ABSTRACT
This phase III, non-randomized, open-label, multi-center study (NCT01827839) evaluated the immunogenicity and safety of an adjuvanted recombinant subunit herpes zoster (HZ) vaccine (HZ/su) in adults aged ≥ 50 y with prior physician-documented history of HZ. Participants (stratified by age: 50–59, 60–69 and ≥ 70 y) received 2 doses of HZ/su 2 months apart and were followed-up for another 12 months. Anti-glycoprotein E (gE) antibodies were measured by enzyme-linked immunosorbent assay before vaccination and 1 month after the second dose (Month 3). Solicited local and general adverse events (AEs) were recorded for 7 d and unsolicited AEs for 30 d after each vaccination. Serious AEs were recorded until study end. The primary immunogenicity objective was met if the lower limit of the 95% confidence interval (CI) of the vaccine response rate (VRR), defined as a 4-fold increase in anti-gE over baseline, at Month 3 was ≥ 60%. 96 participants (32/age group) were enrolled. The primary immunogenicity objective was met, as the VRR at Month 3 was 90.2% (95% CI: 81.7–95.7). Geometric mean anti-gE antibody concentrations at Month 3 were similar across age groups. 77.9% and 71.6% of participants reported local and general solicited AEs, respectively. The most frequent solicited AEs were pain at injection site, fatigue, headache, myalgia and shivering. The HZ/su vaccine was immunogenic in adults aged ≥ 50 y with a physician-documented history of HZ, and no safety concerns were identified.
Introduction
Herpes Zoster (HZ), or shingles, is caused by the symptomatic reactivation of the varicella-zoster virus (VZV) from latency. It typically manifests as a localized, dermatomal rash, which lasts about 2 to 4 weeks and is usually accompanied by pain and pruritus.Citation1 Diminished VZV-specific cell-mediated immunity, as a consequence of advanced age, disease, drug treatment or medical interventions, represents a risk factor for developing HZ.Citation2,3 Although the age of VZV acquisition varies by region, the large majority of the adult population have been infected with VZV by the age of 50.Citation4-7
While HZ is usually considered a once-in-a-lifetime experience, several studies have shown that HZ episodes may occur in immunocompetent individuals with a prior history of HZ,Citation8-10 with rates of recurrence comparable to first occurrence of HZ being reported after a follow-up period of 7.3 y.Citation10 Although this high rate of recurrence could be influenced by the lack of laboratory confirmation for all suspected HZ episodes and by other limitations,Citation11 the results still indicate that there may be a benefit in vaccinating individuals with prior history of HZ.
GSK Vaccines’ candidate vaccine for the prevention of HZ (HZ/su) is a recombinant subunit (su) vaccine consisting of the VZV glycoprotein E (gE) antigen and an adjuvant system (AS01B). Recently, results from a pooled analysis over 2 phase III studies enrolling 16596 participants ≥ 50 y of age (ZOE-50; NCT01165177) and 13,900 participants ≥ 70 y of age (ZOE-70; NCT01165229) showed an overall HZ vaccine efficacy (VE) of > 90%.Citation12 No significant decline in VE was reported in participants aged ≥ 70 y in the pooled analyses (ZOE-50 and ZOE-70) compared with younger age groups.Citation13 In phase II studies, HZ/su was also shown to induce a robust immune response in healthy older adults,Citation14,15 and in immunocompromised populations.Citation16,17
However, previous studies of HZ/su excluded adults with a prior history of HZ, and no information is currently available on the immunogenicity and safety of HZ/su in this population. Therefore, the present study was designed to evaluate the immunogenicity and safety of the HZ/su vaccine in adults ≥ 50 y of age with a prior history of HZ.
