ABSTRACT
Previously we showed that 65-kDa Mycobacterium leprae heat shock protein (Hsp65) is a target for the development of a tuberculosis vaccine. Here we evaluated peripheral blood mononuclear cells (PBMC) from healthy individuals or tuberculosis patients stimulated with two forms of Hsp65 antigen, recombinant DNA that encodes Hsp65 (DNA-HSP65) or recombinant Hsp65 protein (rHsp65) in attempting to mimic a prophylactic or therapeutic study in vitro, respectively. Proliferation and cytokine-producing CD4+ or CD8+ cell were assessed by flow cytometry. The CD4+ cell proliferation from healthy individuals was stimulated by DNA-HSP65 and rHsp65, while CD8+ cell proliferation from healthy individuals or tuberculosis patients was stimulated by rHSP65. DNA-HSP65 did not improve the frequency of IFN-gamma+ cells from healthy individuals or tuberculosis patients. Furthermore, we found an increase in the frequency of IL-10-producing cells in both groups. These findings show that Hsp65 antigen activates human lymphocytes and plays an immune regulatory role that should be addressed as an additional antigen for the development of antigen-combined therapies.
KEYWORDS:
Introduction
During the last twenty years, tuberculosis (TB) has been one of the priorities of World Health Organization (WHO) because the high number of infected individuals, the co-infection TB-HIV (human immunodeficiency virus), which accelerates active TB progression, the abandonment of treatment, and the multi- and extensive-drug resistance contribute to a great number of deaths associated with this disease. There were nearly 1.8 million deaths worldwide and over 10.4 million people developing TB in 2015.Citation1
In attempt to reduce the number of infected individuals and to prevent bacillus transmission, intensive efforts have been ongoing to develop new drugs, diagnostic methods and vaccines.Citation2-4 A new vaccine, beyond conferring protection to uninfected individuals, could also boost the number of antigen-specific responding cells in those who are latently infected.
Currently, different promising vaccine formulations have been evaluated in clinical trials: six viral vectored booster vaccines (MVA85A, Ad5Ag85A, Crucell Ad35/MVA85A, ChAdOx1.85A/MVA85A, MVA85A-IMX313, TB/FLU-04L), five protein adjuvant booster vaccines (H1/IC31, H4/IC31, H56/IC31, M72/AS01E, ID93/GLA-SE) two priming vaccines (VPM1002 and MTBVAC), and two therapeutic vaccines (RUTI® and Mycobacterium vaccae).Citation5-7
Despite promising results, there is a general consensus that the prophylaxis against tuberculosis could not be attributed to a single vaccine.Citation8 Over the last 15 years, we have actively participated in the development of a new vaccine against TB. Our efforts have been concentrated on a recombinant DNA plasmid encoding the Mycobacterium leprae 65-kDa heat shock protein (DNA-HSP65). In experimental TB this preparation exhibited a prophylactic and therapeutic effect.Citation9-12 We have also described additional strategies to optimize the protective efficacy of this vaccine in pre-clinical assays such as prime-boost vaccination using BCG priming and DNA-HSP65 boosting, aggregates of DNA-HSP65 and cationic liposomes, or a single-shot vaccine formulation made up of DNA-HSP65, recombinant Hsp65 protein (rHsp65) and PLGA microspheres.Citation13-16 In an attempt to evaluate the immune stimulatory effects of DNA-HSP65 in human cells, we showed that monocyte-derived macrophages stimulated with DNA-HSP65 had increased production of TNF-α and were able to restrict bacterial growth.Citation17 A phase I clinical trial to establish the safety of DNA-HSP65 immunotherapy in patients with advanced head and neck squamous cell carcinoma has also been completed.Citation18,Citation19 These data prompted us to investigate the activation of human monocytes and circulating lymphocytes in healthy individuals and TB patients following in vitro stimulation with Hsp65 antigen. Our aim was to evaluate whether the DNA-HSP65 vaccine or recombinant Hsp65 protein would be able to modulate T cell proliferation and cytokine production. To that end, we attempted to mimic either an in vitro prophylactic study with healthy donor lymphocytes or a therapeutic effect by evaluating the adaptive immune response in TB patients following in vitro Hsp65 antigen stimulation.
Results
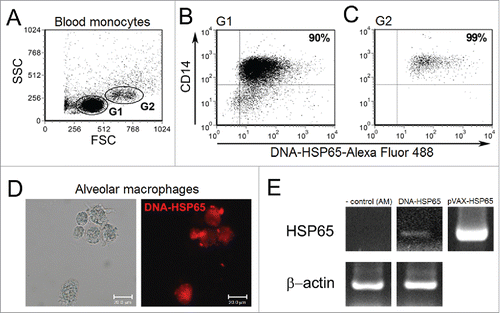
Uptake of DNA vaccine by monocytes and alveolar macrophages
To determine whether human monocytes and alveolar macrophages could uptake naked DNA-HSP65, we stimulated peripheral blood purified CD14+ cells with fluorescent-labeled DNA-HSP65. Flow cytometry analyses showed two distinct CD14+ monocyte populations based on size (FSC) and granularity (SSC), as represented in . Despite a predominant monocyte population characterized by small CD14+ cells, we observed that both small – G1 (B) and large – G2 (C) CD14+ cells were able to uptake naked DNA-HSP65, 77.86 ± 13.46% and 88.30 ± 3.96% respectively. However, there is no significant difference in the DNA-HSP65 uptake between both populations. Previously, we showed that human monocytes could be transfected by DNA-HSP65 plasmid.Citation17 AM were also stimulated with labeled DNA-HSP65 and analyzed by fluorescence microscopy (D). Endocytic vesicles in the cytoplasm showed that AM were able to uptake naked DNA. To confirm that AM were transfected, mRNA for mycobacterial Hsp65 protein was detected 96 hours after DNA-HSP65 stimulation (E).
Figure 1. Uptake of DNA-HSP65 by monocytes and alveolar macrophages. Purified CD14+ cells, from six healthy individuals, and alveolar macrophages (AM) were stimulated for 4 hours with Alexa Fluor labeled DNA-HSP65 and analyzed by flow cytometry or fluorescence microscopy, respectively. (A) Cells were gated by forward (FSC) and side (SSC)-scatter, and analysis was performed on gate 1 (G1), small CD14+ monocytes, and gate 2 (G2), large CD14+ monocytes. (B and C) Percentage of double-positive cells (CD14+/DNA-HSP65-Alexa Fluor 488+) for G1 (B) and G2 (C). (D) AM were analyzed by differential interference contrast microscopy and fluorescence microscopy. By RT-PCR (E) Expression of mycobacterial Hsp65 mRNA by unstimulated AM (negative control), DNA-HSP65-stimulated AM, pVAX-HSP65 (positive control).
