ABSTRACT
Tuberculosis (TB) remains a major health problem worldwide, and the development of effective vaccines is urgently needed. Vaccination strategies based on heterologous prime–boost protocols using Mycobacterium bovis bacillus Calmette-Guérin (BCG) as primer and modified vaccinia virus Ankara strain expressing the mycobacterial antigen Ag85A (MVA85A) as booster may increase the protective efficacy of BCG. In addition, vaccination with the recombinant viral vaccine vesicular stomatitis virus (VSV)-846 (Rv3615c, Mtb10.4, and Rv2660c) can elicit a remarkable T-cell-mediated immune response and provide an effective long-term protection after the BCG challenge. In this study, we used VSV-846 to boost BCG and evaluated its immunogenicity in BALB/c mice. In this prime–boost approach, boosting with VSV-846 significantly enhanced IFN-γ CD4 T cell responses, which are crucial for anti-TB immune responses. Moreover, VSV-846 boosting significantly reduced pathology compared with mock vaccination, and decreased the bacterial loads in lung tissues compared with BCG or VSV-846 vaccination alone. The analysis of vaccine-induced immunity identified that polyfunctional T cells might contribute to the enhanced protection by VSV-846 boosting. This study proved that viral booster VSV-846 in mice improved the protection against mycobacteria infection, which could be helpful in designing an efficient vaccination strategy against TB in humans.
Introduction
Tuberculosis (TB) is a chronic respiratory infectious disease that has been a major health problem in humans for many years. Recent estimates suggest that 1.4 million people die from TB each year, with the majority of cases occurring in Africa and Southeast Asia.Citation1 Bacillus Calmette–Guérin (BCG), a live attenuated strain of Mycobacterium bovis (M. bovis), was developed nearly a century ago. It is widely administered to newborns or young infants in most world areas where TB is endemic.Citation2 BCG protects children against meningeal and military TB efficiently; however, its protective efficacy varies from 0% to 80% in numerous field trials and is unclear in pulmonary TB in adults.Citation3 Nevertheless, BCG is the only licensed vaccine against TB. New TB vaccines are developed via two basic ways. The first aims to replace the current BCG with recombinant BCGCitation4 or genetically attenuated Mycobacterium tuberculosis (M.tb).Citation5 The second intends to create better TB vaccines by developing subunit vaccines, which are mostly based on recombinant proteins mixed with proper adjuvants, or by using attenuated viral vectors.Citation4,Citation6,Citation7 Many people of the world have been immunized with BCG due to the immunization program. However, revaccination strategies, involving homologous BCG boosting have shown limited protective efficacy in several clinical trials.Citation8-11 As a live bacterium, BCG can also be disseminated and cause diseases, especially in patients with primary immunodeficiency; thus, the World Health Organization does not recommend revaccination with BCG.Citation12 Therefore, developing a novel subunit vaccine as booster in addition to BCG priming would be a valuable approach in enhancing BCG protection.
Boosting with a heterologous vector is highly effective for increasing both humoral and cellular immunity.Citation13 Heterologous prime–boost strategies, based on the combination of BCG with DNA vaccines, proteins, or live attenuated viruses, have been developed to improve the efficacy of BCG vaccination against TB.Citation14-16 Boosting with recombinant attenuated viruses with BCG priming has shown potential in animal models. In particular, several boosting vaccines have entered early clinical trials.Citation20 This boosting includes modified vaccinia virus Ankara (MVA) that expresses the mycobacterial protective antigen Ag85A (designated as MVA85A) Citation17,Citation18 However, it failed to generate efficacy against M.tb infection or disease based on a recent clinical trial in healthy adults infected with human immunodeficiency virus (HIV)-1.Citation19 We previously generated a recombinant vesicular stomatitis virus (VSV)-based vaccine VSV-846. This vaccine contains a well-defined triple-antigen fusion TFP846 (Rv3615c, Mtb10.4, and Rv2660c), which has been confirmed to induce robust T-cell-mediated immune responses after vaccination.Citation21 In the current study, we investigated whether VSV-846 boosting can improve the protection imparted by BCG against mycobacterial infection.
