ABSTRACT
This study aimed to assess the 1-y immunogenicity of influenza vaccines and the association between immunogenicity at 1 m and further influenza infections in children aged 6 m to 18 y. Serum hemagglutination inhibition (HI) antibody titers and GMTs were determined for the recommended influenza strains 0, 1, 6, and 12 m post-vaccination. The serological evidence of influenza infections were defined as the increase of HI titer (HI ≥1:40 and 4-fold rise). The seroprotection rates for strains A(H1N1), A(H3N2), and B were 91.2%, 87.6%, and 87.6%, respectively, at 1 month (n = 174). These rates were 76.5%, 64.7%, and 54.6%, respectively, at 12 m. The seroprotection rates and GMTs for influenza A(H1N1) and A(H3N2) were higher at 12 m than at 0 m (p < 0.05) but not for B. There were 39 subjects (42 cases) of serological influenza infections. Subjects with seroprotection at 1 m post-vaccination had showed fewer serologic A(H1N1) (10.1 vs 54.5%) and A(H3N2) (7.2 vs 38.1%) infections than the ones with HI titer <1:40 during follow-up (P < 0.01). In conclusion, influenza vaccines used during the 2008-09 season induced adequate 1-y immunogenicity for A(H1N1) and A(H3N2). The immunogenicity at one month after vaccination influenced further serological influenza infections.
One-year immunogenicity of influenza vaccine
This study aimed to assess the one-year immunogenicity of influenza vaccines and the association between immunogenicity at one month and further influenza infections in children aged 6 months to 18 y. Serum hemagglutination inhibition (HI) antibody titers and GMTs were determined for the recommended influenza strains 0, 1, 6, and 12 months post-vaccination. The serological evidence of influenza infections were defined as the increase of HI titer (HI ≥ 1:40 and 4-fold rise). The seroprotection rates for strains A(H1N1), A(H3N2), and B were 91.2%, 87.6%, and 87.6%, respectively, at 1 month (n = 174). These rates were 76.5%, 64.7%, and 54.6%, respectively, at 12 months. The seroprotection rates and GMTs for influenza A(H1N1) and A(H3N2) were higher at 12 months than at 0 month (p < 0.05) but not for B. There were 39 subjects (42 cases) of serological influenza infections. Subjects with seroprotection at 1 month post-vaccination had showed fewer serological A(H1N1) (10.1 vs 54.5%) and A(H3N2) (7.2 vs 38.1%) infections than the ones with HI titer <1:40 during follow-up (P < 0.01). In conclusion, influenza vaccines used during the 2008–09 season induced adequate 1-year immunogenicity for A(H1N1) and A(H3N2). The immunogenicity at one month after vaccination influenced further serological influenza infections.
Introduction
Influenza causes high morbidity and mortality among infants and young children.Citation1-3 Infants who contract influenza from members of the same household have a high mortality rate and risk of influenza-related hospitalization.Citation4,5 Children who have not been previously exposed to influenza virus, either by vaccination or previous natural infection, are more susceptible to infection. Furthermore, school-age children are considered vectors for the spread of influenza at home and in the community.Citation6,7 Thus, the US Advisory Committee on Immunization Practices recommends routine annual immunization with the influenza vaccine for all children aged >6 months.Citation8 In Korea, the A(H1N1), A(H3N2), and B influenza viruses are prevalent during the influenza season, and the Korean Advisory Committee on Immunization Practices recommends routine annual immunization with the influenza vaccine for children aged 6 to 59 months.
It is well known that influenza vaccine-induced antibodies decline over time, especially in younger children.Citation9 The lower immunogenicity and long-lasting immunity in younger children are supposedly the result of an immature immune system or insufficient immunologic priming by a previous vaccination.Citation10,11 In contrast, immunity, i.e. seroprotection rate and geometric mean titer (GMT), to a specific influenza strain increases with age in children. Natural influenza infection has been proposed as one of the causes of increasing seroprotection rates and GMT in children.Citation1 Alternatively, the persistence of vaccine-induced immune responses has also been suggested as the reason for increasing seroprotection rates and GMT in adults, and this hypothesis has been supported by 1- year study.Citation12,13
In the present study, we assessed the 1-year immunogenicity of 2 commercially available influenza vaccines among Korean children. We assessed the association between immunogenicity at one month and further influenza infections, as well as the influence of influenza vaccination/infections on the immunogenicity at 12 months after vaccination.
