:1543-9; PMID:19303633
- Lopman BA, Pitzer VE, Sarkar R, Gladstone B, Patel M, Glasser J, Gambhir M, Atchison C, Grenfell BT, Edmunds WJ, et al. Understanding reduced rotavirus vaccine efficacy in low socio-economic settings. PLoS One 2012; 7:e41720; PMID:22879893
Appendix - Methods
Animal handling and experimentation was performed in accordance with institutional guidelines (Monash University AEC no. MARP/2014/052). Four-week-old, female C57BL/6 mice (5 per group) were administered via intragastric gavage one of the following: 2.14 mg of nitazoxanide suspended in 0.5% carboxymethylcellulose sodium solution twice daily for 3 d (NTZ only); single dose of 3.21×108 fluorescent-cell-forming-units of Rhesus rotavirus (RRV only); or both the nitazoxanide regime and single dose of Rhesus rotavirus (NTZ+RRV). Serum was collected from the mice at various timepoints throughout the study via cheek bleeding and analyzed for the presence of rotavirus-specific serum IgA by ELISA which yielded either a negative or positive result. Colored bars correspond to the number of mice positive for rotavirus-specific serum IgA within each experimental group on days 1, 22, 29, 36 or 43 post-immunisation (p.i.).