Results
Boosting with VSV-846 increased IFN-γ responses
Cellular immune responses are critical in controlling M.tb infection.Citation22 Groups of BALB/c mice were intranasally inoculated with 106 plaque-forming unit (PFU) of VSV-846 in a single dose after BCG priming (). The IFN-γ immune response induced by the viral vector vaccine VSV-846 was tested at week 18 using IFN-γ enzyme-linked immunosorbent assay (ELISA). Although immunization with BCG or VSV-846 alone induced high levels of IFN-γ compared with phosphate buffered saline (PBS) mock immunization, mice with VSV-846 boosting after BCG priming secreted the most IFN-γ among the groups (). IFN-γ secreting CD4+ T cells are important in M.tb infection and disease prevention.Citation23 We then detected the IFN-γ+CD4+ T cells, and the results showed that they were the most abundant in the spleen of mice with BCG priming and VSV-846-boosting vaccination (). However, the IFN-γ+CD8+ T cells in the VSV-846-boosting group did not increase compared with BCG or VSV-846 immunization by flow cytometry (data not shown). Our results indicated that the viral vaccine VSV-846 might enhance strong cellular immunity after BCG priming.
Figure 1. Timeline of animal vaccination, infection, and detection. Groups of BALB/c mice (n = 6 per group) were intramuscularly administered with BCG (106CFU) or intranasal inoculation of 106 PFU VSV-846 as single-dose vaccination. For prime-boost approach, mice were first primed with 106 CFU BCG at week 0 and then boosted with 106 PFU VSV-846 at week 12. Immune response detection, BCG challenge, bacterial loads, and pathological detection were applied at indicated time points.
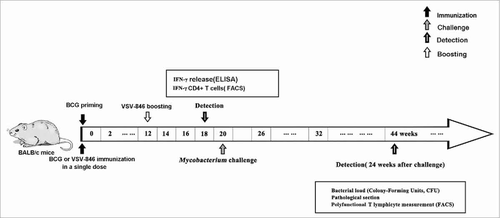
Figure 2. Increased cellular immune responses by VSV-846 boosting. The splenocytes of immunized mice (n = 6 per group) were isolated at week 18. (A) Antigen-specific IFN-γ concentration in cell culture medium was measured by ELISA; and (B) the proportion-specific IFN-γ+ CD4+ T cells in splenocytes were quantified by flow cytometry. The results were expressed as the mean ± SD from three independent experiments. #P < 0.05, ##P < 0.01, ###P < 0.001.
VSV-846 boosting reduced bacterial load and improved pathology after BCG challenge
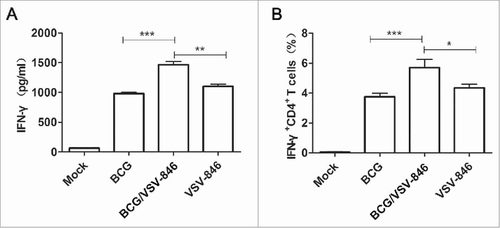
To investigate the protection of different immunization strategies, we further challenged the immunized mice with 107 colony-forming unit (CFU) BCG in the aerosol route and determined the bacterial loads in lungs 24 weeks after the challenge. The pathology of lung tissues was analyzed. Sections from the mock PBS-immunized mice were exhibited, and severe interstitial pneumonia, inflammation, and diffused granuloma-like responses were observed after BCG infection. The mice immunized with VSV-846, BCG priming/VSV-846 boosting, and BCG showed considerably reduced inflammation and intact alveolar morphology, indicating that lung injury was alleviated after the BCG challenge (). The vaccination with VSV-846 boosting efficiently controlled the bacterial growth by ∼199.5-fold lower than the mock PBS vaccination and 3.7-fold lower than the VSV-846 single immunization (, P < 0.001, and P < 0.05). This result suggested that BCG priming/VSV-846 boosting vaccination might induce a better long-term protection against bacterial infection compared with VSV-846 or BCG vaccination.
Figure 3. BCG priming and VSV-846 boosting enhanced long-term protection after BCG infection. (A) Pathology of hematoxylin and eosin-stained lung tissues 24 weeks after the BCG challenge. (B) Bacterial loads in the lung tissues of different groups of mice 24 weeks after the BCG challenge. The results were expressed as the mean ± SD (n = 6) from three independent experiments. #P < 0.05, ##P < 0.01, ###P < 0.001.
