ABSTRACT
Vaccine-enhanced disease has been a major obstacle in developing a safe vaccine against respiratory syncytial virus (RSV). This study demonstrates the immunogenicity, efficacy, and safety of virus-like particle (VLP) vaccines containing RSV F (F VLP), G (G VLP), or F and G proteins (FG VLP) in cotton rats. RSV specific antibodies were effectively induced by vaccination of cotton rats with F VLP or FG VLP vaccines. After challenge, lung RSV clearance was observed with RSV F, G, FG VLP, and formalin inactivated RSV (FI-RSV) vaccines. Upon RSV infection, cotton rats with RSV VLP vaccines were protected against airway hyper-responsiveness and weight loss, which are different from FI-RSV vaccination exhibiting vaccine-enhanced disease of airway obstruction, weight loss, and severe histopathology with eosinophilia and mucus production. FG VLP and F VLP vaccines did not cause pulmonary inflammation whereas G VLP induced moderate lung inflammation with eosinophilia and mucus production. In particular, F VLP and FG VLP vaccines were found to be effective in inducing antibody secreting cell responses in bone marrow and lymphoid organs as well as avoiding the induction of T helper type 2 cytokines. These results provide further evidence to develop a safe RSV vaccine based on VLP platforms.
Introduction
Respiratory syncytial virus (RSV) causes substantial morbidity and mortality, claiming an approximated 160,000 deaths worldwide.Citation1 There is no licensed RSV vaccine since the tragic failure of formalin inactivated RSV (FI-RSV) in 1960s).Citation2,3 Numerous vaccine platforms have been tested, which include subunit soluble RSV F (fusion) and G (attachment) glycoproteins, vectored vaccines, and live attenuated vaccines (reviewed in Citation4). Non-replicating subunit vaccines have the safety concerns of vaccine-enhanced disease after vaccination and infection. Live attenuated vaccines have the difficulty of balancing efficacy and safety. Virus-like particles (VLP) containing RSV F or G proteins represent an attractive approach for developing RSV vaccine candidates. VLP has the structure mimicking infectious viruses but do not have viral genomes. Utilizing the structural proteins (NP, M) of Newcastle disease virus (NDV), chimeric NDV-RSV VLP vaccines were produced in avian cells, which contain the ectodomains of the RSV F and G proteins in a chimeric fusion to the transmembrane and cytoplasmic tail of the NDV proteins.Citation5,6 NDV-RSV VLP vaccines were immunogenic, and capable of inducing neutralizing antibodies and long-lived protection without overt vaccine-enhanced disease in mice,Citation5,7,8 and cotton rats.Citation9 We have previously developed VLP vaccines containing RSV F (F VLP) or RSV G (G VLP) using influenza matrix M1 protein and the recombinant baculovirus / insect cell expression system.Citation10 F VLP was able to induce T helper type 1 (Th1) humoral and cellular immune responses conferring protection against RSV without vaccine-associated lung inflammation in mice.Citation11 In addition, a mixed F VLP and G VLP (FG VLP) was shown to have additive protection by reducing lung viral loads and inducing CD8 T cell responses in mice.Citation12
Cotton rats are considered a more relevant animal model than mice for the efficacy and safety of RSV vaccines and drugs.Citation13,14 Here, we investigated the immunogenicity, efficacy, and safety of F VLP, G VLP, and FG VLP in cotton rats. Mixed FG VLP and F VLP vaccines were effective in conferring protection and in preventing vaccine-enhanced disease of pulmonary inflammation after RSV challenge.
Results
F VLP or F VLP plus G VLP immunization induces RSV F specific antibodies in cotton rats
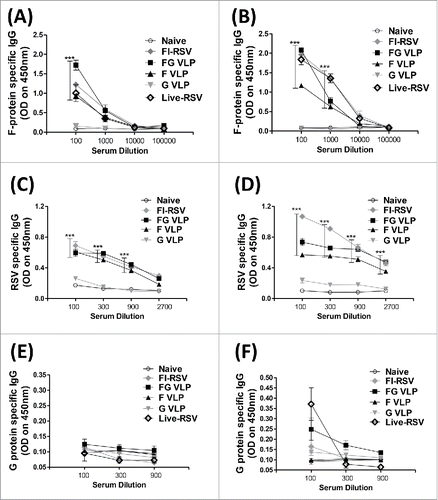
In this study using cotton rats as a relevant animal model, we investigated the protective efficacy of F VLP, G VLP, and mixed F VLP and G VLP (FG VLP) vaccines in comparison with FI-RSV. Cotton rats were intramuscularly immunized with F VLP, G VLP, and FG VLP without adjuvant, or FI-RSV with alum adjuvant. RSV F-protein specific antibodies were determined in sera collected at 3 weeks after prime and boost immunization. Cotton rats with F or FG VLP vaccination induced significant levels of RSV F-specific IgG antibodies after prime (), which were further increased after boost (). The FG VLP group showed higher levels of F-specific antibodies than the F VLP group whereas the G VLP group did not induce F specific antibodies after prime and boost. IgG levels in the FG VLP group were higher than those of the FI-RSV group after prime () and became to be similar as those of the FI-RSV groups after boost (). FI-RSV, FG VLP and F VLP induced considerable amounts of RSV specific antibodies in prime and boost immune sera. G VLP immunization in cotton rat induced low levels of RSV specific antibodies (). RSV G specific antibodies were induced at low levels in FI-RSV, FG VLP, G VLP and live RSV boost immune cotton rats but not detected in prime immune sera (). Serum IgG levels in the live RSV group were found to be similar to that in F VLP group after prime but significantly increased, being similar to those of the FI-RSV and FG VLP groups after boost (). To gain a functional neutralizing activity of immune sera, we determined neutralizing activity of antibodies against A2-K-line19F RSV (Fig. S1). Immune sera from the FI-RSV, FG VLP, F VLP and G VLP immunized groups showed significantly higher levels of neutralizing activity compared with the naïve group. Combination FG or F only VLP vaccines was effective in inducing RSV F specific and neutralizing antibodies after immunization of cotton rats.