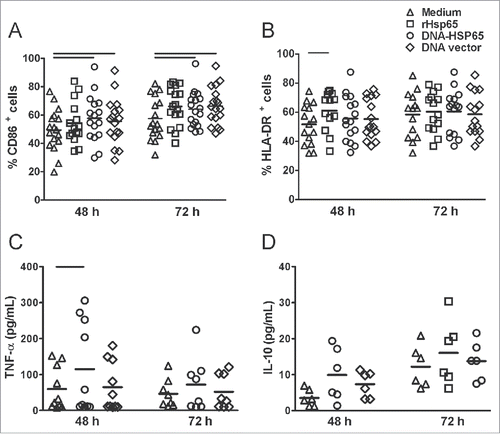
Activation of the innate response induced by recombinant DNA or protein
In order to study the activation of human monocytes by Hsp65 antigen, we evaluated cell phenotype and cytokine production. DNA-HSP65 induced a significant increase in the percentage of CD86-expressing CD14+ cells and rHsp65 induced a significant increase in HLA-DR-expressing CD14+ cells compare with unstimulated cells (A, B). The stimulatory effect in monocytes could not be attributed only to Hsp65 antigen because DNA vector also increased the frequency of CD86-expressing CD14+ cells (A). Significant concentrations of TNF-α were detected 48 hours after stimulation with DNA-HSP65 compare with unstimulated cells (C). IL-10 concentrations were very low and similar among unstimulated, DNA-HSP65-stimulated and DNA vector-stimulated monocytes (D).
Figure 2. Activation of the innate response mediated by DNA-HSP65. Monocytes were stimulated for 48 and 72 hours with Hsp65 antigen (rHsp65 or DNA-HSP65) or DNA vector. (A and B) CD86 and HLA-DR expression on CD14+ monocytes. (C and D) TNF-α and IL-10 secretion in cell culture supernatants. *p < 0.05. Horizontal lines represent the median value. CD86 48 h and 72 h (n = 17), HLA-DR 48 h and 72 h (n = 15) TNF 48 h (n = 9), TNF 72h (n = 7), IL-10 48 h and 72 h (n = 6) were samples from healthy individuals.
Immunological status of TB patients
Next we performed a peripheral blood fast culture with M. tuberculosis antigens (Mtb) to evaluate the immunological status of TB patients. Intracellular cytokine staining showed that Mtb stimulation significantly increased the frequency of IFN-γ-producing CD4+ and CD8+ cells obtained from healthy individuals and TB patients compare with the respective unstimulated cells (A, B). There was not an increase, however, in the frequency of IFN-γ-producing CD4+ or CD8+ cells from TB patients following stimulation compare with healthy individuals. Indeed, the frequency of CD8+IFN-γ+ cells was lower than those in the healthy individuals, although not significant (A, B).
Figure 3. Frequency of IFN-γ- or IL-4-producing CD4+ or CD8+ cells after M. tuberculosis antigen stimulation. Peripheral blood from healthy individuals or TB patients was cultured with Mtb antigens for 24 hours. For the final 4 hours, brefeldin A was added, and intracellular staining was performed. Cells gated as lymphocytes by FSC and SSC and dot plots for double positive cells were analyzed. (A and B) Frequency of IFN-γ -producing CD4+ and CD8+ cells. (C and D) Frequency of IL-4-producing CD4+ and CD8+ cells. Horizontal lines represent the mean value of seven healthy individuals (white squares) and ten TB patients (black squares). *p < 0.05.
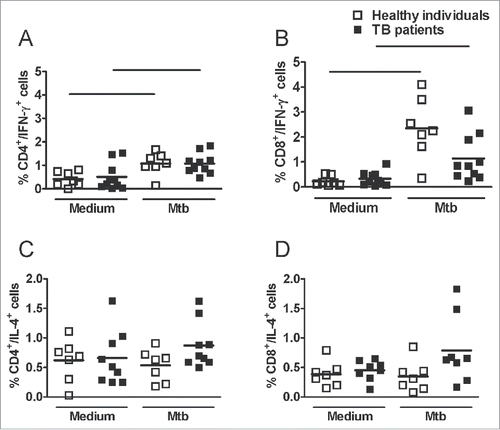
There was no difference between the frequency of IL-4-producing CD4+ cells obtained from healthy individuals and TB patients (C). Although CD8+ cells from TB patients stimulated with Mtb antigens had higher IL-4 production than those from healthy individuals, this difference was not significant (D). These data suggest the impairment of cellular immune responses in TB patients.
Proliferation and cytokine secretion following Hsp65 antigen stimulation
We first evaluated CD4+ and CD8+ cell proliferation, IFN-γ and IL-10 secretion by PBMC of healthy individuals. CFSE-labeled PBMC were stimulated with Hsp65 antigen, and proliferation was analyzed by flow cytometry (A). PHA stimulation was used as a positive control. Hsp65 antigen (rHsp65 or DNA-HSP65) was able to induce significant CD4+ cell proliferation, but only rHsp65 was able to induce significant CD8+ cell proliferation comparing to unstimulated cultures (B, C). Unexpectedly, DNA vector also induced significant CD4+ cell proliferation from healthy individuals (B). The proliferation of CD8+ cells induced by rHsp65 protein was significantly higher than that stimulated by DNA-HSP65 (C).
Figure 4. Healthy individuals cell proliferation response and cytokine secretion after Hsp65 antigen stimulation. CFSE-labeled PBMCs were cultured with Hsp65 antigen (DNA-HSP65 or rHsp65), DNA vector. As a control, cells were stimulated with PHA or Mtb antigens. Cell proliferation was determined by flow cytometry after 12 d in culture. (A) Representative FACS plot of cells gated as lymphocytes by FSC and SSC and dot plots for CD4+ or CD8+ cells were analyzed. Histograms show proliferation rate after different stimulus. (B) CD4+ cell proliferation rate. (C) CD8+ cell proliferation rate. Cytokine concentrations were determined by ELISA 7 d after stimulation. (D) IFN-γ secretion. (E) IL-10 secretion. Horizontal lines represent the mean value of 11 to 18 healthy individuals. *p < 0.05 compare with all stimulus. Bars p < 0.05 compare with linked stimulus.