VSV-846 boosting significantly increased the percentage of polyfunctional Th1 cells
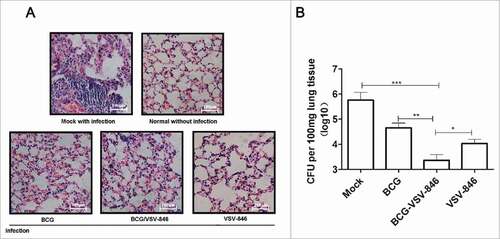
Multifunctional T cells that simultaneously produce IFN-γ, IL-2, and TNF-α are associated with a good clinical outcome of TB.Citation24,Citation25 We investigated the multifunctional T cells induced by VSV-846 boosting through intracellular staining 24 weeks after the challenge. The percentage of single-positive IFN-γ, IL-2, or TNF-α CD4+ T producer did not show a significant difference in the presence or absence of VSV-846 boosting among these groups. However, the total double-positive polyfunctional (IL-2+IFN-γ+, TNF-α+IL-2+ and TNF-α+ IFN-γ+) CD4 T cells (1.09%) were considerably higher after VSV-846 boosting than those after BCG or VSV-846 single immunization. The percentage of triple-positive TNF-α+IL-2+ IFN-γ+ CD4 T cells induced with BCG or BCG priming/VSV-846 boosting was low compared with those of single- or double-positive polyfunctional CD4 T cells (∼0.05%; ).
Figure 4. BCG priming and VSV-846 boosting increased the percentage of polyfunctional (T)cells. Mouse splenocytes 24 weeks after the BCG challenge were isolated and the cells were stained with specific cytokine antibodies and subjected to flow cytometry. (A) Percentage of polyfunctional T cells producing IFN-γ, TNF-a, or IL-2 in total CD4+ T cells. (B) Pie chart analysis of proportions of all single-, double-, and triple-positive CD4+ T cells. Results were expressed as the mean ± SD (n = 6) from three independent experiments. ###P < 0.001.
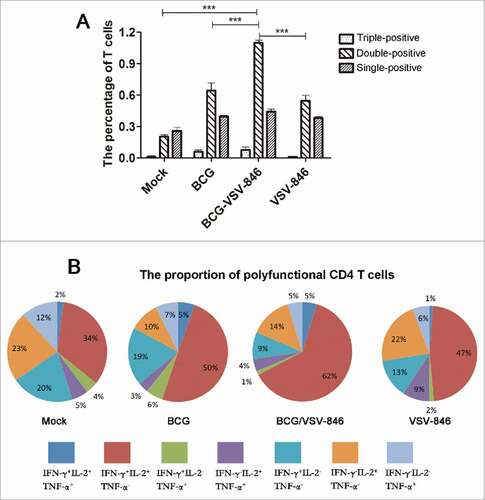
The pie chart analysis of the total percentage of IFN-γ, IL-2, and TNF-α single-, double-, and triple-producing T cells indicated that the proportion of IL-2+IFN-γ+ CD 4 T cells with VSV-846 boosting was markedly increased to 62%, which was higher than the percentage in BCG (50%) or VSV-846single immunization (47%; ). The total percentage of polyfunctional CD4 T cells, including double- and triple-positive CD4 T cells with VSV-846 boosting, was 72%, which was higher than that of the cells with BCG immunization (64%) or VSV-846immunization (59%; ). These results suggested that the polyfunctional T cells might perform an important function in the long-term protection against mycobacterium infection.
Discussion
Reverse genetics has shown that VSV is an excellent vector for delivering foreign antigens inserted in the VSV genome.Citation26 Recombinant VSV has been successfully developed for various vaccine candidates, such as HIV, and severe acute respiratory syndrome, hepatitis B, and influenza viruses.Citation27-30 The VSV viral vector offers certain advantages in vaccine delivery. Our previous study showed that intranasal immunization with a VSV-based vaccine induces preferable mucosal immune responses.Citation31 Specifically, the vector can induce robust humoral and cell-mediated Th1 immunity with a single dose in the absence of additional adjuvants, which is considered crucial in the defense against M.tb.Citation32,Citation33 In the present study, we demonstrated for the first time that VSV-846 boosting after BCG immunization could significantly enhance the immune protection against mycobacterium infection.