Results
Study population
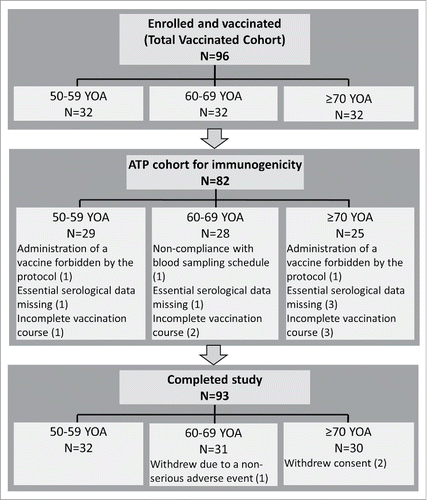
96 participants (32 in each age group) were enrolled and received at least 1 dose of vaccine, and 93 participants (96.9%) completed the study. One participant was withdrawn due to a non-serious adverse event (AE) and 2 participants withdrew consent (not due to an AE). The number of participants included in the according to protocol (ATP) cohort for immunogenicity, along with reasons for elimination are presented in .
The majority of participants in the total vaccinated cohort (TVC) (67.7%, 65/96) reported having a previous episode of HZ within the past 4 years; 32.3% (31/96) reported a previous episode of HZ with an onset of more than 4 y ago ().
Table 1. Characteristics of study participants (Total vaccinated cohort).
The mean age in the TVC at first vaccination was 64.9 y (median: 64 years; range: 50–89 years); 65.6% of participants were female. Most participants were Caucasian (95.8%) (). All participants were seropositive for anti-gE antibodies at baseline.
Immunogenicity
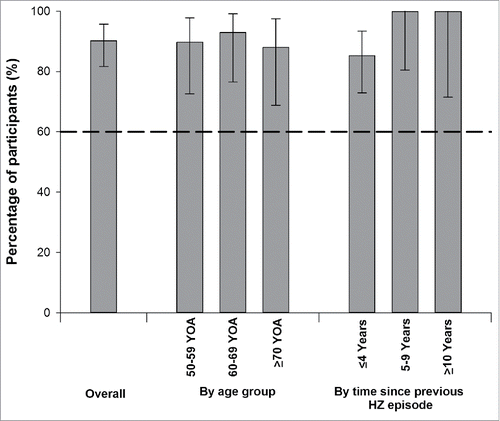
The co-primary immunogenicity objective was met, with a vaccine response rate (VRR) for anti-gE antibodies at Month 3 (1 month after the second vaccine dose) of 90.2% (95% confidence interval [CI]: 81.7–95.7). Within each age group, lower limits of the VRR 95% CIs were also consistently above 60% (). The lowest VRR was observed in participants with the most recent HZ episode history (≤ 4 years).
Figure 2. Vaccine response rates for anti-gE antibody concentrations one month after the second vaccine dose: overall, by age group and by time since previous herpes zoster episode (ATP cohort for immunogenicity).
The observed mean geometric increase (MGI) from pre-vaccination at Month 3 was 19.9. The median fold increase in anti-gE antibody concentrations from pre-vaccination to Month 3 was 25.6 (first and third quartiles [Q1, Q3]: 10.2, 43.8).
The observed post-vaccination anti-gE geometric mean concentrations (GMCs) were comparable for all age groups and between study participants with different timeframes since the previous HZ episode (). A second analysis based on the TVC was performed to complement the ATP analysis because the percentage of vaccinated participants with serological results excluded from the ATP cohort for immunogenicity exceeded 5% overall. Results of this analysis were similar to those based on the ATP cohort for immunogenicity (Table S1).
Table 2. Vaccine response rates and geometric mean concentrations of anti-gE antibodies at Month 0 and Month 3 (ATP cohort for immunogenicity).