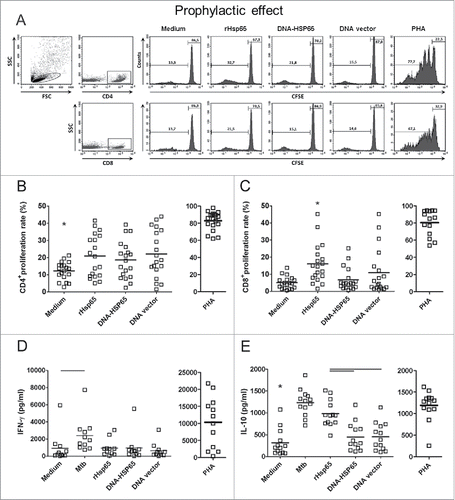
In parallel to the proliferation response, we determined the concentrations of IFN-γ and IL-10 secreted in the supernatants of PBMC cultured under different stimuli. While only Mtb antigens stimulated a significant production of IFN-γ, all stimuli induced a significant IL-10 secretion by cells obtained from healthy individuals compare with unstimulated cells (D, E). Moreover, rHsp65 induced higher IL-10 levels compare with DNA-HSP65. However, when we compared the stimulation of PBMC from healthy individuals with DNA-HSP65 and its control (DNA vector), we observed that both induced similar proliferation rates and levels of IFN-γ and IL-10.
Next, we evaluated whether Hsp65 antigen could upregulate the cell proliferation and the cytokine secretion by PBMC obtained from TB patients by flow cytometry (A). Stimulation with Hsp65 antigen did not induce a significant proliferation of CD4+ cells from TB patients compare with unstimulated PBMC (B). However, rHsp65 protein induced a significant proliferation of CD8+ cells, while DNA-HSP65 did not (C). We did not observe significant IFN-γ concentrations when cells from TB patients were stimulated with Hsp65 antigen compare with unstimulated cells, while cells stimulated with Mtb antigens secreted high concentrations of this cytokine (D). PBMCs from patients stimulated with rHsp65 protein secreted increased levels of IL-10 compare with unstimulated cells, DNA-HSP65 or DNA vector (E).
Figure 5. TB patients cell proliferation response and cytokine secretion after Hsp65 antigen stimulation. PBMC cultures from untreated and treated TB patients were performed as described in . (A) Representative FACS plot of cells gated as lymphocytes by FSC and SSC and dot plots for CD4+ or CD8+ cells were analyzed. Histograms show proliferation rate after different stimulus. (B) CD4+ cell proliferation rate. (C) CD8+ cell proliferation rate. (D) IFN-γ secretion. (E) IL-10 secretion. Horizontal lines represent the mean value of 8 to 12 untreated patients. *p < 0.05 compare with all stimulus. Bars p < 0.05 compare with linked stimulus.
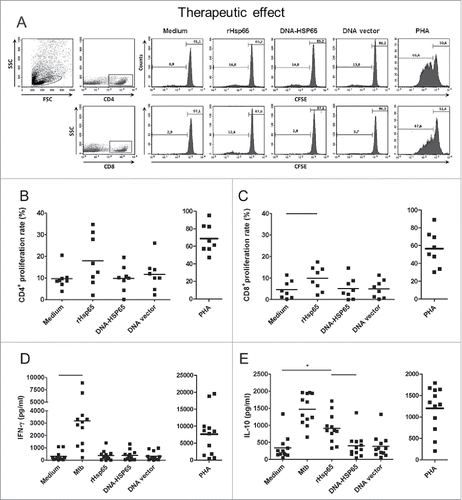
Blood samples were also obtained from ten patients following TB treatment. The polyclonal proliferative response was increased after treatment compare with those patients that were not under treatment (data not shown). Hsp65 antigen was not able to restore the proliferation of CD4+ cells. However, CD8+ cells proliferate significantly after rHsp65 stimulation compared with unstimulated cells (data not shown). This proliferation rate was higher compared with proliferation of CD8+ cells obtained from those patients that were not under treatment.
Therefore, we stimulated cell cultures with both forms of Hsp65 antigen. Our hypothesis was that rHsp65 and DNA-HSP65 could have a synergic effect in the cell proliferation. We did not observe additional CD4+ cell proliferation in the presence of both stimuli for healthy individuals TB patients (A). Similar data were observed for CD8+ cell proliferation, however when cultures were stimulated with rHsp65 plus DNA-HSP65, we observed a significant induction on CD8+ cell proliferation rate in both groups compared with their counterparts cells left unstimulated (B). Taken together, these data show that Hsp65 antigen (rHsp65 or DNA-HSP65) stimulates the proliferation of CD4+ cells from healthy individuals and rHsp65 stimulates the proliferation of CD8+ cells from healthy individuals and TB patients.
Figure 6. Stimulation with rHsp65 plus DNA-HSP65 improves cell proliferation rate. PBMC cultures from health individuals and TB patients (untreated) were performed as described in . (A) CD4+ cell proliferation rate. (B) CD8+ cell proliferation rate. Horizontal lines represent the mean value of 11 to 18 healthy individuals (white squares) and 8 to 12 untreated patients (black squares). *p < 0.05 compare with other groups. Bars p < 0.05 compare with linked stimulus.
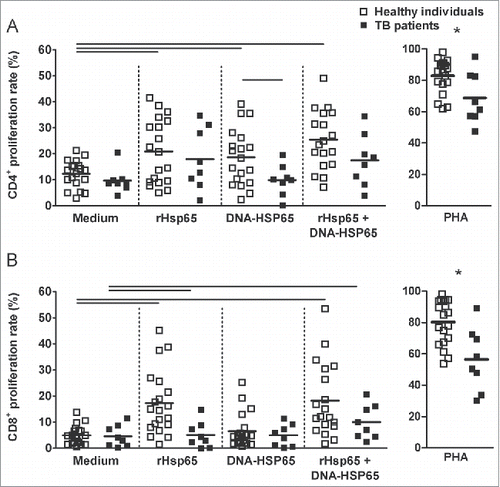
Comparing data from healthy individuals and patients, we observed that the proliferation of CD4+ cells from healthy individuals was significantly higher than the proliferation of CD4+ cells from TB patients following DNA-HSP65 stimulation (19.36 ± 10.60% versus 9.83 ± 5.95%, respectively). In addition, healthy donor cells stimulated with rHsp65 or DNA-HSP65 produced higher IFN-γ levels than cells from TB patients (951.37 ± 962.10 ng/ml vs. 383.56 ± 391.57 ng/ml, and 960.00 ± 1547.00 ng/ml vs. 387.80 ± 402.10, respectively, p > 0.05) (D and D).
Frequency of cytokine-producing cells
Next we investigated the frequency of IFN-γ- and IL-10-producing CD4+ or CD8+ cells stimulated with Hsp65 antigen. shows the results obtained with healthy donor cells (upper panel). A positive response to PHA was detected in all individuals studied (data not shown). We observed that DNA-HSP65 stimulation increased, although not significantly, the frequency of IFN-γ-producing CD4+ cells compare with unstimulated cells, while it had no effect in the CD8+IFN-γ+ population. The rHsp65 did not modify the frequency of IFN-γ-producing CD4+ or CD8+ cells. An increase in the frequency of IL-10-producing CD4+ or CD8+ cells was also observed after stimulation with DNA-HSP65, while rHsp65 had no effect in the frequency of these cell populations.
