ABSTRACT
One hundred and ninety eight females aged 12–15 y were enrolled in an observer-blinded randomized trial to assess the immunogenicity and reactogenicity of the tetravalent HPV vaccine Gardasil® (group 2), in comparison to the bivalent HPV vaccine, Cervarix® (group 1), which was routinely offered in the national vaccination schedule at the time. Participants were blinded to treatment group until all 3 vaccinations had been given, while laboratory staff were masked during testing. For the majority of local and general reactions, recipients of both vaccines reported comparable frequencies. Local and systemic events were rarely of high severity, except for tenderness at the injection site which reached a severe level after at least one of the doses in 24% of the Cervarix® group and 7% of the Gardasil® group (p = 0.001 comparing groups). For most reactions, no dose response was recorded, except for swelling with higher reporting at dose 3 (17.7%) than dose 1 (3.1%) for Cervarix®. SAE reporting was low (n = 3) and considered unrelated to either vaccine. This paper supports the body of evidence that Gardasil® has an acceptable safety profile when compared with Cervarix® and other vaccines given in the national program.
Introduction
Since September 2008, the UK has included vaccination against Human Papillomavirus (HPV) in its immunisation schedule for females aged 12–13 years, as a public health measure to reduce cervical cancers. This was delivered as the bivalent vaccine Cervarix® from September 2008 as a 3-dose schedule, with a catch up campaign. Cervarix® was replaced by the tetravalent vaccine Gardasil® in 2012 which was reduced to a 2-dose schedule in 2014.Citation1 The randomized immunogenicity trial reported here was undertaken before the introduction of Gardasil® in the national program, primarily to determine cross-reactivity of the tetravalent vaccine against non-vaccine types compared with the bivalent vaccine, as well as reporting the safety and immunogenicity of both vaccines.Citation2
The field of vaccine safety and reactogenicity is particularly relevant when addressing parental attitudes to vaccination programmes and the effects on coverage. The results of public concern over vaccine safety have best been demonstrated throughout the MMR controversies, documented via tracking surveys between 1996–2006, and realized through the significant decline in vaccine coverage that followed - coverage in 2006 was still 7% less than in 1998 before the controversy.Citation3 Attitudinal research performed before the introduction of routine HPV vaccination revealed little awareness of HPV among parents (19%), but once provided with information, the majority (84%) said they would consent to vaccination while concerns mainly related to possible side effects and the age at vaccination.Citation4 Parent interviews in another study documented concerns surrounding longer-term effects of new vaccines and their safety, while being confident of vaccines already given.Citation5 The importance of a wealth of evidence for vaccine safety has increasingly gained precedence for the resulting success of vaccination programmes. The HPV program was well-received with coverage rising from 80.9% to 85.9% over 6 y of the 3-dose Cervarix® schedule, reliant on strong evidence for safety such as the data presented in this manuscript and continual surveillance.Citation1
As seen with other vaccines introduced to this age group some potential immune mediated conditions seen in teenage girls (postural orthostatic tachycardia syndrome and complex regional pain syndrome) have been linked to HPV vaccines based on individual case reports, but review of trials and pharmacovigilance data has not supported these claims.Citation6 An unpublished cohort study in France has suggested an increased risk of Guillain–Barré syndrome, which was not seen in other studies such as a cohort study in Finland of potential immune mediated reactions and requires confirmation in further studies.Citation7,8 It was useful to collect safety data throughout this study though the primary objectives were immunological. The original paperCitation2 reported higher moderate/severe tenderness for Cervarix®, but comparable reporting for the other local and general reactions recorded. This manuscript assesses in greater detail the reactogenicity data collected, reporting on dose specific responses and the severity of symptoms.
The efficacy and safety of both vaccines had been demonstrated before this study, with consensus on the need for longer-term follow-up studies to determine correlates of protection against disease, evaluate reduction in disease prevalence and the duration of protection afforded by HPV vaccines.Citation9 This study aimed to assess the broader protection offered by HPV vaccines using evidence of cross-neutralisation of non-vaccine types.Citation10 This paper reports safety data collected from all 198 participants for 7 d following 3 doses of Cervarix® or Gardasil® and throughout the study period for serious adverse events (SAEs).
Results
Study population
The final study population was roughly half of the anticipated 200 per arm across participating surgeries in Hertfordshire and Gloucestershire. Safety data was recorded for all 198 participants at each dose, except 3 participants who provided only 2 diaries. Of the 198 participants, all received 3 doses of vaccine, 96 participants received Cervarix® (group 1) and 102 Gardasil® (group 2). The median age was 12 y old at enrolment.
shows results across all 3 doses for reactions of any severity and results by dose and level of severity.
Local reaction
The most common local reaction was tenderness, at some level of severity for 93.8% and 86.3% of Cervarix® and Gardasil® recipients respectively after a dose (p = 0.10) (). Severe tenderness solely (unwillingness to use arm) was significantly different between vaccines, reported by 24% vs 6.9% of Cervarix® and Gardasil® vaccinees (p = 0.001) (). This difference was greatest after the first dose (p = 0.004). Tenderness was more sustained for Cervarix®, whereas for Gardasil®, tenderness was rarely reported for more than 3 d (). The day whereby maximum tenderness was recorded tended to be on day's 0 and 1 following vaccination ().