Figure 1. FG VLP is effective in inducing RSV (F)protein-specific antibodies in cotton rats. (A) Prime IgG antibodies specific for RSV F protein. (B) Boost IgG antibodies specific for RSV F protein. (C) Prime IgG antibodies specific for RSV. (D) Boost IgG antibodies specific for RSV. (E) Prime IgG antibodies specific for RSV G protein. (F) Boost IgG antibodies specific for RSV G protein. Cotton rats (n = 5 per group) were immunized i.m. with F VLP, G VLP, mixed F VLP and G VLP (FG VLP), FI-RSV (FI-RSV), intranasally incoculated with live RSV (0.3 × 106 PFU). and PBS (Naïve) on days 0 (prime) and 28 (boost). Serum samples were collected at 3 weeks after prime or boost immunization and RSV-specific antibody levels were measured by ELISA. Results are presented as mean ± SEM and statistical significance was performed by one-way ANOVA with Tukey's multiple comparisons post-test in Graph Pad Prism. *** p < 0.001; compared with Naïve group.
RSV VLP vaccination confers protection without airway resistance and body weight loss
To determine protective efficacy, RSV VLP immune cotton rats were intranasally challenged with RSV A2 strain and body weight changes were daily monitored (). The F, G, or FG VLP group did show less than 2% of weight loss after RSV challenge (). The FI-RSV and naïve cotton rat groups showed 5% and 4% of weight loss respectively after RSV challenge. These results suggest that RSV VLP immunization is effective in preventing weight loss against RSV infection.
Figure 2. RSV VLP vaccination prevents weight loss, AHR and clears lung viral loads. (A) Body weight changes after RSV infection. Cotton rats were challenged i.n. with RSV A2 (1 × 106 PFU) on day 56. ** p < 0.01, *** p<0.001; compared with Naïve-Inf., ††† p < 0.001; compared with FI-RSV. (B) Airway hyper responsiveness (AHR). At 4 d post RSV challenge, AHR to increasing concentrations of methacholine (0, 50, 250 mg/ml) was assessed by whole body plethysmography and Penh values were calculated. ** p < 0.01, * p < 0.05; compared with Naïve, †† p < 0.01; compared with Naïve-Inf. (C) RSV titers. Lung viral titers were determined from individual cotton rat with lung lysate at 5 d after RSV challenge. *** p < 0.001; compared with indicated groups. Statistical significance was performed by 2-way ANOVA with Bonferroni post-test to compare replicate mean values in Graph Pad Prism.
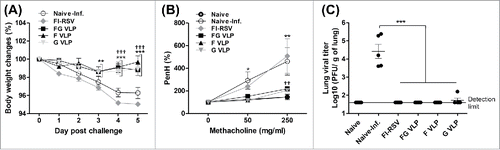
The airway obstruction and bronchoconstriction can be an indicator for severe pulmonary disease due to RSV infection. The airway resistance Penh (%) values were measured day 4 post challenge using plethysmography. RSV infected naïve and FI-RSV immunized cotton rats displayed highest Penh values in response to aerosolized methacholine challenge in a dose responsive manner (). Meanwhile, F, G, or FG VLP immunized cotton rats did not show an increase in Penh values, which are similar to uninfected naïve animals ().
RSV titers were determined in individual lung extracts at 5 d after RSV challenge by an immuno-plaque assay (). The unimmunized naïve cotton rats exhibited high lung viral loads with average titers of 4.5 log10 at day 5 post-challenge. FI-RSV, F and FG VLP immune cotton rats did not show viral titers above the detection limit (1.7 log10). The G VLP group exhibited low viral titers close to the limit of detection. Thus, insect cell-derived VLP vaccination can effectively control RSV replication without airway resistance of hyper-responsiveness and weight loss in cotton rats.