Safety and reactogenicity
Following the first dose, 67.0% (95% CI: 56.6–76.4) of participants reported at least 1 local solicited AE and 51.1% (95%CI: 40.5–61.5) reported at least 1 general solicited AE. Post-dose 2, at least 1 local solicited AE was reported by 69.8% (95%CI: 58.9–79.2) of participants and at least 1 general solicited AE, by 60.5% (95%CI: 49.3–70.8) of participants. Overall, 77.9% (95% CI: 68.2–85.8) of participants reported at least 1 local solicited AE and 71.6% (95% CI: 61.4–80.4) of participants reported at least 1 general solicited AE. The percentage of participants reporting any solicited AE was 87.5% (95% CI: 76.8–94.4) in participants with an HZ episode documented ≤ 4 y before study start, 66.7% (95% CI: 41.0–86.7) in participants with an episode documented between 5–9 y before study start, and 69.2% (95% CI: 38.6–90.9) in participants with an episode documented ≥ 10 y before study start.
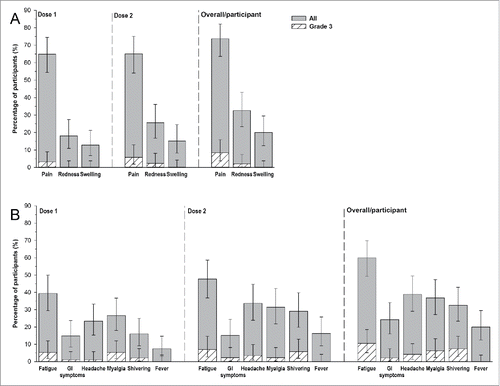
During the 7 d following each vaccine dose, the most common solicited local symptom was pain (73.7%). Pain was reported by 64.9% (95%CI: 54.4–74.5) of participants post-dose 1 and 65.1% (95%CI: 1.9–13.0) of participants post-dose 2. The most frequently reported solicited general symptoms was fatigue, in 39.4% (95%CI: 29.4–50.0) of participants post-dose 1, and 47.7% (95%CI: 36.8–58.7) of participants post-dose 2 (). The most frequently reported solicited general symptoms were fatigue (60.0%), headache (38.9%), myalgia (36.8%), and shivering (32.6%). Among grade 3 solicited local symptoms, pain was most frequently reported by 3.2% (95% CI: 0.7–9.0%) of participants post-dose 1, and 5.8% (95%CI: 1.9–13.0%) of participants post-dose 2. The most commonly reported grade 3 general symptom was fatigue, recorded for 5.3% (95%CI: 1.7–12.0) of participants post-dose 1 and 7.0% (95%CI: 2.6–14.6) of participants post-dose 2 (). All solicited local symptoms had a median duration of 3 days; the median duration of solicited general symptoms ranged between 1 day (gastrointestinal symptoms, shivering, and fever) and 3 d (myalgia) during the 7-day follow-up period post-vaccination.
Figure 3. Incidence of solicited local (A) and general (B) adverse events (total vaccinated cohort, overall/participant).
Thirty (31.3%) participants reported at least 1 unsolicited symptom within 30 d following vaccination; 12 (12.5%) participants reported 17 AEs that were considered related to vaccination by the investigators (Table S2). Eleven (11.5%) participants reported grade 3 unsolicited symptoms; 4 participants reported at least one grade 3 unsolicited symptom that was considered related to vaccination by investigators (Table S2). A total of 5 serious AEs (SAEs) were reported by 3 (3.1%) participants up to study end (Table S3). All SAEs resolved and were considered unrelated to vaccination by the investigators. No potential immune-mediated diseases (pIMDs) were reported up to study end.
For 6 (6.3%) participants, a total of 9 suspected HZ episodes were recorded based either on patient self-reporting or clinical presentation, however no laboratory confirmation was obtained. Three of the participants with suspected HZ episodes did not receive the second dose of HZ/su, 2 of them due to the occurrence of the suspected HZ episode and 1 due to a local AE after the first dose (itchy upper arm). Three of the suspected HZ episodes were moderate while the rest were of mild intensity, and none were considered related to vaccination ().
Table 3. Overview of suspected HZ episodes reported during the study (total vaccinated cohort).