The results obtained with TB patient cells ( lower panel) show that neither rHsp65 protein nor DNA-Hsp65 stimulation induced an increase in the frequency of CD4+IFN-γ+ or CD8+IFN-γ+. In contrast, DNA-HSP65 increased the frequency of CD4+IL-10+ and CD8+IL-10+ from TB patients.
Discussion
In this study, we describe for the first time the interaction of PBMCs obtained from healthy individuals and TB patients with Hsp65 antigen, either as recombinant Hsp65 protein (rHsp65) or recombinant DNA (DNA-HSP65) to evaluate the immunomodulation of T cell response. As alveolar macrophages and TB patient's blood cells are previously activated we also evaluate the blood mononuclear cells modulation from healthy subjects. Blood sample is the most accessible tissue to investigate T cell immune response. Several vaccines researches consider human blood a valuable biological for clinical investigation as different cell populations (B-cell subsets, natural killer cells, monocytes and T-cell subsets) are presented.Citation20-22
First, we confirmed the uptake of DNA-HSP65 by monocytes from healthy individuals, considering that these cells could play a role as antigen presenting cells. We showed, previously, that human monocyte-derived macrophages and dendritic cells were activated by DNA-HSP65.Citation17 The upregulation of CD86 and MHC class II molecule in the monocyte cell surface as well as the production of TNF by these cells after DNA-HSP65 stimulation prompt us to study the activation of adaptive immune response, evaluating the cell proliferation and the cytokine production by lymphocytes after Hsp65 antigen stimulation. In addition, we investigated the uptake of DNA-HSP65 and the mycobacterial Hsp65 gene expression in alveolar macrophages because we reported previously, that after intramuscular administration, DNA-HSP65 had a wide tissue distribution, including lung.Citation23 Considering a possible route of intranasal mucosal immunization, we aimed to study the interaction and activation of cells from the site of the natural route of infection with the Hsp65 antigen. Therefore, we stimulated alveolar macrophages, obtained from induced sputum of healthy individual, with DNA-HSP65 and co-cultured with peripheral CD4+ or CD8+ lymphocytes. However, unstimulated and stimulated cultures produced similar concentrations of cytokines (data not shown).
Our main focus was to mimic an in vitro prophylactic or therapeutic effect of Hsp65 antigen using cells from healthy individuals or TB patients, respectively. Healthy individuals group included positive and negative tuberculin skin test donors and all subjects were BCG vaccinated soon after birth. Besides, there was no significant difference between these groups. Therefore, both samples were included in the same group (data not shown). We found that while CD4+ cells from healthy individuals proliferated significantly after rHsp65 or DNA-HSP65 stimulation compare with unstimulated cells, cells from TB patients did not. However, because the proliferation induced by Hsp65 antigen was not different from the proliferation induced by empty plasmid (DNA vector), apparently, Hsp65 antigen does not stimulate a vigorous clonal expansion. Regarding the expression of surface markers, cell proliferation and cytokine production, the stimulation induced by DNA vector was as effective as DNA-HSP65. This similar response could be attributed to the immunostimulatory properties of CpG ODN present on plasmid back-bone. A vaccine encoding the circumsporozoite protein of Plasmadium yoelli showed comparable immune response to the plasmid back-bone alone, suggesting a CpG-driven immune activation.Citation24
An important issue to be raised is that PBMCs were cultured for 12 d because DNA-HSP65 stimulation requires cell transfection, gene expression and protein production. This long time culture may have affected the magnitude of cell response. However, a significant proliferative response of CD8+ cells after stimulation with soluble Hsp65 protein, even higher than those induced by the stimulation with DNA-HSP65, was particularly intriguing. It has been shown that exogenous proteins can be degraded into peptides that associate with MHC class I molecules and activate CD8+ cells.Citation25 Furthermore, Giodini and Cresswell described that in the cross-presentation pathway, proteins are first unfolded to allow translocation into the cytosol, where they can subsequently undergo cytosolic refolding assisted by the chaperone Hsp90.Citation26 Since Hsp65 of M. leprae has proteolytic activity,Citation27 it is also possible that this protein plays a role in the mechanism of self cross-presentation. Moreover, there is evidence that soluble proteins secreted by M. tuberculosis such as CFP10 are required for the priming of CD8+ T cells in vivo.Citation28
It is well described that TB patients exhibit downregulation of cellular immune responses, which results in impairment of IFN-γ productionCitation29-32 and proliferation of peripheral blood mononuclear cells.Citation33,Citation34 Here we confirmed the downregulation of the cellular immune response of TB patients by the peripheral blood fast culture assay with Mtb antigens and by the lower cell proliferation response after polyclonal stimulation comparing TB patients to healthy individuals. In addition, Hsp65 antigen was not able to increase the secretion or the frequency of IFN-γ-producing CD4+ or CD8+ cells. Furthermore, it is reported that classical antigens (TB10.4, ESAT-6/CFP-10 and PPD) stimulate better whole blood from TB patients than Hsp65 and Ag85A/B.Citation35 In the other hand, healthy individuals, with no clinical signs of TB, have T lymphocytes that recognize M. tuberculosis proteins efficiently.Citation36 It is noteworthy that pre-clinical studies showed that protection induced by DNA-HSP65 immunization was dependent on upregulation of IFN-γ levels, although this vaccine also had stimulated antigen-specific IL-10 production.Citation13,Citation37 Our findings show that rHsp65 induced an increased secretion of IL-10 and DNA-Hsp65 stimulation resulted in an increase of IL-10-producing cells from healthy individuals and from TB patients.
Several authors reported the essential role of IL-10 in regulating inflammatory response during infection, important to limit pathology and reduce mortality, by controlling the excessive production of IFN-γ and TNF-α.Citation38-41 Furthermore, IL-10 also regulates the production of Th2 cytokines.Citation42-46 The abrogation of IL-10 production during L. major infection improves pathogen clearance but reduces the immunity to reinfection,Citation47 suggesting to IL-10 a role in the maintenance of effector memory populations.Citation48 Other experimental models showed that IL-10 absence is associated with inflammatory exacerbation.Citation40,Citation49-52 Thus, combining Hsp65 antigens with an adjuvant components or molecules able to induce IFN-γ in addition to the IL-10 may regulate this delicate balance between suppressing and activating host response against M. tuberculosis.
In summary, HSP65 antigen stimulation was not able to upregulate the frequency of IFN-γ-producing CD4+ or CD8+ cells obtained from TB patients, but increased the proliferation of CD4+ and CD8+ cells obtained from healthy individuals. DNA-HSP65 stimulation resulted in a significant increase in the frequency of IL-10-producing CD4+ and CD8+ cells from healthy individuals and TB patients. Taken together, these current results show the immune regulatory role of Hsp65 antigen in human cells. We suggest that the combination of distinct forms of antigen would be a useful tool in attempting to improve the prophylactic effect of Hsp65 antigen as a tuberculosis vaccine candidate.