FG VLP vaccination induces B cells capable of secreting F-specific IgG antibodies
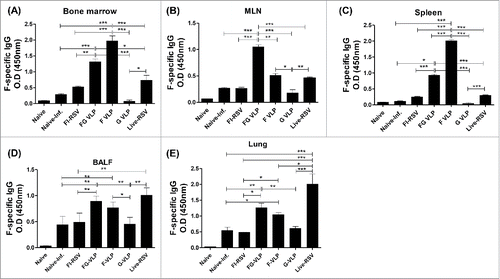
To determine antibody secreting cell responses in vitro, BM, MLN, and spleen were cultured and RSV F protein-specific IgG antibodies determined by ELISA (). FG VLP or F VLP immunized cotton rats induced higher levels of F specific antibody secreting cell responses in BM, MLN, and spleens than those from the FI-RSV, G VLP or naïve group with infection (). RSV F specific IgG antibodies were determined in bronchoalveolar lavage fluids (BALF) and lung extract samples collected at day 5 post challenge (). RSV specific antibody levels in BALF samples were not significantly higher in intramuscularly immunized FG VLP cotton rats before challenge compared with those in naïve cotton rats (Data not shown). After RSV challenge, higher levels of RSV F specific IgG antibodies were induced in the BALF and lungs from the FG VLP or F VLP group compared with those in FI-RSV, G VLP immune or naïve cotton rats (). It is speculated that RSV challenge of the FG and F VLP groups resulted in further increasing IgG antibody levels in BALF and lungs. The live-RSV group showed highest levels of IgG antibody response compared with other vaccine groups in BALF and lung samples but not in bone marrow, draining lymph nodes, and spleens (). These results suggest that FG or F VLP immunization of cotton rats efficiently induces IgG antibody responses as well as antibody secreting cells and long-lived B cells that can differentiate into F-specific antibody secreting cells in MLN, spleens, and BM.
Figure 3. FG VLP or (F)VLP vaccination is effective in inducing RSV F-specific antibodies and antibody secreting cells. (A) BM cells secreting F specific IgG antibodies. (B) MLN cells secreting F specific IgG antibodies. (C) Spleen cells secreting F specific IgG antibodies. Cells from BM, spleens, and MLN were incubated in the culture plates coated with RSV F protein (400 ng/ml) for 2 d. Secreted antibodies were detected by ELISA analysis. RSV F-protein specific l IgG antibody responses in BALF (D) and lung extracts (E) were determined by ELISA. Results are presented as mean ± SEM and statistical significance was performed by one-way ANOVA with Tukey's multiple comparisons post-test in Graph Pad Prism; *** p < 0.001, ** p < 0.01, * p < 0.05.
FG VLP vaccination does not cause pulmonary histopathology and eosinophilia after challenge
To determine pulmonary histopathology, lung tissues at day 5 post RSV challenge were stained and evaluated for peribronchiolitis, perivasculitis, interstial pneumonitis, and histopathological scores (). The FI-RSV group showed highest influx of inflammatory cells around the airways and alveolar septa (B, 2.3), blood vessels (C, 1.8), and interstitial spaces (D, 1.9) as well as cell thickening of airway linings (). RSV infection of naïve cotton rats also caused moderate levels of pulmonary inflammation in the airways (1.2), blood vessels (0.7), and interstitial spaces (0.6). The lowest histopathology scores around the airways, blood vessels, and interstitial spaces were observed with FG VLP or F VLP vaccination (). The G VLP group displayed a certain degree of inflammation around the airways, a higher level than the FG or F VLP group.
Figure 4. FG VLP does not cause pulmonary histopathology in cotton rats after RSV challenge. (A) Representative histology pictures with H&E, PAS, and H&CR. Lung tissues were collected from individual cotton rats at day 5 after RSV A2 challenge and tissue section stained with H&E, PAS and H&CR to assess pulmonary histopathologic changes. H&E stained images indicate peribronchiolar, perivascular, and alveolar pneumonia. (B-D) Histopathology scores. Inflammation response on H&E stained tissue section were scored in the airways (B), blood vessels (C), and interstitial spaces (D) on a scale of 0 to 3 according to diagnostic criteria. (E) Eosinophils. Pulmonary eosinophils per 40X field were counted in 2 different regions of each cotton rats. (F) Mucus producing PAS positive area (%). Bronchiolar mucus expression was stained with PAS (A, F) and scored as percentages of positive from 5 individual airways of each cotton rat. Scale bars indicate 100 µm in H&E and PAS staining and 20 µm in H&CR staining. Results are presented as mean ± SEM and statistical significance was performed by one-way ANOVA with Tukey's multiple comparisons post-test in Graph Pad Prism; *** p < 0.001, ** p < 0.01, * p < 0.05.
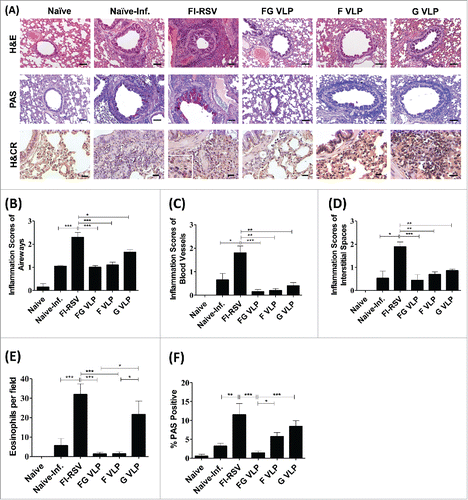
In addition to histological inflammation, we measured eosinophilic infiltrates in the lung sections by H&CR staining. The FG and F VLP groups did not have eosinophil infiltrations similar to naïve uninfected cotton rats (). It is notable that G VLP immune cotton rats showed a higher level of eosinophils than that of FG VLP or F VLP immune animals.