No safety concerns were identified in this study regarding the administration of HZ/su to adults aged ≥ 50 y with prior history of HZ.
Discussion
A prior history of HZ does not confer complete protection against subsequent HZ episodes.Citation8-10 Thus, vaccination of adults aged ≥ 50 y with a prior or unknown history of HZ may be beneficial, provided that immunogenicity and safety of the vaccine are not adversely affected by a previous HZ episode.
This phase III study showed that 2 doses of HZ/su administered 2 months apart elicited a strong humoral immune response in adults aged ≥ 50 y with a prior history of HZ. Both the VRR and the anti-gE antibody concentrations were high at 1 month after the second dose.
The humoral immune response to HZ/su elicited in adults with history of HZ in this study was robust and of similar magnitude to that seen in adults without a documented history of HZ in previous studies.Citation15 Although we found that observed VRRs appeared to be lowest in participants who had an HZ episode within the last 4 y before vaccination, there was no apparent difference at 1 month post dose 2 in anti-gE antibody concentrations of HZ/su. Lower observed VRRs (85.2% versus 100%) in the participants with the most recent history of HZ (≤ 4 years) are likely to be the result of the observed higher pre-vaccination anti-gE antibody concentrations in this population compared with those observed in participants with more remote HZ episodes (≥ 4 years), which were similar to those of adults without a documented history of HZ.Citation15
In this study, participants reported local and general AEs with comparable observed incidences (grade 3 and any severity) to those reported following HZ/su vaccination in other studies in adults ≥ 50 YOA.Citation13-15,18 In a study assessing the safety of different formulations of HZ/su vaccine in adults ≥ 50 YOA, higher incidences of solicited local AEs were observed for AS01-adjuvanted formulations when compared with unadjuvanted ones.Citation14 An increased incidence of local solicited symptoms has previously been reported for other vaccines adjuvanted with AS01 systems.Citation20 However, both local and general solicited AEs in our study were transient, and mostly of mild or moderate intensity, in line with previous data in HZ/su vaccinees.Citation13-15,18 In addition, none of the 5 SAEs that occurred during the study were considered related to vaccination by the investigators. Our results indicate that HZ/su has a clinically acceptable safety profile in adults aged ≥ 50 years, and that vaccine reactogenicity and safety are not impacted by a prior history of HZ. During this study, 6 participants (6.3%) reported a total of 9 suspected HZ cases. Considering that HZ/su has recently been shown to have >90% efficacy against HZ in adults aged 50 y and older, the number of subjects with previous history of HZ reporting suspected episodes of HZ in our study is unexpected.Citation12,13
The main objectives of the study were to evaluate HZ/su immunogenicity and safety, and no assessment of efficacy was planned. Also, although recurrence rates of up to 13.6%Citation10 have been reported, literature dataCitation10,20 suggested that 2 or fewer HZ cases would occur during the course of the study due to the small sample size and relatively brief follow-up period. Given the small number of expected HZ cases, a rigorous HZ case ascertainment procedure was not mandated in the protocol.
We speculate that some of the suspected HZ cases may not have been true HZ episodes since laboratory confirmation was not required per protocol. Some suspected cases (n = 3) were only based on self-reporting of the participants. Study participants were educated to recognize and report symptoms of HZ during the initial visit, which may have contributed to a heightened awareness of HZ signs and increased reports of non-HZ symptoms as suspected HZ. In support of this, in phase III clinical trials, between 24.8% and 40.2% of self-reported cases were confirmed as false positives after more rigorous testing.Citation13,21 In addition, the geographic distribution of suspected HZ cases supports the possibility of biased self-reporting as all 9 suspected HZ cases were reported at the 2 Canadian sites (by 4 and 2 study participants, respectively), which enrolled a total of 48 participants. Conversely, no suspected HZ cases were reported in Russia (48 participants enrolled at 2 sites) even though epidemiological data suggest that HZ incidence does not differ between regions.Citation20 Based on the known pathophysiology of HZ, the composition of HZ/su, the robust humoral and cellular immune responses elicited following vaccination with HZ/su, and the high efficacy observed against HZ during the phase III studies,Citation12,13 we were unable to identify a plausible biologic basis to suspect that HZ/su would increase the risk of developing HZ in individuals with prior HZ episode, while protecting against the disease in those without a history of HZ.