Results
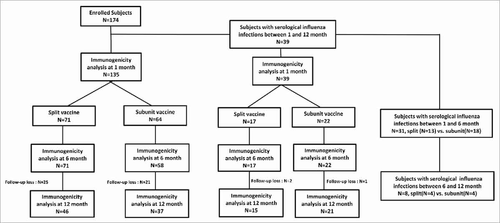
A total of 174 subjects were recruited. The median age of the subjects was 6.3 years, and 50% (n = 87) of the subjects were males. The proportion of subjects who were given a single dose was 145 and 2 doses was 29 (). Thirty-nine subjects (42 cases) had serological evidence of influenza infections and were analyzed separately. Fifty-five subjects withdrew from the study mainly because of follow-up losses caused by reluctance to provide samples, transportation difficulties and migration to other places ().
Table 1. Demographic findings of study subjects
Figure 1. Flow chart of study. Subjects with serological infections were analyzed separately to evaluate the pure vaccine effect.
Overall immunogenicity in all study subjects
The overall immunogenicity in all the subjects was as follows: The seroprotection rates for A(H1N1) at 0, 1, 6, and 12 months were 48.8%, 91.2%, 83.5%, and 76.5%, respectively. Their GMTs for A(H1N1) at 0, 1, 6, and 12 months were 29.7, 161.9, 133.4, and 87.3, respectively. The seroprotection rates for A(H3N2) at 0, 1, 6, and 12 months were 41.2%, 87.6%, 73.8%, and 64.7%, respectively. Their GMTs for A(H3N2) at 0, 1, 6, and 12 months were 21.2, 171.9, 72.8, and 51.1, respectively. The seroprotection rates for B at 0, 1, 6, and 12 months were 51.8%, 87.6%, 68.9%, and 54.6%, respectively. Their GMTs for B at 0, 1, 6, and 12 months were 28.3, 155.6, 42.7, and 32.2, respectively (). We also analyzed 119 subjects who completed 4 rounds of sampling throughout the study period (). The median age of the subjects was 7.0 years, and 52.1% (n = 62) of the subjects were males. The seroprotection rates and GMTs for A(H1N1) at 0, 1, 6, and 12 months were 47.9%/29.4, 90.8%/135.9, 81.5%/130.5, and 76.5%/87.3, respectively. The seroprotection rates and GMTs for A(H3N2) at 0, 1, 6, and 12 months were 42.0%/21.2, 88.2%/168.6, 73.9%/81.4, and 64.7%/51.1, respectively. The seroprotection rates and GMTs for B at 0, 1, 6, and 12 months were 53. 8%/30.8, 89.1%/161.9, 69.7%/46.5, and 54.6%/32.2, respectively. The seroprotection rates and GMTs for influenza A(H1N1) and A(H3N2) were higher at 12 months than at 0 months (pre-vaccination). However, there was no difference in the seroprotection rate and GMT between 0 months and 12 months for B.
Table 2. Immunogenicityof split and subunit influenza vaccines in all subjects
Table 3. Immunogenicity of split and subunit influenza vaccines in all subjects who completed 12-month follow-up
Immunogenicity in the subjects of serological evidence of influenza infections
Thirty-nine subjects (42 cases) had serological influenza infections. Twenty-two subjects showed a ≥ 4 folds increase in influenza A(H1N1) titer (18 during months 1–6, 4 during months 6–12). Nineteen subjects showed a ≥ 4-fold increase in A(H3N2) titer (15 during months 1–6, 4 during months 6–12), and 1 subject showed a ≥ 4-fold increase in B titer. The seroprotection rates for A(H1N1) in the 22 subjects with serological A(H1N1) infection at 0, 1, 6, and 12 months were 50.0%, 72.7%, 86.4%, and 90.5%, respectively. Their GMTs for H1N1 at 0, 1, 6, and 12 months were 31.1, 51.5, 219.3, and 140.2, respectively. The seroprotection rates for A(H3N2) in the 19 subjects with serological A(H3N2) infection at 0, 1, 6, and 12 months were 0.0%, 57.9%, 78.9%, and 82.4%, respectively. Their GMTs for A(H3N2) at 0, 1, 6, and 12 months were 6.0, 26.8, 178.5, and 73.7, respectively ().