We showed that the triple-antigen fusion in a naked DNA plasmid could elicit a robust immune response against mycobacterium infection.Citation21 The selection and use of Rv3615c, M.tb10.4, and Rv2660c antigens was based on previous studies.Citation34-36 Both Rv3615c and M.tb10.4 are abundant in T cell epitopes that promote strong T cell immune responses, including effector T cell subsets secreting IFN-γ and IL-234. Rv2660c is stably expressed in the multistage of M.tb infection, and it enhances protective immunity significantly characterized by a high proportion of multifunctional CD4+ T cells against M.tb.Citation35,Citation37 A strong cellular immune response was induced even with a single dose of VSV-846 immunization, as shown by increased IFN-γ release and IFN-γ+ CD4 T cells. VSV-846 boosting significantly enhanced BCG-elicited cellular immune responses. Accordingly, the bacterial load in the lung tissues with VSV-846 boosting decreased by ∼199.5-fold compared with that of mock PBS-immunized mice 24 weeks after the challenge.
Introducing other heterologous vaccines as boosters is one of the approaches to induce efficient cellular immunity by enhancing the apparent waning immunological memory.Citation38 The antigen-specific IL-2/TNF-a/IFN-γ- producing polyfunctional CD4+T cells are reportedly implicated in anti-TB immune protection.Citation39-41 In our study, the subset of IL-2+IFN-γ+ double-positive CD-4 T cells was increased in the VSV-846 boosting-group compared with other groups. This increase might be associated with the enhanced protection, because a recent study has shown that the enhanced protective efficacy against M.tb, achieved by the BCG prime–DNA boost regimen, is associated with increased splenic IL-2-producing CD4 T cells.Citation42 These results are expected because CD4 T cells that secrete IL-2 may serve as a reservoir of memory T cells with effector potential for a rapid recall response.Citation43,Citation44 Recently, a different prime-boost anti-TB vaccine model has also confirmed that IL-2-secreting CD4+ T cell subsets represent central memory T cells that can continuously replenish T cell populations and are associated with enhanced control of bacterial growth.Citation45 Thus, the VSV-846 vaccine appears to boost the BCG-primed immune responses by enhancing the multifunctional IL-2/IFN-γ-secreting CD4+ T cells, which might improve the protection against TB.
Bladder cancer patients treated with intravesical BCG therapy might be infected with systemic BCGitis—a severe systemic BCG infection, suggesting that M. bovis BCG might cause mycobacterial dissemination similar to M.tb in human.Citation46 BCG has been used in mycobacterial-infected animal models in multiple reports because of its safety and practicality.Citation47,Citation48 However, our results would be more convincing if mice had been challenged using M.tb strain in the study. Additionally, although we found IFN-γ immune response was enhanced by VSV-846 boosting and contributed to the protection against BCG infection, we could not exclude the function of other cytokines such as IL-17, which primarily attracts and activates neutrophils to the site of granuloma formation and contribute to protection against TB at early stages.Citation46,Citation48
In conclusion, our results indicated that heterologous boosting with VSV-846 after BCG immunization enhanced the cellular immune responses and protection against mycobacterium infection. BCG priming/VSV-846 boosting should be further investigated as a potential vaccine immunization strategy against M.tb.
Materials and methods
Immunization/challenge protocols
Six- to eight-week-old female BALB/c mice were purchased from the Experimental Animal Center of the Chinese Academy of Sciences and reared under pathogen-free conditions. All animal experiments were performed in accordance with the guidelines of the Laboratory Animal Ethics Commission of Soochow University.
The vaccination schedules of mice are shown in . Prior to all immunizations, groups of mice (n = 6) were lightly anesthetized with 30% isoflurane (Baxter) diluted in propylene glycol. For the prime-boost strategy, the mice were first intramuscularly immunized with a single dose of 1 × 106 CFU BCG and then intranasally inoculated with 106 PFU VSV-846 in a 25 µL volume 12 weeks after BCG priming. PBS was used as a mock group, but administrated in the same way above. For BCG or VSV-846 single-dose immunization, the group of mice was either intramuscularly immunized with 1 × 106 CFU BCG or intranasally inoculated with 106 PFU VSV-846 once.
Bacterial strains and culture conditions
Escherichia coli (E. coli) strain DH5α was grown in a Luria–Bertani medium for cloning. M. bovis BCG (Denmark strain 1331) was provided by the Center for Disease Control of Suzhou and cultivated in a Middlebrook 7H9 medium or enumerated on 7H11 agar supplemented with 10% oleic acid–albumin–dextrose–catalase, 0.5% glycerol, and 0.05% Tween 80.