Although the study was adequately designed and powered to assess the overall immunogenicity and safety endpoints, no formal comparisons by age group or by time from the previous HZ episode were performed. Another limitation was that clinical diagnosis by the investigator and confirmatory laboratory testing were not compulsory in the process of recording suspected HZ episodes.
Conclusion
In adults ≥50 YOA with a history of previous HZ, both the immune responses to HZ/su and its safety profile were consistent with those observed in other trials evaluating HZ/su in similarly aged population without a prior history of HZ.
Materials and methods
Objectives of the study
The co-primary objectives of the study were: (i) to evaluate the anti-gE VRR at Month 3 (1 month following a 2-dose administration of HZ/su vaccine) in all study participants ≥ 50 y of age with a prior physician-documented history of HZ; and (ii) to evaluate the safety and reactogenicity following administration of HZ/su vaccine from the first vaccination up to 30 d after the last vaccination. The first primary objective was met if the lower limit of the 95% CI of the VRR for anti-gE enzyme-linked immunosorbent assay antibody concentrations at Month 3 was at least 60%.
The secondary objectives of the study were: (i) to characterize the anti-gE immune response before first vaccination (Month 0) and at Month 3 within each of the following age ranges: 50–59, 60–69 and ≥ 70 y of age and (ii) to evaluate safety following administration of HZ/su vaccine from 30 d after the last vaccination until study end.
Study design
This was a phase III, non-randomized, open-label, multi-center study with a single group (Clinicaltrials.gov: NCT01827839) conducted in 2 centers in Canada and 2 centers in the Russian Federation between June 2013 and November 2014.
The duration of the study was approximately 14 months for each participant, (approximately 12 months after the second dose), including 3 study site visits (at Month 0, Month 2 and Month 3) and 2 telephone contacts (at Months 8 and 14).
Participants
This study enrolled male and female participants aged ≥ 50 y with a prior physician-documented history of HZ.
Participants were stratified by age: 50–59, 60–69 and ≥ 70 y of age, ensuring an equal distribution of the study population across the 3 age strata.
Exclusion criteria comprised active HZ infection (an episode was considered no longer active when all lesions had at least turned to crusts), previous vaccination against VZV or HZ and/or planned administration during the study of an HZ vaccine (including an investigational or non-registered vaccine) other than the study vaccine, any confirmed or suspected immunosuppressive or immunodeficient condition resulting from disease or immunosuppressive/cytotoxic therapy. Time frame since the prior HZ episode was recorded for all participants.
Ethics and informed consent
The study protocol was approved by the appropriate independent ethics committee or institutional review board at each study center. Written informed consent was obtained from all participants before study entry.
The study was conducted in accordance with the provisions of the Declaration of Helsinki and the International Conference on Harmonization–Good Clinical Practice guidelines.
Vaccination
Participants received 2 doses of the investigational vaccine, HZ/su, approximately 2 months apart, by intra-muscular injection into the deltoid muscle of the non-dominant arm. HZ/su contains 50 μg of lyophilized recombinant VZV gE antigen reconstituted with 0.5 ml of the liposome-based AS01B adjuvant system containing 50 μg of monophosphoryl lipid A and 50 μg of Quillaja saponaria Molina, fraction 21 (licensed by GSK from Antigenics Inc., a wholly owned subsidiary of Agenus Inc., a Delaware, USA corporation).
Evaluation of immunogenicity
Blood samples were collected from all eligible participants at Month 0 and Month 3 to assess gE-specific humoral immune responses by an in-house enzyme-linked immunosorbent assay (ELISA). The characterization of the background signal for the ELISA was performed using VZV naïve samples, and based on these experiments; the cut-off for seropositivity was established at 97 mIU/mL.