Methods
Subjects
Healthy Brazilian volunteers, without clinical or laboratory evidence of active TB were recruited for this study: 13 individuals were recruited from Belo Horizonte, Minas Gerais and 12 were from Ribeirão Preto, São Paulo. Healthy individuals and TB patients were HIV-negative. None of the individuals was taking immunosuppressant medications. A tuberculin skin test (TST) was done in all subjects. All of them showed BCG vaccinal scars in the right forearm. The study was approved at the Research Ethical Committee of Ribeirão Preto Clinical Hospital (12401/2004), and at the Research Ethical Committee of School of medicine from Federal University of Minas Gerais (228/03), Brazil, and informed consent was obtained from individual subjects and healthy individuals.
Twelve Brazilian adult patients who were diagnosed with active pulmonary TB but untreated were recruited from three different governmental hospitals in Belo Horizonte. Pulmonary TB was defined by clinical history, sputum-positive smears by Ziehl-Neelsen staining, and chest X-rays consistent with TB. Blood samples were also obtained from ten patients following TB treatment. summarizes the clinical parameters of the study participants.
Table 2. Clinical parameters of the study participants.
Table 1. Frequency of cytokine-producing CD4+ or CD8+ cells of healthy individuals and TB patients.
DNA vaccine and recombinant Hsp65
The DNA-HSP65 vaccine was derived from the pVAX1 vector (Invitrogen, V26020) digested with BamHI and NotI (Invitrogen, 15201049 and 15441017), and a 3.3-kb fragment (corresponding to the M. leprae HSP65 gene) was inserted. The pVAX vector was used as control. Plasmids were purified as previously described.Citation17 Escherichia coli BL21 cells transformed with the peT28A plasmid encoding the M. leprae HSP65 gene were cultured in LB containing ampicillin (100 μg/μL), induced with 0.1 M isopropylthiogalactoside (IPTG; Gibco, 15529019), and purified according to the protocol of Portaro et al.Citation27 Endotoxin levels in plasmids and recombinant Hsp65 were determined using a QCL-1000 Limulus amoebocyte lysate kit (Cambrex Company, QCL-1000). Endotoxin levels were less than 0.1 endotoxin units (EU)/μg DNA and less than 0.19 EU/μg protein. To neutralize endotoxin levels, 30 μg/ml of polimixin B (Sigma, 1405–20–5) was added to the culture medium.
DNA labeling
The DNA vaccine was labeled with Alexa Fluor 488 or Alexa Fluor 594 by the Universal Linkage System (ULS™) using the ULYSIS nucleic acid labeling kit (Molecular Probes, U21652), as previously described.Citation53
M. tuberculosis antigens (Mtb)
M. tuberculosis H37Rv was grown in Sauton's medium, harvested after 14 d of culture, heat killed for 2 hours at 80ºC, washed twice with PBS and suspended in 50 mL of PBS. The suspension was sonicated (3 pulses, 5 min) and then centrifuged at 5000 x g for 30 minutes. Supernatants were sterilized through a 0.22-μm filter, and the protein concentration was determined with a Coomassie plus assay Kit (Pierce, 23236).
Monoclonal antibodies
Mouse anti-human monoclonal antibodies (mAb), conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), Cy-chrome or tri-color (TC) were used in different combinations for flow cytometry assays. The mAb anti-CD19-FITC (clone 4G7, 347543), anti-CD14-PE (clone MφP9, 562335) and anti-CD3-Cy-chrome (clone UCHT1, 555334) were used to evaluate the percentage of purified CD14+ cells. The mAb anti-CD14-FITC (clone M5E2, 557153), anti-CD86-PE (clone IT2.2, 555665) and anti-HLA-DR-PE (clone G46–6, 562304) were used to evaluate the monocyte phenotype. The mAb anti-CD4-PE (clone RPA-T4, 555347) and anti-CD8-TC (clone RPA-T8, 555368) were used for proliferation assays. For intracellular cytokine staining, mAb anti-IFN-γ (clone 4S.B3, 557074), anti-IL-4 (clone 8D4–8, 559333), and anti-IL-10 (clone JES3–19F1, 559330) all conjugated with PE were used simultaneously with mAb anti-CD4-TC (clone SK3, 347324) and anti-CD8-FITC (clone G42–8, 551347). Isotype-matching antibodies were used as negative controls. All mAb were purchased from BD (Becton Dickinson) and used according to the manufacturer's instructions.
Monocyte assays
Peripheral blood mononuclear cells (PBMC) were separated by density gradient centrifugation using Ficoll-Paque (GE Life Sciences, 17–1440–02). CD14+ monocytes were purified from healthy volunteers by positive selection with immunomagnetic microbeads, according to the manufacturer's instruction (Miltenyi Biotec, 130–050–201). The mean purity of CD14+ cells was around 90%. Purified monocytes were resuspended in RPMI 1640 (Sigma-Aldrich, R6504–10L) supplemented with 10% fetal bovine serum (FBS) (Gibco, 26140–079), streptomycin/ampicillin (Gibco, 15140122) and gentamicin (Gibco, 15750060) at a concentration of 2 × 105 cells in 96-well flat-bottom microtiter plates. Cells were then stimulated with 5 μg of Alexa Fluor 488-labeled DNA-vaccine for 4 hours and analyzed by flow cytometry. To evaluate activation of the innate response, PBMC (2 × 106 /mL) were plated in a 48-well plate. Adherent cells were stimulated with Hsp65 antigen (20 μg/mL of DNA-HSP65 or 25 μg/mL rHsp65) or empty DNA vector (20 μg/mL) for 48 and 72 hours. TNF-α and IL-10 concentrations were determined by ELISA (Becton Dickinson, 555212 and 555157). Phenotype evaluation was done with a biparametric gate in the FSC (forward scatter) and SSC (side scatter) dot plot drawn around the monocyte population. CD14+ cells were acquired using a FACSCan® Flow Cytometer (Becton Dickinson). CD86 and HLA-DR expression was analyzed by histograms. CELLQuestTM software was used for data acquisition and analysis.
Blood and PBMC culture
We cultured 1 ml of total peripheral blood from healthy individuals and TB patients in a 15 ml polypropylene tube in RPMI 1640 supplemented with streptomycin/ampicillin and gentamicin at 37°C in a 5% CO2 humidified incubator. Blood was stimulated for 24 hours with Mtb antigens (10 μg/mL).