To determine mucus production, lung tissue sections stained with PAS were used to quantify PAS positive area in the airway epithelium (). The FI-RSV group exhibited high PAS positivity in 10 randomly selected airways in each rat. In contrast, the FG VLP group showed lowest PAS positivity similar to naïve uninfected cotton rats. G VLP immune cotton rats exhibited substantial levels of mucus production as indicated by PAS positivity. Overall these results suggest that FG VLP and F VLP can prevent RSV vaccine-enhanced pulmonary inflammation whereas G VLP can cause a certain level of lung inflammation upon RSV infection.
RT-PCR was applied to determine cytokine and chemokine mRNA expression levels in lung tissue samples collected from FG VLP vaccinated cotton rats at day 5 post challenge (Fig. S2). Th1 IFN- γ mRNA levels were observed at the highest levels in the FG VLP group (Fig. S2B). Naïve cotton rats also showed a moderate level of IFN- γ mRNA upon RSV infection. In contrast, Th2 type IL-4 mRNA levels were highest in the FI-RSV group (Fig. S2C). It is notable that FI-RSV immune cotton rats induced the highest level of tumor necrosis factor (TNF)-α mRNA expression after RSV challenge whereas the FG VLP group showed a background level of TNF-α mRNA (Fig. S2D). The chemokine interferon-γ inducible protein-10 (IP-10) mRNA expression was detected at the highest level in naïve cotton rats with RSV infection (Fig. S2E). Interestingly, the naïve group showed high levels of IL-6 and IL-10 mRNA expression but these cytokine mRNAs were not detected in other groups (Fig. S2F, G), suggesting a correlation with high viral loads in cotton rats after RSV infection. These results suggest that FG VLP vaccination modulates the induction of Th1 and Th2 cytokines, and chemokines possibly relating to the prevention of RSV vaccine-enhanced disease.
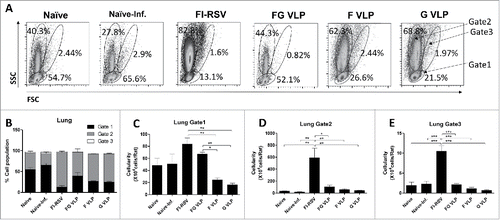
FG VLP vaccines differentially modulates infiltration of granulocytes and lymphocytes into the lungs upon RSV challenge
To better understand infiltrating cell populations in the lungs, we analyzed the side-scattering (SSC) and forward-scattering (FSC) profiles of cells (), by following a similar gating strategy reported.Citation15,16 SSC and FSC gating of cotton rat lung cells displayed 3 distinct cellular populations: the gate 1 cells (small lymphocyte like cells), the large SSC gate 2 cells (monocyte, eosinophil, neutrophil like cells), and the large SSC and FSC gate 3 cells (dendritic, macrophage-like cells) respectively. The FI-RSV group showed the large granular size gate 2 cells in the lungs at the highest percentages and cellularity, which are 5 to 10-fold higher than those in other groups (naïve, naïve-inf, FG, F, G VLP) (). The FG VLP group displayed a similar pattern of cellular distributions as observed in uninfected naïve cotton rats (). F or G VLP immune cotton rats showed a relatively low cellularity in the small size lymphocyte-like gate 1 cells compared with other groups (). Therefore these results suggest that RSV F, G, or FG VLP immunization of cotton rats does not induce abnormal infiltration of inflammatory innate and lymphocyte immune cells into the lungs.
Figure 5. Flow cytometric analysis of cellular infiltrates into the lungs after RSV challenge of cotton rats. (A) Flow cytometry profiles of lung cells based on forward (size, FSC) and side (granularity, SSC) scattering in lung cells. Percentages of cell populations of regional gates 1, 2, and 3 in lung cells. (B) Cellularity of the gate 1 (lymphocytes). (B) Cellularity of the gate 2 (monocytes, neutrophils, eosinophils). (C) Cellularity of the gate 3 (Dendritic, granular/myeloid and macrophage cells). (B-D) Cellularity was presented from the results of total lung cell numbers per cotton rats multiplied by percentages of each population. Gate 1: Small lymphocyte-like cells, Gate 2: Large monocytes, neutrophils, and eosinophils-like cells, Gate 3: Dendritic, granular/myeloid and macrophage-like cells. Results are presented as mean ± SEM and statistical significance was performed by one-way ANOVA with Tukey's multiple comparisons post-test in Graph Pad Prism; *** p < 0.001, ** p < 0.001, * p < 0.05.