Table 4. Immunogenicity against influenza strains in the subjects with serological infections
Immunogenicity in the subjects without serological evidence of infections
The median age of the subjects was 7.8years, and 51.8% (n = 43) of the subjects were males. Immunogenicity in the 83 subjects who completed 4 rounds of sampling among the 135 subjects without serological evidence of infection was as follows. The seroprotection rates for A(H1N1) at 0, 1, 6, and 12 months were 50.6%, 96.4%, 86.8%, and 73.0%, respectively. Their GMTs for A(H1N1) at 0, 1, 6, and 12 months were 32.5, 190.7, 140.0, and 93.8, respectively. The seroprotection rates for A(H3N2) at 0, 1, 6, and 12 months were 50.6, 94.0, 72.3, and 59.0%, respectively. Their GMTs for A(H3N2) at 0, 1, 6, and 12 months were 26.1, 240.9, 69.4, and 43.1, respectively. The seroprotection rates for B at 0, 1, 6, and 12 months were 59.0%, 92.8%, 72.3%, and 56.6%, respectively. Their GMTs for B at 0, 1, 6, and 12 months were 38.4, 187.5, 52.7, and 36.8, respectively (). Subjects who were vaccinated by the split (n = 46) and subunit (n = 37) influenza vaccines showed comparably moderate immunogenicity without significant differences for A(H1N1), A(H3N2) and B. (). The seroprotection rates and GMTs for influenza A(H1N1) were still higher at 12 months than at 0 month (pre-vaccination) in both vaccine groups (p<0.05). Against A(H3N2) and B, there was no significant difference in the seroprotection rates between 0 months and 12 months, and between the 2 vaccines. GMTs for influenza A(H3N2) at 12 months post-vaccination were higher than that at 0 month, totally but no difference between the 2 vaccine groups.
Table 5. Immunogenicity of influenza vaccines in subjects who completed 12-month follow-up excluding the subjects with serological infections
Comparison of immunogenicity between subjects with and without serological infections
Among all study subjects, subjects with seroprotection at 1 month post-vaccination for A(H1N1) had fewer serological evidence of A(H1N1) influenza infections (16/159 = 10.1%) than those with HI titer <1:40 (6/15 = 40%) during follow-up (P < 0.01). For A(H3N2), subjects with seroprotective titer at 1 month had fewer serological evidence of influenza infections than the subjects with HI titer <1:40 (11/153 = 7.2% vs 8/21 = 38.1%, P < 0.01). We compared the seroprotection rate and GMT at 12 months for A(H1N1) and A(H3N2) between the subjects with and without serological infections. Although the immunogenicity was higher in the subjects with infections against both strains (seroprotection rate: 90.5 vs 73.0% for A(H1N1), 82.4 vs 59.0% for A(H3N2); GMT: 140.2 vs 93.7 for A(H1N1), 73.7 vs 43.1 for A(H3N2)), there was no statistical difference (p > 0.05).
Comparison of immunogenicity by age in subjects without serological infections
The subjects were divided into 3 groups according to their age (6 months to 35 months, 3–8 years, and 9–18 years). At 6 months post vaccination, the seroprotection rates for A(H1N1), A(H3N2), and B in children below 3 y were 61.8%, 38.2%, and 26.5%, respectively, whereas the seroprotection rates in children aged 3–8 y were 94.0%, 88.0%, and 78.0%, respectively. At 6 months post vaccination, the GMTs for A(H1N1), A(H3N2), and B in children below 3 y were 66.6, 22.2, and 14.7, respectively, whereas the seroprotection rates in children aged 3–8 y were 178.8, 88.2, and 55.8, respectively. Overall, the seroprotection rates for A(H1N1) 12 months post-vaccination were higher than the baseline values (0 month) in all age groups (P < 0.01). The seroprotection rates for A(H3N2) 12 months post-vaccination were higher than the baseline values in all age groups without any statistical significance. Throughout the study, the children who were below 3 y showed lower seroprotection rates than children aged ≥ 3 y ().
Table 6. Immunogenicity of influenza vaccines by age groups excluding the subjects with serological infections
Discussion
In this study, we found that commercially available influenza vaccines provided an adequate long-term immunity to influenza A(H1N1) and A(H3N2). One-year immunity was declined but higher compare with baseline. However, the immunogenicity for influenza B (Yamagata lineage) after one year were not higher than the baseline. A possible explanation for this could be that the study subjects might have had less exposure to Yamagata lineage because Victoria lineage was included as the vaccine strain for 2 consecutive seasonsCitation14 (2006–2007 and 2007–2008) with the same components of A(H1N1) and A(H3N2). A lesser exposure and stimulation against the strain may influence on long-term immunity.