PBMCs from healthy individuals and TB patients (5 × 105/mL)were stained with 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE; Invitrogen, Molecular Probes, C34554) and plated in 96-well round bottom culture plates in RPMI 1640 supplemented with 10% autologous serum, streptomycin/ampicillin and gentamicin. Hsp65 antigen (50 μg/mL of DNA-HSP65 or 25 μg/mL of rHsp65), DNA vector (50 μg/mL), rHsp65 plus DNA vaccine or plus vector were then added. Phytohemagglutin mitogen was used as a positive control (1% PHA, Gibco, 10576015). After 12 days, the cells were harvested and stained for CD4 and CD8 receptors.
In parallel, PBMCs from healthy individuals and TB patients were also cultured in the same way over 7 d for intracellular cytokine assays. Mtb antigens (10 μg/mL) were also used as an additional stimulus. Brefeldin A (Sigma, B5936–200UL) was added (10 μg/mL) at the final 4 hours of culture. CD4 or CD8 receptor and intracellular cytokine staining was performed as described previously.Citation54 Data were acquired using a FACSCalibur® Flow Cytometer (Becton Dickinson). Selective analysis of lymphocytes was performed by establishing a specific scatter gate in addition to positive staining for the CD4 or CD8 receptor. CFSE content (proliferation) and intracellular cytokines were expressed and analyzed by histograms and dot plots, respectively.
Supernatants of PBMC cultures were collected for IFN-γ and IL-10 detection by ELISA (BD PharMingen, 555142 and 555157) according to the manufacturer's protocols. The detection limit was 9.76 pg/mL and 7.81 pg/mL, respectively.
Fluorescence microscopy and HSP65 mRNA expression
Alveolar macrophages (AM) (5 × 104 cells) from healthy individuals induced sputum were stimulated with 5 μg of Alexa Fluor 594-labeled DNA vaccine for 4 hours, as previously described, as well as RNA extraction and RT-PCR for HSP65 mRNA detection was performed according to Franco et al. 2008.Citation17
Statistical analysis
Data are expressed as mean ± SEM. Statistical analyses were performed using PRISM software (version 6.0; GraphPad, San Diego, CA). Statistical significance was determined by a paired nonparametric test (Wilcoxon) among different stimulations in the same group and an unpaired nonparametric test (Mann-Whitney) for different groups. Values of p < 0.05 were considered statistically significant.
Abbreviations
| AM | = | alveolar macrophages |
| BCG | = | Bacillus Calmette Guérin |
| CFSE | = | 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester |
| DNA-HSP65 | = | recombinant DNA that encodes Hsp65 |
| EU | = | endotoxin units |
| FBS | = | fetal bovine serum |
| FITC | = | fluorescein isothiocyanate |
| FSC | = | forward scatter |
| HIV | = | human immunodeficiency virus |
| Hsp65 | = | heat shock protein |
| IPTG | = | isopropylthiogalactoside |
| mAb | = | monoclonal antibodies |
| Mtb | = | M. tuberculosis antigens |
| PBMC | = | peripheral blood mononuclear cells |
| PE | = | phycoerythrin |
| PHA | = | Phytohemagglutin |
| rHsp65 | = | recombinant Hsp65 protein |
| SSC | = | side scatter |
| TB | = | tuberculosis |
| TC | = | tri-color |
| TST | = | tuberculin skin test |
| WHO | = | World Health Organization |
Disclosure of potential conflicts of interest
The authors declare no financial or commercial conflicts of interest.
Acknowledgments
The authors thank Mrs. Ana Flávia Gembre, Mrs. Izaíra T. Brandão and Mrs. Ana Paula Masson for technical assistance, Mr. Walter Miguel Turato and Mrs. Fabiana Rossetto Morais for flow cytometry analysis and the Program for Technological Development in Tools for Health - PDTIS - FIOCRUZ for use of its facilities. We also thank Dr. David Jamil Hadad for induced sputum clinical supervision.
Funding
This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 04/13465–2 and 07/02407–0), and Conselho Nacional de Pesquisa (CNPq).
References
- WHO. Fact sheet NO 104. 2015; http://wwwhoint/mediacentre/factsheets/fs104/en/
- Karp CL, Wilson CB, Stuart LM. Tuberculosis vaccines: barriers and prospects on the quest for a transformative tool. Immunol Rev 2015; 264:363-81; PMID:25703572; http://dx.doi.org/10.1111/imr.12270
- Nathan C, Barry CE, 3rd. TB drug development: immunology at the table. Immunol Rev 2015; 264:308-18; PMID:25703568; http://dx.doi.org/10.1111/imr.12275
- Orme IM. Tuberculosis vaccine types and timings. Clin Vacc Immunol 2015; 22:249-57; PMID:25540272; http://dx.doi.org/10.1128/CVI.00718-14
- Frick M. The Tuberculosis Vaccine Pipeline: A New Path to the Same Destination? UK HIV i-Base/Treatment Action Group 2015; 2015; http://www.pipelinereport.org/2015/tb-vaccines
- Weiner J, 3rd, Kaufmann SH. Recent advances towards tuberculosis control: vaccines and biomarkers. J Int Med 2014; 275:467-80; PMID:24635488; http://dx.doi.org/10.1111/joim.12212
- O'Shea MK, McShane H. A review of clinical models for the evaluation of human TB vaccines. Hum Vacc Immunother 2016; 12:1177-87; PMID:26810964; http://dx.doi.org/10.1080/21645515.2015.1134407
- Montagnani C, Chiappini E, Galli L, de Martino M. Vaccine against tuberculosis: what's new? BMC Infect Dis 2014; 14 Suppl 1:S2; PMID:24564340; http://dx.doi.org/10.1186/1471-2334-14-S1-S2
- Lowrie DB, Silva CL, Colston MJ, Ragno S, Tascon RE. Protection against tuberculosis by a plasmid DNA vaccine. Vaccine 1997; 15:834-38; PMID:9234527; http://dx.doi.org/10.1016/S0264-410X(97)00073-X
- Bonato VL, Lima VM, Tascon RE, Lowrie DB, Silva CL. Identification and characterization of protective T cells in hsp65 DNA-vaccinated and Mycobacterium tuberculosis-infected mice. Infect Immun 1998; 66:169-75.