Figure 1. Local reactions. (A) Displays the duration of solicited local reactions following vaccination, on average across all 3 doses of each study vaccine as a percentage of vaccinated participants. Events displayed represent any severity of symptom. (B) Presents the average day after vaccination whereby the maximum local reaction was first experienced for each vaccine over all 3 doses, as a percentage of vaccinees. Day 0 is the day of vaccination.
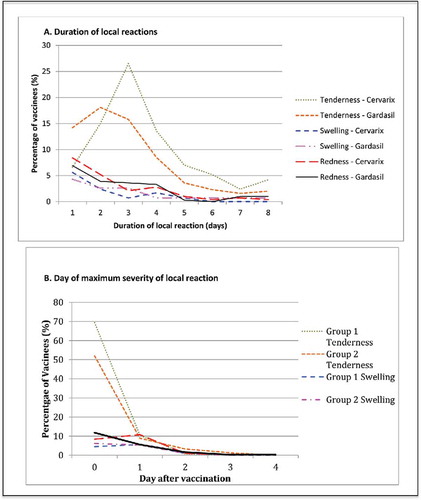
Proportions reporting redness at the injection site were comparable for both vaccines (), with most reporting redness of mild severity (< 30 mm diameter) and rarely moderate or severe redness (). The onset of maximum redness tended to be on the day of vaccination or the day following, lasting 1–2 d and rarely exceeding 4 d ().
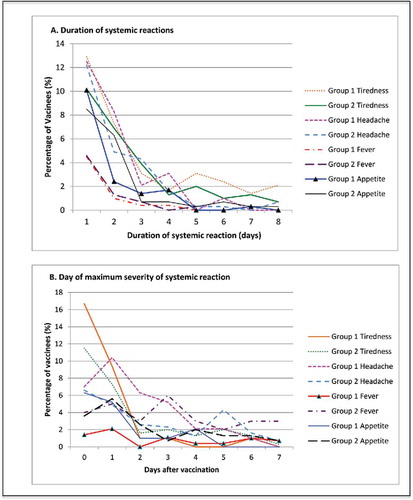
Figure 2. Systemic reactions. (A) Displays the duration of solicited systemic reactions following vaccinations on average across all 3 doses of each study vaccine, as a percentage of vaccinated participants. Events displayed represent any severity of sympton. (B) Presents the average day after vaccination whereby the maximum systemic reaction was first experienced for each vaccine over all 3 doses, as a percentage of vaccines. Day 0 is the day of vaccination.
Swelling was the most infrequently reported local reaction, experienced by 25% of Cervarix® and 24.5% of Gardasil® vaccinees.() The onset of maximum swelling tended to be on day 0 or 1 and the duration was rarely beyond 4 d ().
When comparing 95% confidence intervals for proportions with any severity of each local reaction across doses (data not shown), only swelling demonstrated a dose specific response between dose1 and dose3, with 3/96 vs. 17/96 reporting this reaction in the Cervarix arm.
Systemic reactions
Systemic symptoms were most commonly reported at day 0 or 1 () and rarely lasted beyond 4 d (). When comparing 95% confidence intervals for systemic reactions, no dose specific differences were observed at any level of severity (data not shown).
Tiredness was frequently documented with similar proportions for both vaccines (, ), except at dose 3, with 11.5% (11/96) of Cervarix® vs 3% (3/101) of Gardasil® vaccinees reporting moderate/severe tiredness (p = 0.026). Severe tiredness was rare in both groups and any level didn't often last beyond 4 d (). The highest level of tiredness was first recorded on days 0/1 ().
Headache was reported by 49% of Cervarix® and 47.1% of Gardasil® recipients (). However, headache was generally mild, with 4.2% and 1% of participants reporting severe headache for Cervarix® and Gardasil® respectively (). The groups were comparable at each dose, except for moderate/severe headache after dose 2; in 9.5% of Cervarix® and 1% of Gardasil® vaccinees (p = 0.008) (). Duration of headache was rarely beyond 3/4 d (). The majority of the onset of maximum headache was at day 0 or 1 ().
Loss of appetite was less common than headache or tiredness, with similar proportions reporting this between the vaccines administered and was generally mild, with only 8.3 and 8.8% reporting moderate/severe loss of appetite (). Appetite loss was, as per other symptoms, greatest at days 0 and 1 () and generally lasted no longer than 2 d ().
Temperature >37.5°C was reported similarly by both vaccine groups () and was rarely moderate or severe (). The day of maximum temperature experienced was variable across the 7 d post-vaccination (). Fever was generally resolved after 1 day ().
Visits to GP
In both groups there were 4 GP visits. For the Cervarix® group this was due to, hemorrhoids, sore throats, leg muscle tenderness and ear inflammation. In those who received Gardasil®, flu-like symptoms, arm tenderness, a twisted ankle and blackouts with loss of sight were experienced. The Chief Investigator deemed all unrelated except for arm tenderness.