Discussion
Cotton rats are considered an appropriate animal model that can reliably predict the clinical outcomes.Citation17,18 Recently, the RSV F nanoparticles produced in insect cells were reported to be safe in healthy individuals and young women of childbearing age in phase I and II clinical trials.Citation19,20 Protection was reported without causing RSV disease by immunization of mice with mixed F VLP and G VLP (FG VLP) vaccines with DNA plasmids expressing F proteins as evidenced by clearing lung viral loads and preventing pulmonary inflammation.Citation21 However, the efficacy and safety of RSV F, G, or F+G VLP vaccine platforms have not been studied in cotton rats, which is the main focus in this study. We have investigated the immunogenicity, RSV disease assessment (weight loss, AHR), efficacy, cytokine patterns to indicate the type of immune response upon RSV infection, and vaccine-associated safety of F, G, and FG VLP vaccines in cotton rats. F, G, and FG VLP vaccines did not cause RSV disease of weight loss and AHR in addition to conferring protection against RSV replication. Mixed FG VLP was found to be more immunogenic in inducing RSV F specific antibody responses and less PAS positive mucus production compared with other F or G VLPs.
RSV is an enveloped virus. Therefore, enveloped non-replicating VLPs would provide an attractive approach to mimic the virus. Chimeric NDV-RSV VLPs in avian cells by transiently transfecting DNA plasmids expressing multiple NDV proteins and chimeric RSV G plus F proteins were not effective in producing F alone VLPs.Citation5 In the insect cell expression system, full length RSV F or G proteins were effectively incorporated into VLPs.Citation10 In cotton rats, this study demonstrated that F VLP was more immunogenic and effective in clearing lung viral titers than G VLP although both F VLP and G VLP did not cause weight loss and AHR lung disease after RSV challenge. FI-RSV immune and naïve cotton rats showed high AHR representing RSV disease mimicking the RSV pathogenesis in humans. Some RSV vaccine candidates (FI-RSV, purified-F, -G proteins, and recombinant live vectors expressing RSV F or G) are likely to have safety concerns of inducing vaccine-enhanced respiratory disease. The safety issue of vaccine-enhanced pulmonary inflammation in developing RSV vaccines should be a high priority to be addressed. Vaccination of mice with recombinant vaccinia virus (vv) expressing RSV G (vv-G) but not with vv-F was reported to induce severe pulmonary eosinophilia after RSV challenge, closely mimicking the vaccine-enhanced disease as observed with FI-RSV.Citation22-24 Both vv-G and vv-F vaccination of mice were shown to induce significant RSV disease of weight loss and histopathology after RSV challenge.Citation25 G-specific T cells were known to cause more severe RSV disease than F-specific T cells in mice.Citation26 Cotton rats with G VLP presented a tendency of causing a certain degree of lung inflammation around the airways, blood vessels, and interstitial spaces as well as eosinophils and mucus production compared with F VLP or FG VLP but much less than FI-RSV. Cotton rats with F VLP did not show RSV disease after challenge, which is consistent with the results in mice.Citation11 Interestingly, cotton rats with FG VLP significantly suppressed RSV disease of eosinophila and mucus production, compared with G VLP alone and even better than F VLP. This result of additive effects on preventing RSV disease in cotton rats by FG VLP is similarly observed in a mouse model.Citation21
FI-RSV immune or naïve cotton rats exhibited RSV disease such as weight loss and AHR after RSV infection. Cotton rats with FI-RSV showed high levels of IL-4 and inflammatory TNF-α cytokines compared with FG VLP immune cotton rats. Mice primed with vv-G vaccines induced high levels of both Th2 IL-4 and Th1 IFN-γ cytokines at high levels while vv-F primed mice showing high levels of IFN-γ producing cells.Citation25 IFN-γ has a dual role of RSV protection and RSV disease with high IFN-γ T cell responses.Citation27 Th2 cytokines such as IL-4 have been implicated in the development of lung immunopathology whereas TNF-α inflammatory cytokine was shown to be responsible for weight loss in mice.Citation28 Naïve cotton rats with RSV infection induced higher levels of IP-10 and IL-6 cytokines which were also shown to be associated with acute RSV infection.Citation29,30 Thus, high levels of Th2, Th1, and inflammatory cytokines as well as chemokine IP-10 might be involved in causing RSV disease, which are modulated to low levels by FG VLP vaccination preventing RSV disease.
Cotton rats are more susceptible to RSV infection than mice so that histopathological pulmonary inflammation may closely mimic that observed in RSV infected humans. Nonetheless, cotton rats are difficult to handle. Cellular immunology of mechanism studies of vaccines in cotton rats is limited and also difficult compared with mice. Considering the major target population for RSV vaccination, efficacy studies of single vaccination in neonatal and infant cotton rat models will provide valuable information, which remains to be determined. It is also unclear that the vaccine efficacy in cotton rats will be predictable in humans. Scale-up manufacturing and purification processes of RSV VLP vaccines should be developed.
In conclusion, F VLP and FG VLP vaccines were immunogenic and able to confer protection without causing RSV disease and pulmonary inflammation in cotton rats. Importantly, inclusion of F VLP in the G VLP vaccination of cotton rats could prevent eosinophilia and mucus production after RSV challenge. This study provides evidence that F VLP and FG VLP can be developed into safe RSV vaccine candidates.