- Lowrie DB, Tascon RE, Bonato VL, Lima VM, Faccioli LH, Stavropoulos E, Colston MJ, Hewinson RG, Moelling K, Silva CL. Therapy of tuberculosis in mice by DNA vaccination. Nature 1999; 400:269-71; PMID:10421369; http://dx.doi.org/10.1038/22326
- Bonato VL, Goncalves ED, Soares EG, Santos Junior RR, Sartori A, Coelho-Castelo AA, Silva CL. Immune regulatory effect of pHSP65 DNA therapy in pulmonary tuberculosis: activation of CD8+ cells, interferon-gamma recovery and reduction of lung injury. Immunology 2004; 113:130-38; PMID:15312144; http://dx.doi.org/10.1111/j.1365-2567.2004.01931.x
- Goncalves ED, Bonato VL, da Fonseca DM, Soares EG, Brandao IT, Soares AP, Silva CL. Improve protective efficacy of a TB DNA-HSP65 vaccine by BCG priming. Genet Vacc Ther 2007; 5:7; PMID:17714584; http://dx.doi.org/10.1186/1479-0556-5-7
- Rosada RS, de la Torre LG, Frantz FG, Trombone AP, Zarate-Blades CR, Fonseca DM, Souza PR, Brandao IT, Masson AP, Soares EG, et al. Protection against tuberculosis by a single intranasal administration of DNA-hsp65 vaccine complexed with cationic liposomes. BMC Immunol 2008; 9:38; PMID:18647414; http://dx.doi.org/10.1186/1471-2172-9-38
- de Paula L, Silva CL, Carlos D, Matias-Peres C, Sorgi CA, Soares EG, Souza PR, Blades CR, Galleti FC, Bonato VL, et al. Comparison of different delivery systems of DNA vaccination for the induction of protection against tuberculosis in mice and guinea pigs. Genet Vacc Ther 2007; 5:2; PMID:17250766; http://dx.doi.org/10.1186/1479-0556-5-2
- Souza PR, Zarate-Blades CR, Hori JI, Ramos SG, Lima DS, Schneider T, Rosada RS, Torre LG, Santana MH, Brandao IT, et al. Protective efficacy of different strategies employing Mycobacterium leprae heat-shock protein 65 against tuberculosis. Exp Opin Biol Ther 2008; 8:1255-64; PMID:18694348; http://dx.doi.org/10.1517/14712598.8.9.1255
- Franco LH, Wowk PF, Silva CL, Trombone AP, Coelho-Castelo AA, Oliver C, Jamur MC, Moretto EL, Bonato VL. A DNA vaccine against tuberculosis based on the 65 kDa heat-shock protein differentially activates human macrophages and dendritic cells. Genet Vacc Ther 2008; 6:3; PMID:18208592; http://dx.doi.org/10.1186/1479-0556-6-3
- Michaluart P, Abdallah KA, Lima FD, Smith R, Moyses RA, Coelho V, Victora GD, Socorro-Silva A, Volsi EC, Zarate-Blades CR, et al. Phase I trial of DNA-hsp65 immunotherapy for advanced squamous cell carcinoma of the head and neck. Cancer Gene Ther 2008; 15:676-84; PMID:18535616; http://dx.doi.org/10.1038/cgt.2008.35
- Victora GD, Socorro-Silva A, Volsi EC, Abdallah K, Lima FD, Smith RB, Moyses RA, Zarate-Blades CR, Michaluart P, Silva CL, et al. Immune response to vaccination with DNA-Hsp65 in a phase I clinical trial with head and neck cancer patients. Cancer Gene Ther 2009; 16:598-608; PMID:19197326; http://dx.doi.org/10.1038/cgt.2009.9
- Smith SG, Zelmer A, Blitz R, Fletcher HA, Dockrell HM. Polyfunctional CD4 T-cells correlate with in vitro mycobacterial growth inhibition following Mycobacterium bovis BCG-vaccination of infants. Vaccine 2016; 34:5298-305; PMID:27622301; http://dx.doi.org/10.1016/j.vaccine.2016.09.002
- Smith SG, Smits K, Joosten SA, van Meijgaarden KE, Satti I, Fletcher HA, Caccamo N, Dieli F, Mascart F, McShane H, et al. Intracellular cytokine staining and flow cytometry: considerations for application in clinical trials of novel tuberculosis vaccines. PloS One 2015; 10:e0138042; PMID:26367374; http://dx.doi.org/10.1371/journal.pone.0138042
- Al-Attiyah RJ, Mustafa AS. Mycobacterial antigen-induced T helper type 1 (Th1) and Th2 reactivity of peripheral blood mononuclear cells from diabetic and non-diabetic tuberculosis patients and Mycobacterium bovis bacilli Calmette-Guerin (BCG)-vaccinated healthy subjects. Clin Exp Immunol 2009; 158:64-73; PMID:19737232; http://dx.doi.org/10.1111/j.1365-2249.2009.04000.x
- Coelho-Castelo AA, Trombone AP, Rosada RS, Santos RR, Jr., Bonato VL, Sartori A, Silva CL. Tissue distribution of a plasmid DNA encoding Hsp65 gene is dependent on the dose administered through intramuscular delivery. Genet Vacc Ther 2006; 4:1; PMID:16445866; http://dx.doi.org/10.1186/1479-0556-4-1
- Mor G, Klinman DM, Shapiro S, Hagiwara E, Sedegah M, Norman JA, Hoffman SL, Steinberg AD. Complexity of the cytokine and antibody response elicited by immunizing mice with Plasmodium yoelii circumsporozoite protein plasmid DNA. J Immunol 1995; 155:2039-46; PMID:7636255
- Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol Rev 2005; 207:145-57; PMID:16181333; http://dx.doi.org/10.1111/j.0105-2896.2005.00316.x
- Giodini A, Cresswell P. Hsp90-mediated cytosolic refolding of exogenous proteins internalized by dendritic cells. EMBO J 2008; 27:201-11; PMID:18046456; http://dx.doi.org/10.1038/sj.emboj.7601941
- Portaro FC, Hayashi MA, De Arauz LJ, Palma MS, Assakura MT, Silva CL, de Camargo AC. The Mycobacterium leprae hsp65 displays proteolytic activity. Mutagenesis studies indicate that the M. leprae hsp65 proteolytic activity is catalytically related to the HslVU protease. Biochemistry 2002; 41:7400-6; PMID:12044173; http://dx.doi.org/10.1021/bi011999l
- Woodworth JS, Fortune SM, Behar SM. Bacterial protein secretion is required for priming of CD8+ T cells specific for the Mycobacterium tuberculosis antigen CFP10. Infect Immun 2008; 76:4199-205; PMID:18591224; http://dx.doi.org/10.1128/IAI.00307-08
- Sodhi A, Gong J, Silva C, Qian D, Barnes PF. Clinical correlates of interferon gamma production in patients with tuberculosis. Clin Infect Dis 1997; 25:617-20; PMID:9314449; http://dx.doi.org/10.1086/513769
- Dlugovitzky D, Bay ML, Rateni L, Urizar L, Rondelli CF, Largacha C, Farroni MA, Molteni O, Bottasso OA. In vitro synthesis of interferon-gamma, interleukin-4, transforming growth factor-beta and interleukin-1 beta by peripheral blood mononuclear cells from tuberculosis patients: relationship with the severity of pulmonary involvement. Scand J Immunol 1999; 49:210-17; PMID:10075027; http://dx.doi.org/10.1046/j.1365-3083.1999.00492.x