The protective immunity induced by influenza vaccination may persist for up to 1 y after vaccination in a subset of children and adolescents. There are several vaccine studies which show 1-year immunity after influenza vaccination. One of the study proved virosomal influenza vaccines may induce protective immunity for at least 1 y after vaccination in elderly subjects.Citation12 There have been few studies on long-lasting immunity of influenza vaccines in children. Pandemic influenza vaccines were suggested to provide seroprotective antibody levels against A/H1N1 influenza disease for up to 1 y after immunization in children.Citation13,15 This paper is the first to study and compare the immunogenicity of 2 different inactivated influenza vaccines for one year in healthy children. When we considered pure vaccine effects, there was no difference of immunity between split and subunit vaccines in the subjects without serological infections during a one year follow up. We found that subjects with a seroprotective antibody titer exhibited fewer A(H1N1) and A(H3N2) serological evidence of influenza infections (A(H1N1), 10.7% vs. 40.0%; A(H3N2), 7.2% vs. 38.1%). Vaccine-induced hemagglutination inhibition (HI) antibody titers, measured against influenza antigens from strains causing disease, are good surrogate markers of clinical efficacy. HI antibody titers of ≥ 1:40 can be considered as the protection threshold beyond which it is unlikely that development of serious illness will occur.Citation16 In a meta-analysis of randomized clinical trials evaluating the efficacy of influenza vaccine in healthy children, the reductions in the risk of laboratory-confirmed influenza cases were estimated to be 60–82% from vaccination and 33% from clinical influenza-like illness.Citation17 Effectiveness of inactivated influenza vaccines could be varied with antigenic match.Citation18 and vaccine efficacy can be as high as 86% when the vaccine and circulating viruses are well matched.Citation19 In our study, each vaccine was well matched with the circulating influenza viruses.Citation20
The seroprotection rates for A(H1N1) and A(H3N2) in this study increased by 27.7% and 23.5%, respectively, in the year after vaccination (). These increases may be due to natural influenza infections and immunogenic persistence of influenza vaccines. Infections were not due to pandemic H1N1 2009 influenza virus because this study was performed before the virus was emerged in 2009. Natural influenza infection, defined by serological testing, occurred in 22.4% (39/174) of study subjects, most of whom showed seroprotection for each strain at 12 months. Although common HI antibody titers are measured at 3 or 4 weeks after vaccination generally, HI titers may increase up to 6 weeks post-vaccination.Citation21 Therefore rising in HI titer might partly cause higher infection rate in this study. The overall annual influenza infection rate is currently estimated to be 10–20% of the world's population, and approximately 10% of children acquire seroprotection by infection annually.Citation1 The seroconversion rate of the pandemic H1N1 2009 influenza by natural infection in community was 12.2%.Citation22 Furthermore, only 3.5% and 7% of infected patients had ≤ 4-fold decline of antibody titer as measured by HI assays.Citation23 The durability of the antibody may be because of the prolonged circulation of antibody-secreting cells.Citation24
Our results show that children below 3 y showed lower seroprotection rates and GMTs 1 month post-vaccination and decreased immunogenic persistence thereafter compare with children over 3 y. The seroprotection rates and GMTs of children below 3 y declined significantly within 5 month after 1 month post-vaccination. It is generally accepted that vaccine-induced seroprotection and GMTs decrease over time and that lower seroprotection rates and GMTs are observed in young children and elderly people. Palache et al. suggested that the lower seroprotection rates in children below 3 y might be due to insufficient immunologic priming by a previous vaccination.Citation11 The higher basal seroprotection rates and GMTs in children ≥ 3 y in this study might be influenced by the cumulative effect of previous natural influenza infections and influenza vaccination.
There are several limitations in this study. First, we could not confirm influenza infection by culture or by PCR. However, it is commonly accepted that seroconversion or an HI titer increase greater than 4-fold is indicative of a natural influenza infection. There is a probability of exaggeration in infection rate by delayed serological response to the vaccine. Second, we could not compare the immunogenicity including basal HI titers between vaccine-primed subjects and vaccine naïve subjects because of higher coverage rates of influenza vaccination in 2007–08. Only 33 (18.9%) of the subjects in this study were influenza vaccine naïve, as the influenza vaccine coverage rates in South Korea had been quite high (84.8%) in the risk group.Citation25
In conclusion, influenza vaccines in this study provided an adequate 1-year immunity to influenza H1N1 and H3N2 without differences between split and subunit vaccines in children and adolescents. The immunogenicity at one month after vaccination influenced further serological influenza infections. This study helps to understand the influence of influenza vaccinations and infections on one-year immunity, and the relationship between HI titer at one month after vaccination and further influenza infections. In the future, a study of large scale to fully evaluate the impact of influenza vaccination/natural infections on the long-term immunity in children is needed.