Material and methods
Preparation of RSV vaccines
RSV F (F-VLP) and RSV G (G-VLP) VLP vaccines were produced in insect cells as described previously.Citation10 RSV (A2 strain) grown in HEp-2 cells was inactivated with formalin (1:4000 vol/vol) for 3 d at 37°C and purified using ultra centrifugation as described previously.Citation10 FI-RSV vaccine was adsorbed to aluminum hydroxide adjuvant (4mg/ml) for immunization.Citation31
Immunization and challenge in cotton rats
Cotton rats (Sigmodon hispidis, 4–5 weeks old) were purchased from the Harlan Laboratories (Indianapolis, IN, USA). Cotton rats were randomly assigned to each vaccine group (n = 5) and intramuscularly (i.m.) immunized with F VLP (20 μg), G VLP (20 μg), mixed RSV F (20 μg) and G VLP (20 μg) (designated FG VLP), FI-RSV (2 μg), or intranasally (i.n.) inoculated with live RSV (0.3 × 106 plaque-forming units, PFU) at week 0 (prime) and 4 (boost). Cotton rats were i.n. challenged with 1 × 106 PFU of RSVA2 at 4 weeks after boost and daily monitored to record body weight changes. At day 5 post challenge, cotton rats were killed to collect lungs, spleens, bone marrows (BM), mediastinal lymph nodes (MLN) and broncho-alveolar lavage fluid (BALF) samples. BALF samples were collected by infusing 1 ml of phosphate buffered saline (PBS) into the lungs via trachea using a 25 gauge catheter. After BALF collection, the lung tissues were obtained and homogenized to collect lung extracts for measuring IgG antibodies. Experimental procedures were conducted with the approval by Institutional Animal Care and Use Committee of Georgia State University and were in full compliance with the Committee's guidelines.
Assays for serum antibodies, neutralizing activity, and antibody secreting cells
RSV F specific antibodies and G specific antibodies (IgG) in primed and boosted sera were determined by ELISA using RSV F (BEI, NIAID, NIH) or G protein (Sino Biological, PA) as a coating antigen. The antibody responses were detected using horseradish peroxidase (HRP) -conjugated goat anti-rat IgG (eBioscience). RSV-specific neutralizing antibody titers were measured using RSV expressing the red fluorescent monomeric Katushka 2 protein (A2-K-line19F) as described.Citation16 The mean percentages of red-fluorescent reduction by sera from vaccinated rats compared with medium control. RSV F specific IgG antibodies were determined in BALF and lung lysate collected at day 5 post challenge. To determine IgG antibody secreting cell responses, 1 × 106 cells from MLN, spleens, and BM were added to the 96 well plates coated with RSV F proteins and then incubated for 2 d. The antibodies secreted into the culture supernatants were determined with HRP conjugates of anti-rat IgG, then developed with 3,3′,5,5′-tetramethylbenzidine (TMB, Sigma Aldrich) and stopped with 1M H3PO4. The optical density at 450 nm was read using an ELISA reader (Bio-Tek, EL800).
Airway hyper-responsiveness and lung viral titers
Before RSV challenge, baseline airway hyper-responsiveness (AHR) to methacholine (Sigma, St Louis, MO, USA) was evaluated using a whole-body plethysmograph (EMKA Technologies). The AHR data presented as the percent increases above the baseline enhanced pause (Penh) measurements. Viral titers in individual lungs at day 5 after challenge were determined using an immune plaque assay as previous described.Citation10
Lung histopathology and flow cytometry
The lungs collected at 5 d after challenge were fixed with 10% formalin in PBS, embedded in paraffin blocks. Lung tissue sections were stained with hematoxylin and eosin (H&E), periodic acid-schiff stain (PAS), and hematoxylin and congo red (H&CR) to assess histopathological changes, mucin expression, and eosinophils infiltration respectively as described previously.Citation21,32 Numerical assessment of histopathology was performed on a scale of 0–3 by blinded observers with the severity scoring system.Citation21,33 The mucin expression was detected with PAS-positive area. The degrees of pulmonary eosinophilia were indicated by eosinophil numbers present per 400× field using H&CR stain. Lung cells were isolated by layering cell suspensions in percoll density gradient medium (44% and 67%) followed by red blood cell lysis.Citation16 Using Flow cytometry, relative size and granularity for lung cells were analyzed based on forward (FSC) and side (SSC) scattering.
Reverse transcriptase (RT) polymerase chain reaction (PCR)
Total RNA from homogenized lung tissues was isolated by using Tryzol (Ambion, Fisher Scientific), according to the manufacturer's instructions. Cytokine or chemokine specific cDNA synthesis and semiquantitative RT-PCR were determined by the described previously method.Citation34 In brief, total RNA (1µg) from each sample was used for the cDNA synthesis by using oligo dT primers and Taq reverse transcription reagent (applied biosystem). The primers and conditions for PCR-amplified cotton rat cytokine and chemokine cDNA were described previously.Citation34
Statistical analysis
The data are shown as means ± standard errors of means (SEM). Statistical analysis was performed by a one-way ANOVA with Tukey's post hoc test or 2-way ANOVA with Bonferroni post-test multiple comparison test (Graph Pad Software Inc.).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary files
Download Zip (1.4 MB)Acknowledgments
The following reagent was obtained through BEI Resources, NIAID, NIH: Soluble Fusion Glycoprotein with C-Terminal His Tag from RSV A2, recombinant produced in 293F cells, NR-28908.