Evaluation of safety and reactogenicity
Solicited local and general AEs were recorded for 7 d (Days 0–6) after each vaccination. Unsolicited AEs were recorded for 30 d (Days 0–29) after each vaccination, according to the Medical Dictionary for Regulatory Activities classification. Grade 3 redness and swelling were defined as having >100-mm diameter, grade 3 fever as oral temperature >39.0°C, and all other grade 3 AEs as preventing normal daily activity. SAEs and pIMDs were recorded throughout the study.
Intercurrent medical conditions (IMCs) that could potentially impact a participant's immune response to HZ/su were recorded until Month 3, and participants presenting with such an IMC were eliminated from the ATP cohort for immunogenicity.
Recording of suspected HZ episodes
Suspected HZ episodes were considered as IMCs and were reported during the entire study period. A suspected HZ case was defined as a new rash characteristic of HZ (i.e., unilateral, dermatomal and accompanied by pain broadly defined to include allodynia, pruritus or other sensations). At the first study visit, participants were educated to recognize the typical HZ symptoms. Suspected cases of HZ were recorded by the investigator and were based either on clinical presentation or patient self-reporting of characteristic symptoms.
Statistical analysis
The primary analysis of immunogenicity was based on the ATP cohort for immunogenicity, which included all participants who complied with the procedures and intervals defined in the protocol and for whom immunogenicity results were available until Month 3. If ≥ 5% of vaccinated participants with serological results were excluded from the ATP cohort for immunogenicity, a second analysis based on the TVC was to be performed to complement the ATP analysis. The TVC included all participants who received at least one dose of HZ/su vaccine.
Participants were eliminated from the ATP cohort for immunogenicity if, during the study, they incurred a condition that had the capability of confounding their immune response (e.g., episodes of HZ before the last immunogenicity assessment at Month 3, or other IMCs that may influence a participant's immune response).
The VZV gE-specific VRR was calculated with an exact 95% CI. The VRR was defined as the percentage of initially seropositive participants with a 4-fold increase in the anti-gE antibody concentration at Month 3 compared with pre-vaccination concentrations. This threshold was selected based on receiver operating characteristics curve analyses performed in earlier studies. The post-vaccination over baseline ratio in the placebo (saline) group was used to define a non-responder, while the post-vaccination over baseline ratio in the gE adjuvanted group was used to define a responder at 1 month post-dose 2. The optimal observation associated to the best couple (specificity and sensitivity) was chosen to define the threshold for a vaccine-induced immune response. The 95% CI for GMCs was obtained for each group separately. First, the 95% CI for the mean of log-transformed concentrations was obtained, assuming that log-transformed values were normally distributed with unknown variance. The 95% CI for GMCs were then calculated by anti-log transformation of the 95% CI for the mean of log-transformed concentrations.
The primary objective was met if the lower limit of the 95% CI of the gE specific VRR was at least 60%. We calculated that a sample size of 84 evaluable participants would allow us to demonstrate the primary objectives with at least 97% power. Assuming 10% non-evaluable participants, a target enrollment population of 96 participants was calculated.
The following immunogenicity parameters were calculated: seropositivity rates for anti-gE antibodies with exact 95% CIs; anti-gE antibody GMCs with 95% CIs; MGI, defined as the geometric mean of the within participant ratio of the post-vaccination concentration to the pre-vaccination concentration; descriptive statistics of fold increase from baseline for anti-gE antibody concentrations. The same tabulations were done per age group, as pre-defined in the protocol, and also per time frame from the previous HZ episode (≤ 4 years, 5–9 y and ≥ 10 y since previous HZ episode), as a post-hoc exploratory analysis. For each parameter, overlapping 95% CIs were used to suggest comparability between age groups. However, no formal comparisons between different categories (age groups, time since vaccination) were made as no adjustment for multiplicity of endpoints was considered.