Materials and methods
Study design and participants
Between October 2008 and January 2010, we conducted an observational open label multi-center study to assess the immunogenicity of the influenza vaccine and its persistence in children up to 18 y of age. The study was performed at 6 hospitals in Seoul and its adjacent area, Gyeonggi-do. All participants were healthy, without chronic medical disease or acute febrile illness. The study protocol was approved by the institutional review board of each hospital. Written informed consent was obtained from parents of all subjects before participation in the study. For each subject, serum samples were obtained prior to vaccination (baseline) and at 30 days, 6 months, and 12 months after vaccination. Blood samples were immediately stored at −70°C until required for testing.
Vaccines
Healthy children aged 6 months to 18 y randomly received split or subunit vaccines according to vaccine assignment to each hospital. Subjects were vaccinated with either a trivalent inactivated split influenza vaccine (Vaxigrip®, Aventis Pasteur, Lyon, France) or a trivalent inactivated subunit influenza vaccine (SK influenza trivaccine®, Agrippal S1, Chiron Vaccines, Siena, Italy). Vaccines were injected a single dose or as 2 doses with a 1-month interval for unprimed subjects younger than 9 y getting vaccinated for the first time and those who have only previously gotten one dose of vaccine, as a dose of 0.25 ml for those aged 6 months to 3 years, or as a dose of 0.5 ml for those aged 3–18 y. Both vaccines contained the A/Brisbane/59/2007IVR-148 (H1N1), A/Uruguay/716/2007NYMCX-175C (H3N2), and B/Florida/4/2006 (B Yamagata lineage), as recommended by the WHO for the 2008–2009 influenza season in the northern hemisphere.
Immunogenicity assessments
All samples were subjected to a HI test using chicken erythrocytes for the A/Brisbane/59/2007 IVR-148 (H1N1), A/Uruguay/716/2007 NYMCX-175C (H3N2), and B/Florida/4/2006 strains.Citation26 For the HI test, serum was treated with a receptor-destroying enzyme (RDEII, Denka Seiken Co., Ltd.) at an initial dilution of 1:10. The samples were titrated to determine absolute endpoint titers. HI antibody was titered to a maximum of 1:2,560. Seroprotection was defined as a titer of ≥ 1:40.Citation27 Recently, cutoff value of 1:110 was recommended to predict 50% clinical protection rate in ≤ 6-year-old children.Citation28 However, we used cutoff value of 1:40 as seroprotective titer in this study because older children and adolescents were included. GMT and titer increases were calculated with 95% confidence intervals (CIs).
The serological evidence of influenza infections were defined as either a change from a titer of < 1:10 at 1 month or 6 months post-vaccination to a titer of ≥ 1:40 at the 6-month or 12-month endpoint, or a 4-fold or greater increase in titer in those with a 1-month or 6-month titer of ≥ 1:10. After completing HI testing of the collected samples, we divided the participants into a serological evidence of influenza infection group and a control group (no serological influenza infection) to assess the change in immunogenicity due to vaccination.
Statistical analysis
Descriptive data are reported as numbers of subjects or geometric means with 95% CIs. To compare the seroprotection rates between the 2 vaccine types (split vaccine vs subunit vaccine) and the 2 periods (0 month vs 12 months), Chi-squared test was used. GMT was compared between them using the Student-t test. The analysis of variance for repeated measures was used to assess the variation of the HI antibody titers over time. Seroprotection rates at 1 and 6 months and at 6 and 12 months after vaccination were compared using the McNemar test and paired t-test (based on an increase in GMT). Group differences for serological evidence of influenza infections were analyzed using the t-test. P-values of < 0.05 were considered to indicate statistical significance (2-tailed test). Seroprotection rates and GMTs were compared among 3 age groups using ANOVA multiple comparison test. SPSS version 12.0 (SPSS Inc., Chicago, IL) was used for the statistical analyses.