- Sahiratmadja E, Alisjahbana B, de Boer T, Adnan I, Maya A, Danusantoso H, Nelwan RH, Marzuki S, van der Meer JW, van Crevel R, et al. Dynamic changes in pro- and anti-inflammatory cytokine profiles and gamma interferon receptor signaling integrity correlate with tuberculosis disease activity and response to curative treatment. Infect Immun 2007; 75:820-9; PMID:17145950; http://dx.doi.org/10.1128/IAI.00602-06
- Jurado JO, Pasquinelli V, Alvarez IB, Pena D, Rovetta AI, Tateosian NL, Romeo HE, Musella RM, Palmero D, Chuluyan HE, et al. IL-17 and IFN-gamma expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. J Leukocyte Biol 2012; 91:991-1002; PMID:22416258; http://dx.doi.org/10.1189/jlb.1211619
- Hirsch CS, Toossi Z, Othieno C, Johnson JL, Schwander SK, Robertson S, Wallis RS, Edmonds K, Okwera A, Mugerwa R, et al. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis 1999; 180:2069-73; PMID:10558973; http://dx.doi.org/10.1086/315114
- Al-Attiyah R, Mustafa AS, Abal AT, Madi NM, Andersen P. Restoration of mycobacterial antigen-induced proliferation and interferon-gamma responses in peripheral blood mononuclear cells of tuberculosis patients upon effective chemotherapy. FEMS Immunol Med Microbiol 2003; 38:249-56; PMID:14522460; http://dx.doi.org/10.1016/S0928-8244(03)00166-4
- Kassa D, Ran L, Geberemeskel W, Tebeje M, Alemu A, Selase A, Tegbaru B, Franken KL, Friggen AH, van Meijgaarden KE, et al. Analysis of immune responses against a wide range of Mycobacterium tuberculosis antigens in patients with active pulmonary tuberculosis. Clin Vacc Immunol 2012; 19:1907-15; PMID:23015647; http://dx.doi.org/10.1128/CVI.00482-12
- Munk ME, Schoel B, Kaufmann SH. T cell responses of normal individuals towards recombinant protein antigens of Mycobacterium tuberculosis. Eur J Immunol 1988; 18:1835-8; PMID:2462503; http://dx.doi.org/10.1002/eji.1830181128
- Zarate-Blades CR, Rodrigues RF, Souza PR, Rios WM, Soares LS, Rosada RS, Brandao IT, Masson AP, Floriano EM, Ramos SG, et al. Evaluation of the overall IFN-gamma and IL-17 pro-inflammatory responses after DNA therapy of tuberculosis. Hum Vacc Immunother 2013; 9:1093-103; PMID:23324590; http://dx.doi.org/10.4161/hv.23417
- Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol 1996; 157:798-805; PMID:8752931
- Wilson EH, Wille-Reece U, Dzierszinski F, Hunter CA. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J Neuroimmunol 2005; 165:63-74; PMID:16005735; http://dx.doi.org/10.1016/j.jneuroim.2005.04.018
- Hunter CA, Ellis-Neyes LA, Slifer T, Kanaly S, Grunig G, Fort M, Rennick D, Araujo FG. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol 1997; 158:3311-6; PMID:9120288
- Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol 2004; 172:4123-32; PMID:15034024; http://dx.doi.org/10.4049/jimmunol.172.7.4123
- Schandene L, Alonso-Vega C, Willems F, Gerard C, Delvaux A, Velu T, Devos R, de Boer M, Goldman M. B7/CD28-dependent IL-5 production by human resting T cells is inhibited by IL-10. J Immunol 1994; 152:4368-74; PMID:7512591
- Joss A, Akdis M, Faith A, Blaser K, Akdis CA. IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway. Eur J Immunol 2000; 30:1683-90; PMID:10898505; http://dx.doi.org/10.1002/1521-4141(200006)30:6%3c1683::AID-IMMU1683%3e3.0.CO;2-A
- Grunig G, Corry DB, Leach MW, Seymour BW, Kurup VP, Rennick DM. Interleukin-10 is a natural suppressor of cytokine production and inflammation in a murine model of allergic bronchopulmonary aspergillosis. J Exp Med 1997; 185:1089-99; PMID:9091582; http://dx.doi.org/10.1084/jem.185.6.1089
- Wilson MS, Elnekave E, Mentink-Kane MM, Hodges MG, Pesce JT, Ramalingam TR, Thompson RW, Kamanaka M, Flavell RA, Keane-Myers A, et al. IL-13Ralpha2 and IL-10 coordinately suppress airway inflammation, airway-hyperreactivity, and fibrosis in mice. J Clin Investigat 2007; 117:2941-51; PMID:17885690; http://dx.doi.org/10.1172/JCI31546
- Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol 2000; 164:6406-16; PMID:10843696; http://dx.doi.org/10.4049/jimmunol.164.12.6406
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 2002; 420:502-7; PMID:12466842; http://dx.doi.org/10.1038/nature01152
- Redford PS, Murray PJ, O'Garra A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol 2011; 4:261-70; PMID:21451501; http://dx.doi.org/10.1038/mi.2011.7
- Li C, Sanni LA, Omer F, Riley E, Langhorne J. Pathology of Plasmodium chabaudi chabaudi infection and mortality in interleukin-10-deficient mice are ameliorated by anti-tumor necrosis factor alpha and exacerbated by anti-transforming growth factor beta antibodies. Infect Immun 2003; 71:4850-6; PMID:12933825; http://dx.doi.org/10.1128/IAI.71.9.4850-4856.2003
- Edelmann W, Schleiss K, Joss A. Ecological, energetic and economic comparison of anaerobic digestion with different competing technologies to treat biogenic wastes. Water Sci Technol 2000; 41:263-73; PMID:11382001
- Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun 1999; 67:4435-42; PMID:10456884
- Suzuki Y, Sher A, Yap G, Park D, Neyer LE, Liesenfeld O, Fort M, Kang H, Gufwoli E. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J Immunol 2000; 164:5375-82; PMID:10799901; http://dx.doi.org/10.4049/jimmunol.164.10.5375
- Coelho-Castelo AA, Santos Junior RR, Bonato VL, Jamur MC, Oliver C, Silva CL. B-lymphocytes in bone marrow or lymph nodes can take up plasmid DNA after intramuscular delivery. Hum Gene Ther 2003; 14:1279-85; PMID:12952599; http://dx.doi.org/10.1089/104303403767740812
- Sathler-Avelar R, Vitelli-Avelar DM, Massara RL, de Lana M, Pinto Dias JC, Teixeira-Carvalho A, Eloi-Santos SM, Martins-Filho OA. Etiological treatment during early chronic indeterminate Chagas disease incites an activated status on innate and adaptive immunity associated with a type 1-modulated cytokine pattern. Microbes Infect 2008; 10:103-13; PMID:18248755; http://dx.doi.org/10.1016/j.micinf.2007.10.009