The primary analysis for safety was based on the TVC. The percentage of participants reporting each local or general solicited AE during the 7-day follow-up period was tabulated with exact 95% CIs after each vaccine dose and overall; the same tabulation was done by age groups as pre-defined in the protocol, and also per time frame from the previous HZ episode, as a post-hoc exploratory analysis. The proportion of participants with at least one report of unsolicited AE reported up to 30 d after each vaccination was tabulated with exact 95% CIs; SAEs and withdrawal due to AE(s) were described in detail; and suspected HZ episodes reported during the study were listed. The statistical analyses were performed using SAS version 9.2 on Windows and StatXact-8.1 procedure for SAS.
Abbreviations
| AE | = | adverse events |
| ATP | = | according to protocol cohort |
| CI | = | confidence interval |
| gE | = | glycoprotein E |
| GMC | = | geometric mean concentration |
| HZ | = | herpes zoster |
| HZ/su | = | adjuvanted recombinant subunit herpes zoster vaccine |
| IMC | = | intercurrent medical conditions |
| MGI | = | mean geometric increase |
| pIMDs | = | potentially immune-mediated diseases |
| SAE | = | serious adverse events |
| TVC | = | total vaccinated cohort |
| VRR | = | vaccine response rate |
| VZV | = | varicella-zoster virus |
| YOA | = | years of age |
Disclosure of potential conflicts of interest
At the time of the study, OG, MK, KG, MD, TCH and HL were employed by the GSK group of companies. OG, TCH and HL received shares in the GSK group of companies as part of their employee remuneration.
Author contributions
Conceived and designed the experiments: OG, TCH, HL; Performed the experiments: OG, MK, DS; Analyzed and/or interpreted the data: OG, MK, KG, LC, MD, TCH, HL; Wrote the paper: OG, MK, DS, KG, LC, MD, TCH, HL.
Supplemental_Material.docx
Download MS Word (34.6 KB)Acknowledgements
The authors thank all the participants, study nurses and staff members who contributed to this study. The authors also thank the following people at GSK vaccines: Lidia Oostvogels (clinical development team), Stéphanie Ravault (GVCL), Karolien Peeters (general study support), Ilse Vastiau (regulatory affairs) and Toufik Zahaf (statistician). We are indebted to the investigators, Janzen Jeannette, Romanenko Victor and Osipova Irina. The authors would like to thank Andrew Darrow and Francine Lowry for writing the report and Bhakthi Pereira for writing the protocol, Timea Kiss (XPE Pharma & Science on behalf of GSK Vaccines) for medical writing assistance, Amelie Fassbender and Jarno Jansen (XPE Pharma & Science on behalf of GSK Vaccines) for editing and coordinating the publication development.
Funding
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of the present manuscript. All authors had full access to the data and agreed with the submission of the publication.
References
- Fashner J, Bell AL. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician 2011; 83(12):1432-7; PMID:21671543.
- Cohen JI. VZV: molecular basis of persistence (latency and reactivation). In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K, eds. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press 2007, 2007.
- Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, Betts RF, Gershon AA, Haanpaa ML, McKendrick MW, et al. Recommendations for the management of herpes zoster. Clin Infect Dis 2007; 44(Suppl 1):S1-26; PMID:17143845; http://dx.doi.org/10.1086/510206
- Lee BW. Review of varicella zoster seroepidemiology in India and Southeast Asia. Trop Med Int Health 1998; 3(11):886-90; PMID:9855401; http://dx.doi.org/10.1046/j.1365-3156.1998.00316.x
- Kilgore PE, Kruszon-Moran D, Seward JF, Jumaan A, Van Loon FP, Forghani B, McQuillan GM, Wharton M, Fehrs LJ, Cossen CK, et al. Varicella in Americans from NHANES III: implications for control through routine immunization. J Med Virol 2003; 70(Suppl 1):S111-8; PMID:12627498; http://dx.doi.org/10.1002/jmv.10364
- Araujo LQ, Macintyre CR, Vujacich C. Epidemiology and burden of herpes zoster and post-herpetic neuralgia in Australia, Asia and South America. Herpes 2007; 14(Suppl 2):40-4; PMID:17939895.