Production of recombinant viruses
Recombinant VSV-846 was plaque purified and expanded according to a previously described method.Citation26 Briefly, BHK-21 cells grown to 50% confluence were infected with 10 multiplicity of infection recombinant vaccinia virus, expressing T7 RNA polymerase and incubated for 2 h in serum-free Dulbecco's modified Eagle's medium. Vaccinia virus-infected cells were then co-transfected with the generated plasmid, expressing the recombinant VSV anti-genome VSV-XN2–846 and other plasmids for VSV N, P, and L protein expressions. Supernatants were collected 48 h after transfection, sieved through a 0.22-μm-pore filter to remove vaccinia virus, and passaged onto fresh BHK-21 cells. The medium was collected immediately after cytopathic effects were observed (2 d) and sieved through a 0.22-μm-pore filter. Subsequently, the viruses were plaque purified, and seed stocks were amplified in BHK-21 cells. The viral titer was determined by a plaque assay performed in Vero E6 cells, and the recombinant virus was stored at −80 °C until use.
IFN-γ ELISA
Splenocytes were isolated from immunized mice at week 18 and then plated (5 × 105cells/well) under TFP846 protein stimulation at a final concentration of 10 μg/mL for 60 h. The TFP846 protein was expressed by pET28a vector in E. coli BL21 and purified by Ni-nitrilotriacetic acid–metal ion affinity chromatography as previously described.Citation21 INF-γ concentrations in culture medium were determined using an ELISA kit (BD PharMingen). Plates were coated with captured anti-IFN-γ monoclonal antibodies (mAbs) at 4 °C overnight and then blocked with the splenocyte culture medium at room temperature for 2 h. After the plates were washed with deionized water and PBS with Tween 20, biotinylated anti-IFN-γ mAb was added at room temperature for 2 h. Streptavidin–alkaline phosphatase (AP) was added to the plates, which were incubated for 1 h. The color was developed by an AP colorimetric substrate. An immunospot analyzer (Cellular Technology) was used to enumerate spots.
Mice challenge and bacterial load detection
The immunized mice were intranasally infected with 1 × 107 CFU of BCG at week 20. The individual lung tissue homogenates were prepared 24 weeks after the challenge and plated on a Middlebrook 7H11 medium in serial dilutions in triplicate. The bacterial loads were measured according to colony numbers on the plates six weeks after plating.
Flow cytometry
To detect IFN-γ secreting CD4 T cells, splenocytes were isolated at week 18 and then cultured the under stimulation of TFP846 protein with a final concentration of 10 μg/mL at 37 °C for 60 h. After centrifugation for 5 min at 1,000 × g, the cells were then washed in staining buffer containing 1% fetal bovine serum and stained with PerCP-Cy5.5-anti-mouse CD4 (Biolegend). The surface-stained cells were washed, fixed, and permeabilized using a commercially available Cytofix/Cytoperm kit (BD Biosciences). The cells were then stained for 30 min on ice for intracellular cytokine expression using FITC-conjugated anti-mouse IFN-γ antibody (Biolegend). The stained cells were analyzed using FACSCanto II flow cytometer with FACSDiva software.
Splenocytes were prepared 24 weeks after BCG challenge to detect polyfunctional T cells. Cells were co-stained with PerCP-Cy5.5-anti-mouse CD4 (Biolegend), FITC-anti-mouse IFN-γ (Biolegend), PE-anti-mouse IL-2 (Biolegend), and APC-Cy7-anti-mouse TNF-α (Biolegend). The stained cells were analyzed with a FACSCanto II flow cytometer with FACSDiva software.
Statistical analysis
Statistical analyses were performed with GraphPad Prism. All data were given as mean and standard deviation. Data were statistically analyzed by two-tailed independent Student's t-test through SPSS 12.0. The level of statistical significance was set to P < 0.05.
Abbreviations
| TB | = | Tuberculosis |
| BCG | = | bacillus Calmette–Guérin |
| CFU | = | colony-forming unit |
| ELISA | = | enzyme-linked immuno sorbent assay |
| VSV | = | vesicular stomatitis virus |
| M. tb | = | Mycobacterium tuberculosis |
| PBS | = | phosphate buffered saline |
| PFU | = | plaque forming unit |
Disclosure of potential conflicts of interest
The authors declare that there is no conflict of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
The authors would like to thank Prof. John K Rose at Yale University for providing the VSV reverse genetic system. The authors also thank Dr. Bernard Moss at NIH for providing vTF7–3 vaccinia virus expressing T7 RNA polymerase.