Abbreviations
| GMT | = | Geometric mean titer |
| HI | = | Hemagglutination inhibition |
| CI | = | Confidence interval |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author's contributions
EKK, BWE, NHK and YKK participated in data collection and data analysis. EKK, JSL and DHK have been involved in design of the study.
Funding
This study was supported by the Korea Food & Drug Administration (09122KFDA426).
References
- Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000; 342:225-31; PMID:10648763; https://doi.org/http://dx.doi.org/10.1056/NEJM200001273420401
- Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, Black S, Shinefield H, Fukuda K. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000; 342:232-9; PMID:10648764; https://doi.org/http://dx.doi.org/10.1056/NEJM200001273420402
- Neuzil KM, Zhu Y, Griffin MR, Edwards KM, Thompson JM, Tollefson SJ, Wright PF. Burden of interpandemic influenza in children younger than 5 years: a 25-year prospective study. J Infect Dis 2002; 185:147-52; PMID:11807687; https://doi.org/http://dx.doi.org/10.1086/338363
- Chiu SS, Lau YL, Chan Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, Simmerman JM, Gordon A, Sato M, Howie S, Krishnan A, Ope M, Lindblade KA, Carosone-Link P, Lucero M, Ochieng W, Kamimoto L, Dueger E, Bhat N, Vong S, Theodoratou E, Chittaganpitch M, Chimah O, Balmaseda A, Buchy P, Harris E, Evans V, Katayose M, Gaur B, O'Callaghan-Gordo C, Goswami D, Arvelo W, Venter M, Briese T, Tokarz R, Widdowson MA, Mounts AW, Breiman RF, Feikin DR, Klugman KP, Olsen SJ, Gessner BD, Wright PF, Rudan I, Broor S, Simões EA, Campbell H. Global burden of respiratory infections due to seasonal influenza in young children: A systematic review and meta-analysis Lancet. 2011; 378(9807):1917-30; https://doi.org/https://doi.org/10.1016/S0140-6736(11)61051-9. Epub 2011 Nov 10.
- Chiu SS, Lau YL, Chan KH, Wong WH, Peiris JS. Influenza related hospitalizations among children in Hong Kong. N Engl J Med 2002; 347:2097-103; PMID:12501221; https://doi.org/http://dx.doi.org/10.1056/NEJMoa020546
- Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med 2001; 344:889-96; PMID:11259722; https://doi.org/http://dx.doi.org/10.1056/NEJM200103223441204
- Wright P. Influenza in the family. N Engl J Med 2000; 343:1331-2; PMID:11058679; https://doi.org/http://dx.doi.org/10.1056/NEJM200011023431808
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, et al. Centers for Disease Control and Prevention (CDC), Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1-62; PMID:20689501
- Zuccotti G, Amendola A, Viganò A, Pariani E, Zappa A, Pogliani L, Giacomet V, Savarino A, Podestà A, Rottoli A, et al. Long-term immunogenicity of a virosomal subunit inactivated influenza vaccine in children with asthma. Vaccine 2007; 25:6692-8; PMID:17697730; https://doi.org/http://dx.doi.org/10.1016/j.vaccine.2007.07.013
- Clerici M, DePalma L, Roilides E, Baker R, Shearer GM. Analysis of T helper and antigen-presenting cell functions in cord blood and peripheral blood leukocytes from healthy children of different ages. J Clin Invest 1993; 91:2829-36; PMID:8514891; https://doi.org/http://dx.doi.org/10.1172/JCI116526
- Palache AM, Beyer WE, Luchters G, Volker R, Sprenger MJ, Masurel N. Infuenza vaccines : the effect of vaccine dose on antibody response in primed populations during the ongoing interpandemic period. A review of the literature. Vaccine 1993; 11:892-908
- de Bruijn IA, Nauta J, Cramer WC, Gerez L, Palache AM. Clinical experience with inactivated, virosomal influenza vaccine. Vaccine 2005; 23:S39-49; https://doi.org/http://dx.doi.org/10.1016/j.vaccine.2005.04.020
- Nassim C, Christensen S, Henry D, Holmes S, Hohenboken M, Kanesa-Thasan N. Identification of antigen and adjuvant doses resulting in optimal immunogenicity and antibody persistence up to 1 year after immunization with a pandemic A/H1N1 influenza vaccine in children 3 to < 9 years of age. Pediatr Infect Dis J 2012; 31:e59-65; PMID:22418661; https://doi.org/http://dx.doi.org/10.1097/INF.0b013e31824b9545