Funding
This work was supported by NIH/NIAID grants AI105170 (S.M.K.), AI119366 (S.M.K.), and AI093772 (S.M.K.).
References
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545-55; PMID:20399493; http://dx.doi.org/10.1016/S0140-6736(10)60206-1
- Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 1969; 89:405-21; PMID:4305197
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89:422-34; PMID:4305198
- van Drunen Littel-van den Hurk S, Mapletoft JW, Arsic N, Kovacs-Nolan J. Immunopathology of RSV infection: prospects for developing vaccines without this complication. Rev Med Virol 2007; 17:5-34; PMID:17004293; http://dx.doi.org/10.1002/rmv.518
- McGinnes LW, Gravel KA, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Schmidt MR, Morrison TG. Assembly and immunological properties of Newcastle disease virus-like particles containing the respiratory syncytial virus F and G proteins. J Virol 2011; 85:366-77; PMID:20980510; http://dx.doi.org/10.1128/JVI.01861-10
- Murawski MR, McGinnes LW, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Heaton PM, Fraire AE, Morrison TG. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. J Virol 2010; 84:1110-23; PMID:19889768; http://dx.doi.org/10.1128/JVI.01709-09
- Schmidt MR, McGinnes-Cullen LW, Kenward SA, Willems KN, Woodland RT, Morrison TG. Modification of the respiratory syncytial virus f protein in virus-like particles impacts generation of B cell memory. J Virol 2014; 88:10165-76; PMID:24965456; http://dx.doi.org/10.1128/JVI.01250-14
- Schmidt MR, McGinnes LW, Kenward SA, Willems KN, Woodland RT, Morrison TG. Long-term and memory immune responses in mice against Newcastle disease virus-like particles containing respiratory syncytial virus glycoprotein ectodomains. J Virol 2012; 86:11654-62; PMID:22896618; http://dx.doi.org/10.1128/JVI.01510-12
- Cullen LM, Blanco JC, Morrison TG. Cotton rat immune responses to virus-like particles containing the pre-fusion form of respiratory syncytial virus fusion protein. J Transl Med 2015; 13:350; PMID:26541285; http://dx.doi.org/10.1186/s12967-015-0705-8
- Quan FS, Kim Y, Lee S, Yi H, Kang SM, Bozja J, Moore ML, Compans RW. Viruslike particle vaccine induces protection against respiratory syncytial virus infection in mice. J Infect Dis 2011; 204:987-95; PMID:21881112; http://dx.doi.org/10.1093/infdis/jir474
- Kim KH, Lee YT, Hwang HS, Kwon YM, Kim MC, Ko EJ, Lee JS, Lee Y, Kang SM. Virus-like particle vaccine containing the F protein of respiratory syncytial virus confers protection without pulmonary disease by modulating specific subsets of dendritic cells and effector t cells. J Virol 2015; 89:11692-705; PMID:26355098; http://dx.doi.org/10.1128/JVI.02018-15
- Lee S, Quan FS, Kwon Y, Sakamoto K, Kang SM, Compans RW, Moore ML. Additive protection induced by mixed virus-like particles presenting respiratory syncytial virus fusion or attachment glycoproteins. Antiviral Res 2014; 111:129-35; PMID:25239522; http://dx.doi.org/10.1016/j.antiviral.2014.09.005
- Boukhvalova MS, Prince GA, Blanco JC. The cotton rat model of respiratory viral infections. Biologicals 2009; 37:152-9; PMID:19394861; http://dx.doi.org/10.1016/j.biologicals.2009.02.017
- Niewiesk S, Prince G. Diversifying animal models: the use of hispid cotton rats (Sigmodon hispidus) in infectious diseases. Lab Anim 2002; 36:357-72; PMID:12396279; http://dx.doi.org/10.1258/002367702320389026
- Hotard AL, Shaikh FY, Lee S, Yan D, Teng MN, Plemper RK, Crowe JE, Jr., Moore ML. A stabilized respiratory syncytial virus reverse genetics system amenable to recombination-mediated mutagenesis. Virology 2012; 434:129-36; PMID:23062737; http://dx.doi.org/10.1016/j.virol.2012.09.022
- Hwang HS, Lee YT, Kim KH, Park S, Kwon YM, Lee Y, Ko EJ, Jung YJ, Lee JS, Kim YJ, et al. Combined virus-like particle and fusion protein-encoding DNA vaccination of cotton rats induces protection against respiratory syncytial virus without causing vaccine-enhanced disease. Virology 2016; 494:215-24; PMID:27123586; http://dx.doi.org/10.1016/j.virol.2016.04.014
- Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, Dormitzer M, O'Grady J, Koenig S, Tamura JK, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis 1997; 176:1215-24; PMID:9359721; http://dx.doi.org/10.1086/514115