Additional information
Funding
References
- Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health 2013; 34:271–86; PMID:23244049; https://doi.org/https://doi.org/10.1146/annurev-publhealth-031912-114431
- Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol 2005; 3:656–62; PMID:16012514; https://doi.org/https://doi.org/10.1038/nrmicro1211
- WHO. WHO vaccine-preventable dieases:monitoring system 2004 global summary. 2004
- Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Nasser Eddine A, Mann P, Goosmann C, Bandermann S, Smith D, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest 2005; 115:2472–9; PMID:16110326; https://doi.org/https://doi.org/10.1172/JCI24617
- Brodin P, Majlessi L, Brosch R, Smith D, Bancroft G, Clark S, Williams A, Leclerc C, Cole ST. Enhanced protection against tuberculosis by vaccination with recombinant Mycobacterium microti vaccine that induces T cell immunity against region of difference 1 antigens. J Infect Dis 2004; 190:115–22; PMID:15195250; https://doi.org/https://doi.org/10.1086/421468
- Hogarth PJ, Hewinson RG, Vordermeier HM. Development of vaccines against bovine tuberculosis. J Pharm Pharmacol 2006; 58:749–57; PMID:16734976; https://doi.org/https://doi.org/10.1211/jpp.58.6.0005
- McShane H, Hill A. Prime-boost immunisation strategies for tuberculosis. Microbes Infect 2005; 7:962–7; PMID:15890555; https://doi.org/https://doi.org/10.1016/j.micinf.2005.03.009
- Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Karonga Prevention Trial Group. Lancet 1996; 348:17–24; PMID:8691924; https://doi.org/https://doi.org/10.1016/S0140-6736(96)02166-6
- Roth AE, Benn CS, Ravn H, Rodrigues A, Lisse IM, Yazdanbakhsh M, Whittle H, Aaby P. Effect of revaccination with BCG in early childhood on mortality: randomised trial in Guinea-Bissau. BMJ 2010; 340:c671; PMID:20231251; https://doi.org/https://doi.org/10.1136/bmj.c671
- Leung CC, Tam CM, Chan SL, Chan-Yeung M, Chan CK, Chang KC. Efficacy of the BCG revaccination programme in a cohort given BCG vaccination at birth in Hong Kong. Int J Tuberc Lung Dis 2001; 5:717–23; PMID:11495262
- Barreto ML, Pereira SM, Pilger D, Cruz AA, Cunha SS, Sant'Anna C, Ichihara MY, Genser B, Rodrigues LC. Evidence of an effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: second report of the BCG-REVAC cluster-randomised trial. Vaccine 2011; 29:4875–7; PMID:21616115; https://doi.org/https://doi.org/10.1016/j.vaccine.2011.05.023
- Global tuberculosis programme and global programme on vaccines. Statement on BCG revaccination for the prevention of tuberculosis. Wkly Epidemiol Rec 1995; 70:229–31; PMID:7669527
- Dalmia N, Ramsay AJ. Prime-boost approaches to tuberculosis vaccine development. Expert review of vaccines 2012; 11:1221–33; PMID:23176655; https://doi.org/https://doi.org/10.1586/erv.12.94
- Feng CG, Palendira U, Demangel C, Spratt JM, Malin AS, Britton WJ. Priming by DNA immunization augments protective efficacy of Mycobacterium bovis Bacille Calmette-Guerin against tuberculosis. Infect Immun 2001; 69:4174–6; PMID:11349095; https://doi.org/https://doi.org/10.1128/IAI.69.6.4174-4176.2001
- McShane H, Brookes R, Gilbert SC, Hill AV. Enhanced immunogenicity of CD4(+) t-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect Immun 2001; 69:681–6; PMID:11159955; https://doi.org/https://doi.org/10.1128/IAI.69.2.681-686.2001
- McShane H, Pathan AA, Sander CR, Goonetilleke NP, Fletcher HA, Hill AV. Boosting BCG with MVA85A: the first candidate subunit vaccine for tuberculosis in clinical trials. Tuberculosis (Edinb) 2005; 85:47–52; PMID:15687027; https://doi.org/https://doi.org/10.1016/j.tube.2004.09.015
- Zika virus: A new global threat for 2016. Lancet 2016; 387:96