- World Health Organization. WHO recommendations on the composition of influenza virus vaccines in the 2006–2007 season and 2007–2008 season. Available at: http://www.who.int/influenza/vaccines/2007northreport.pdf, http://www.who.int/influenza/vaccines/20078anorthreport.pdf [accessed on 07 Dec 2016]
- de Whalley P, Walker W, Snape MD, Oeser C, Casey M, Moulsdale P, Harrill C, Andrews N, Hoschler K, Thompson B, et al. A 1-year follow-on study from a randomised, head-to-head, multicentre, open-label study of two pandemic influenza vaccines in children. Health Technol Assess 2011; 15:1-128; https://doi.org/http://dx.doi.org/10.3310/hta15450
- Brydak LB, Machala M. Humoral immune response to influenza vaccination in patients from high risk groups. Drugs 2000; 60:35-53; PMID:10929929; https://doi.org/http://dx.doi.org/10.2165/00003495-200060010-00004
- Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2008; Apr 16(2):CD004879; PMID:18425905; https://doi.org/https://doi.org/10.1002/14651858.CD004879.pub3
- Belongia EA, Kieke BA, Donahue JG, Greenlee RT, Balish A, Foust A, Lindstrom S, Shay DK; Marshfield Influenza Study Group. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J Infect Dis 2009; 199:159-67; PMID:19086915; https://doi.org/http://dx.doi.org/10.1086/595861
- Bridges CB, Thompson WW, Meltzer MI, Reeve GR, Talamonti WJ, Cox NJ, Lilac HA, Hall H, Klimov A, Fukuda K. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trial. JAMA 2000; 284:1655-63; PMID:11015795; https://doi.org/http://dx.doi.org/10.1001/jama.284.13.1655
- Korea Centers for Disease Control and Prevention. Korean Influenza Surveillance Report, 2008–2009. Available at: http://cdc.go.kr/CDC/cms/content/mobile/77/12377_view.html [accessed on 07 Dec 2016]
- Rastogi S, Gross PA, Bonelli J, Dran S, Levandowski RA, Russo C, Weksler ME, Kaye D, Levison M, Abrutyn E, et al. Time to peak serum antibody response to influenza vaccine. Clin Diagn Lab Immunol 1995; 2:120-1; PMID:7719904
- Bone A, Guthmann JP, Assal A, Rousset D, Degeorges A, Morel P, Valette M, Enouf V, Jacquot E, Pelletier B, et al. Incidence of H1N1 2009 virus infection through the analysis of paired plasma specimens among blood donors, France. PLoS One 2012; 7:e33056; PMID:22457734; https://doi.org/http://dx.doi.org/10.1371/journal.pone.0033056
- Chan KH, To KK, Hung IF, Zhang AJ, Chan JF, Cheng VC, Tse H, Che XY, Chen H, Yuen KY. Differences in antibody responses of individuals with natural infection and those vaccinated against pandemic H1N1 2009 influenza. Clin Vaccine Immunol 2011; 18:867-73; PMID:21411604; https://doi.org/http://dx.doi.org/10.1128/CVI.00555-10
- Jones PD, Ada GL. Persistence of influenza virus-specific antibody-secreting cells and B-cell memory after primary murine influenza virus infection. Cell Immunol 1987; 109:53-64; PMID:3498544; https://doi.org/http://dx.doi.org/10.1016/0008-8749(87)90291-7
- Kee SY, Lee JS, Cheong HJ, Chun BC, Song JY, Choi WS, Jo YM, Seo YB, Kim WJ. Influenza vaccine coverage rates and perceptions on vaccination in South Korea. J Infect 2007; 55:273-81; PMID:17602750; https://doi.org/http://dx.doi.org/10.1016/j.jinf.2007.04.354
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2008–2009 influenza season. Available at: http://apps.who.int/iris/bitstream/10665/241112/1/WER8309_81-87.PDF [accessed on 07 Dec 2016]
- Committee for Proprietary Medicinal Products (CPMP). Note for guidance on harmonisation of requirements for influenza vaccines (CHMP/BWP/214/96); adopted March 1997. London, UK: The European Agency for the Evaluation of Medicinal Products 1997
- Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, Tsai T, Clemens R, Rappuoli R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011; 30:1081-5; PMID:21983214; https://doi.org/http://dx.doi.org/10.1097/INF.0b013e3182367662