- Siber GR, Leombruno D, Leszczynski J, McIver J, Bodkin D, Gonin R, Thompson CM, Walsh EE, Piedra PA, Hemming VG, et al. Comparison of antibody concentrations and protective activity of respiratory syncytial virus immune globulin and conventional immune globulin. J Infect Dis 1994; 169:1368-73; PMID:8195619; http://dx.doi.org/10.1093/infdis/169.6.1368
- Glenn GM, Fries LF, Thomas DN, Smith G, Kpamegan E, Lu H, Flyer D, Jani D, Hickman SP, Piedra PA. A Randomized, Blinded, Controlled, Dose-ranging study of a respiratory syncytial virus recombinant fusion (F) nanoparticle vaccine in healthy women of childbearing age. J Infect Dis 2015; 213(3):411-22; PMID:26259809
- Glenn GM, Smith G, Fries L, Raghunandan R, Lu H, Zhou B, Thomas DN, Hickman SP, Kpamegan E, Boddapati S, et al. Safety and immunogenicity of a Sf9 insect cell-derived respiratory syncytial virus fusion protein nanoparticle vaccine. Vaccine 2013; 31:524-32; PMID:23153449; http://dx.doi.org/10.1016/j.vaccine.2012.11.009
- Hwang HS, Kwon YM, Lee JS, Yoo SE, Lee YN, Ko EJ, Kim MC, Cho MK, Lee YT, Jung YJ, et al. Co-immunization with virus-like particle and DNA vaccines induces protection against respiratory syncytial virus infection and bronchiolitis. Antiviral Res 2014; 110:115-23; PMID:25110201; http://dx.doi.org/10.1016/j.antiviral.2014.07.016
- Johnson TR, Johnson JE, Roberts SR, Wertz GW, Parker RA, Graham BS. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol 1998; 72:2871-2880; PMID:9525607
- Olson MR, Varga SM. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J Immunol 2007; 179:5415-24; PMID:17911628; http://dx.doi.org/10.4049/jimmunol.179.8.5415
- Varga SM, Wissinger EL, Braciale TJ. The attachment (G) glycoprotein of respiratory syncytial virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4+ T cell responses. J Immunol 2000; 165:6487-95; http://dx.doi.org/10.4049/jimmunol.165.11.6487
- Monick MM, Powers LS, Hassan I, Groskreutz D, Yarovinsky TO, Barrett CW, Castilow EM, Tifrea D, Varga SM, Hunninghake GW. Respiratory syncytial virus synergizes with Th2 cytokines to induce optimal levels of TARC/CCL17. J Immunol 2007; 179:1648-58; PMID:17641031; http://dx.doi.org/10.4049/jimmunol.179.3.1648
- Alwan WH, Kozlowska WJ, Openshaw PJ. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med 1994; 179:81-9; PMID:8270885; http://dx.doi.org/10.1084/jem.179.1.81
- Castilow EM, Olson MR, Meyerholz DK, Varga SM. Differential role of gamma interferon in inhibiting pulmonary eosinophilia and exacerbating systemic disease in fusion protein-immunized mice undergoing challenge infection with respiratory syncytial virus. J Virol 2008; 82:2196-207; PMID:18094193; http://dx.doi.org/10.1128/JVI.01949-07
- Hussell T, Pennycook A, Openshaw PJ. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur J Immunol 2001; 31:2566-73; PMID:11536154; http://dx.doi.org/10.1002/1521-4141(200109)31:9%3c2566::AID-IMMU2566%3e3.0.CO;2-L
- Matsuda K, Tsutsumi H, Sone S, Yoto Y, Oya K, Okamoto Y, Ogra PL, Chiba S. Characteristics of IL-6 and TNF-alpha production by respiratory syncytial virus-infected macrophages in the neonate. J Med Virol 1996; 48:199-203; PMID:8835355; http://dx.doi.org/10.1002/(SICI)1096-9071(199602)48:2%3c199::AID-JMV13%3e3.0.CO;2-A
- Roe MFE, Bloxham DM, Cowburn AS, O'Donnell DR. Changes in helper lymphocyte chemokine receptor expression and elevation of IP-10 during acute respiratory syncytial virus infection in infants. Pediatr Allergy Immunol 2011; 22:229-34; PMID:20561238; http://dx.doi.org/10.1111/j.1399-3038.2010.01032.x
- Prince GA, Curtis SJ, Yim KC, Porter DD. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J Gen Virol 2001; 82:2881-8; PMID:11714962; http://dx.doi.org/10.1099/0022-1317-82-12-2881
- Meyerholz DK, Griffin MA, Castilow EM, Varga SM. Comparison of histochemical methods for murine eosinophil detection in an RSV vaccine-enhanced inflammation model. Toxicol Pathol 2009; 37:249-55; PMID:19181630; http://dx.doi.org/10.1177/0192623308329342
- Stokes KL, Chi MH, Sakamoto K, Newcomb DC, Currier MG, Huckabee MM, Lee S, Goleniewska K, Pretto C, Williams JV, et al. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J Virol 2011; 85:5782-93; PMID:21471228; http://dx.doi.org/10.1128/JVI.01693-10
- Blanco JC, Richardson JY, Darnell ME, Rowzee A, Pletneva L, Porter DD, Prince GA. Cytokine and chemokine gene expression after primary and secondary respiratory syncytial virus infection in cotton rats. J Infect Dis 2002; 185:1780-5; PMID:12085325; http://dx.doi.org/10.1086/340823