- Williams A, Goonetilleke NP, McShane H, Clark SO, Hatch G, Gilbert SC, Hill AV. Boosting with poxviruses enhances Mycobacterium bovis BCG efficacy against tuberculosis in guinea pigs. Infect Immun 2005; 73:3814–6; PMID:15908420; https://doi.org/https://doi.org/10.1128/IAI.73.6.3814-3816.2005
- Ndiaye BP, Thienemann F, Ota M, Landry BS, Camara M, Dieye S, Esmail H, Goliath R, Huygen K, January V, et al. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2015; 3:190–200; PMID:25726088; https://doi.org/https://doi.org/10.1016/S2213-2600(15)00037-5
- Brennan MJ, Clagett B, Fitzgerald H, Chen V, Williams A, Izzo AA, Barker LF. Preclinical evidence for implementing a prime-boost vaccine strategy for tuberculosis. Vaccine 2012; 30:2811–23; PMID:22387630; https://doi.org/https://doi.org/10.1016/j.vaccine.2012.02.036
- Kong H, Dong C, Xiong S. A novel vaccine p846 encoding Rv3615c, Mtb10.4, and Rv2660c elicits robust immune response and alleviates lung injury induced by Mycobacterium infection. Hum Vaccin Immunother 2014; 10:378–90; PMID:24280763; https://doi.org/https://doi.org/10.4161/hv.27121
- Kaufmann SH, Hussey G, Lambert PH. New vaccines for tuberculosis. Lancet 2010; 375:2110–9; PMID:20488515; https://doi.org/https://doi.org/10.1016/S0140-6736(10)60393-5
- Yuk JM, Jo EK. Host immune responses to mycobacterial antigens and their implications for the development of a vaccine to control tuberculosis. Clin Exp Vaccine Res 2014; 3:155–67; PMID:25003089; https://doi.org/https://doi.org/10.7774/cevr.2014.3.2.155
- Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di Carlo P, Titone L, Galati D, Bocchino M, Matarese A, Salerno A, et al. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol 2010; 40:2211–20; PMID:20540114; https://doi.org/https://doi.org/10.1002/eji.201040455
- Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MO. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol 2009; 39:723–9; PMID:19224636; https://doi.org/https://doi.org/10.1002/eji.200838693
- Roberts A, Buonocore L, Price R, Forman J, Rose JK. Attenuated vesicular stomatitis viruses as vaccine vectors. J Virol 1999; 73:3723–32; PMID:10196265
- Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM, Montefiori D, Roberts A, Buonocore L, Rose JK. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 2001; 106:539–49; PMID:11551502; https://doi.org/https://doi.org/10.1016/S0092-8674(01)00482-2
- Kapadia SU, Rose JK, Lamirande E, Vogel L, Subbarao K, Roberts A. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology 2005; 340:174–82; PMID:16043204; https://doi.org/https://doi.org/10.1016/j.virol.2005.06.016
- Cobleigh MA, Wei X, Robek MD. A vesicular stomatitis virus-based therapeutic vaccine generates a functional CD8 T cell response to hepatitis B virus in transgenic mice. J Virol 2013; 87:2969–73; PMID:23269785; https://doi.org/https://doi.org/10.1128/JVI.02111-12
- Roberts A, Kretzschmar E, Perkins AS, Forman J, Price R, Buonocore L, Kawaoka Y, Rose JK. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J Virol 1998; 72:4704–11; PMID:9573234
- Wu F, Fan X, Yue Y, Xiong S, Dong C. A vesicular stomatitis virus-based mucosal vaccine promotes dendritic cell maturation and elicits preferable immune response against coxsackievirus B3 induced viral myocarditis. Vaccine 2014; 32:3917–26; PMID:24874923; https://doi.org/https://doi.org/10.1016/j.vaccine.2014.05.052
- Tyagi AK, Nangpal P, Satchidanandam V. Development of vaccines against tuberculosis. Tuberculosis (Edinb) 2011; 91:469–78; PMID:21334259; https://doi.org/https://doi.org/10.1016/j.tube.2011.01.003
- Publicover J, Ramsburg E, Rose JK. A single-cycle vaccine vector based on vesicular stomatitis virus can induce immune responses comparable to those generated by a replication-competent vector. J Virol 2005; 79:13231–8; PMID:16227246; https://doi.org/https://doi.org/10.1128/JVI.79.21.13231-13238.2005