- Nardone A, de Ory F, Carton M, Cohen D, van Damme P, Davidkin I, Rota MC, de Melker H, Mossong J, Slacikova M, et al. The comparative sero-epidemiology of varicella zoster virus in 11 countries in the European region. Vaccine 2007; 25(45):7866-72; PMID:17919788; http://dx.doi.org/10.1016/j.vaccine.2007.07.036
- Tseng HF, Chi M, Smith N, Marcy SM, Sy LS, Jacobsen SJ. Herpes zoster vaccine and the incidence of recurrent herpes zoster in an immunocompetent elderly population. J Infect Dis 2012; 206(2):190-6; PMID:22669900; http://dx.doi.org/10.1093/infdis/jis334
- Weitzman D, Shavit O, Stein M, Cohen R, Chodick G, Shalev V. A population based study of the epidemiology of Herpes Zoster and its complications. J Infect 2013; 67(5):463-9; PMID:23872209; http://dx.doi.org/10.1016/j.jinf.2013.06.016
- Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc 2011; 86(2):88-93; PMID:21220354; http://dx.doi.org/10.4065/mcp.2010.0618
- Volpi A, Gatti A, Pica F. Frequency of herpes zoster recurrence. Mayo Clin Proc 2011; 86(6):586; author reply -7; PMID:21628622; http://dx.doi.org/10.4065/mcp.2011.0096
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Diez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barbera J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016; 375(11):1019-32; PMID:27626517; http://dx.doi.org/10.1056/NEJMoa1603800
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barbera J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372(22):2087-96; PMID:25916341; http://dx.doi.org/10.1056/NEJMoa1501184
- Chlibek R, Bayas JM, Collins H, de la Pinta ML, Ledent E, Mols JF, Heineman TC. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults >=50 years of age. J Infect Dis 2013; 208(12):1953-61; PMID:23904292; http://dx.doi.org/10.1093/infdis/jit365
- Chlibek R, Smetana J, Pauksens K, Rombo L, Van den Hoek JA, Richardus JH, Plassmann G, Schwarz TF, Ledent E, Heineman TC. Safety and immunogenicity of 3 different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. Vaccine 2014; 32(15):1745-53; PMID:24508036; http://dx.doi.org/10.1016/j.vaccine.2014.01.019
- Berkowitz EM, Moyle G, Stellbrink HJ, Schurmann D, Kegg S, Stoll M, El Idrissi M, Oostvogels L, Heineman TC. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis 2015; 211(8):1279-87; PMID:25371534.
- Stadtmauer EA, Sullivan KM, Marty FM, Dadwal SS, Papanicolaou GA, Shea TC, Mossad SB, Andreadis C, Young JA, Buadi FK, et al. A phase 1/2 study of an adjuvanted varicella-zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood 2014; 124(19):2921-9; PMID:25237196; http://dx.doi.org/10.1182/blood-2014-04-573048
- Leroux-Roels I, Leroux-Roels G, Clement F, Vandepapeliere P, Vassilev V, Ledent E, Heineman TC. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein e subunit vaccine candidate in young and older adults. J Infect Dis 2012; 206(8):1280-90; PMID:22872734; http://dx.doi.org/10.1093/infdis/jis497
- Garcon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines 2011; 10(4):471-86; PMID:21506645; http://dx.doi.org/10.1586/erv.11.29
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4(6):e004833; PMID:24916088; http://dx.doi.org/10.1136/bmjopen-2014-004833
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352(22):2271-84; PMID:15930418; http://dx.doi.org/10.1056/NEJMoa051016