- Millington KA, Fortune SM, Low J, Garces A, Hingley-Wilson SM, Wickremasinghe M, Kon OM, Lalvani A. Rv3615c is a highly immunodominant RD1 (Region of Difference 1)-dependent secreted antigen specific for Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A 2011; 108:5730–5; PMID:21427227; https://doi.org/https://doi.org/10.1073/pnas.1015153108
- Lin PL, Dietrich J, Tan E, Abalos RM, Burgos J, Bigbee C, Milk L, Gideon HP, Rodgers M, Cochran C, et al. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J Clin Invest 2012; 122:303–14; PMID:22133873; https://doi.org/https://doi.org/10.1172/JCI46252
- Hoang T, Aagaard C, Dietrich J, Cassidy JP, Dolganov G, Schoolnik GK, Lundberg CV, Agger EM, Andersen P. ESAT-6 (EsxA) and TB10.4 (EsxH) based vaccines for pre- and post-exposure tuberculosis vaccination. PLoS One 2013; 8:e80579; PMID:24349004; https://doi.org/https://doi.org/10.1371/journal.pone.0080579
- Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, Schoolnik GK, Cassidy JP, Billeskov R, Andersen P. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med 2011; 17:189–94; PMID:21258338; https://doi.org/https://doi.org/10.1038/nm.2285
- Agger EM, Andersen P. A novel TB vaccine; towards a strategy based on our understanding of BCG failure. Vaccine 2002; 21:7–14; PMID:12443657; https://doi.org/https://doi.org/10.1016/S0264-410X(02)00447-4
- Derrick SC, Yabe IM, Yang A, Morris SL. Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4 T cells. Vaccine 2011; 29:2902–9; PMID:21338678; https://doi.org/https://doi.org/10.1016/j.vaccine.2011.02.010
- Lindenstrom T, Agger EM, Korsholm KS, Darrah PA, Aagaard C, Seder RA, Rosenkrands I, Andersen P. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol 2009; 182:8047-55; ; https://doi.org/https://doi.org/10.4049/jimmunol.0801592
- Sweeney KA, Dao DN, Goldberg MF, Hsu T, Venkataswamy MM, Henao-Tamayo M, Ordway D, Sellers RS, Jain P, Chen B, et al. A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nature medicine 2011; 17:1261–8; PMID:21892180; https://doi.org/https://doi.org/10.1038/nm.2420
- Kang H, Yuan Q, Ma H, Hu ZD, Han DP, Wu K, Lowrie DB, Fan XY. Enhanced protective efficacy against Mycobacterium tuberculosis afforded by BCG prime-DNA boost regimen in an early challenge mouse model is associated with increased splenic interleukin-2-producing CD4 T-cell frequency post-vaccination. Immunology 2014; 143:661–9; PMID:24965530; https://doi.org/https://doi.org/10.1111/imm.12348
- Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nature reviews Immunology 2008; 8:247–58; PMID:18323851; https://doi.org/https://doi.org/10.1038/nri2274
- Dooms H, Kahn E, Knoechel B, Abbas AK. IL-2 induces a competitive survival advantage in T lymphocytes. Journal of immunology 2004; 172:5973-9; ; https://doi.org/https://doi.org/10.4049/jimmunol.172.10.5973
- Lindenstrom T, Knudsen NP, Agger EM, Andersen P. Control of chronic mycobacterium tuberculosis infection by CD4 KLRG1- IL-2-secreting central memory cells. Journal of immunology 2013; 190:6311-9; ; https://doi.org/https://doi.org/10.4049/jimmunol.1300248
- Desel C, Dorhoi A, Bandermann S, Grode L, Eisele B, Kaufmann SH. Recombinant BCG DeltaureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J Infect Dis 2011; 204:1573–84; PMID:21933877; https://doi.org/https://doi.org/10.1093/infdis/jir592
- Lee J, Brehm MA, Greiner D, Shultz LD, Kornfeld H. Engrafted human cells generate adaptive immune responses to Mycobacterium bovis BCG infection in humanized mice. BMC Immunol 2013; 14:53; PMID:24313934; https://doi.org/https://doi.org/10.1186/1471-2172-14-53
- Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 2007; 8:369–77; PMID:17351619; https://doi.org/https://doi.org/10.1038/ni1449