SAEs
Three unrelated SAEs were reported in the trial concerning 2 subjects in group 1 (Cervarix® arm), one presented with abdominal pain and was found to have mesenteric adenitis and the other was admitted to hospital with cervicalgia, migraine, dizziness, nausea and labyrinthitis associated with chronic fatigue. The participant reporting an SAE in the Gardasil® arm was diagnosed with diabetes on admission to hospital with ketoacidosis.
Discussion
This manuscript documents the reactogenicity and safety profiles of the Cervarix® and Gardasil® vaccines when given to healthy UK 12–15 y olds in a 3 dose schedule. The only consistently observed local or systemic reaction difference between vaccines was tenderness, more frequently reported for Cervarix® than Gardasil® at doses 1 and 2 for any level of tenderness and for moderate/severe tenderness at all doses (p = <0.001). Pain at the injection site has been widely reported for Cervarix® but of low severity with few grade 3 events, (defined as symptoms preventing normal activity, redness/swelling >50 mm and fever >39°C).Citation11-13 Studies comparing vaccination with Gardasil® have shown Cervarix® to elicit more local site reactions, though similar reporting of general symptoms and transient reactions with <2% lasting more than 7 d.Citation14
There is evidence to suggest that the increased reactogenicity of Cervarix® can be attributed to the vaccine adjuvant. AS04 is associated with greater reactogenicity as it stimulates superior immunogenicity compared with aluminum salt adjuvants only, giving rise to higher HPV16/18 GMTs, via innate immunity, through interaction with Toll-like receptors and downstream cytokine signaling pathways.Citation15 Cervarix® has been deemed safe for use in national programmes based on results from a large number of trials, whereby tenderness was outweighed by the benefit of vaccination. Such studies have evaluated safety outcomes in seronegative African females aged 10–25,Citation16 in older women >25 y of age,Citation17 HIV positive females across 3 continents,Citation13 males aged 10–18,Citation18 Korean girls, adolescents and women (age 10–25),Citation12,19 Chinese females aged 9–45Citation20 and many other diverse population groups in terms of age, ethnicity, location and gender. They all reported adequate safety profiles for the Cervarix® vaccine, for example; when compared with pooled safety analysis of 11 Phase II/III studies across all age groups,Citation21 also with studies in healthy women, aluminum-based placebos and other vaccine controls such as HepA/B and Boostrix vaccines. Additionally, the safety of the Cervarix® vaccine has since been demonstrated in pregnant women, a group of particular concern due the potential association between the increased reactogenicity and immunogenicity with miscarriage. This study found no increase in risk of miscarriage if vaccination was within 90 d of conception when compared with the unexposed groups of no vaccination or Hepatitis A vaccination.Citation22
The majority of local and systemic symptoms were mild, of short duration and similar between vaccines. Although proportions of participants reporting mild reactions were high for symptoms such as headache, loss of appetite and tiredness, these are common side effects associated with vaccinations. For example, Cervarix® given with diphtheria-tetanus-acellular pertussis-inactivated poliovirus (dTpa-IPV) or both alone was comparable in terms of reporting of local and systemic symptoms.Citation23 Gardasil® administered with Meningococcal ACWY and Tetanus, diphtheria, and acellular pertussis (Tdap) or when given alone was found to be associated with less pain at the HPV site than the Tdap site and systemic symptoms to be less frequent for HPV given alone.Citation24 Cervarix® compared with MenC and/or Tdap, had comparable proportions of general symptoms, local reactions, SAEs and unsolicited AEs reported.Citation25 Hepatitis B vaccination given at the same time as Cervarix®, compared with when given separately demonstrated higher proportions of local symptoms at the HPV site, though short-lived and with low grade 3 reporting (as previously defined), largely comparable proportions of solicited general symptoms and unrelated SAEs, therefore considered safe when administered concomitantly,Citation26,27 as for Hepatitis A and B and Cervarix® combined or given separately.Citation28
Higher proportions of Cervarix® vs Gardasil® recipients reported a raised temperature at dose 1, moderate/severe tiredness at dose 3 and headache at dose 2, however, this was not consistent across all 3 doses (), and may be due to small numbers or confounding factors such as vaccination coinciding with menstrual cycles or examinations. Therefore, as these symptoms were expected, not consistently observed and because both vaccines gave rise to comparable proportions of severe reactions, they did not raise specific safety concerns. In a study comparing the vaccines in 21 centers across 4 countries assessing dosing,Citation14 >1000 18–45 y old American women in a comparison studyCitation9 and HIV seropositive Danish females and males,Citation29 no significant safety concerns arose, supporting the use of Gardasil® in diverse groups. The fact that Gardasil® has a similar or lower reactogenicity for the majority of local and systemic symptoms in this study supports the potential use of both vaccines in the UK vaccination program. Full coverage has not yet been reported for Gardasil® as it is given over 2 school years; however, 89.4% of the target population has received a priming dose.Citation30
A limitation of collecting safety data is the subjectivity of self-assessment. For example, high numbers of participants reporting tenderness may be due to the scale used as our diary considered being ‘unwilling to use arm’ of high severity, which may be open to exaggeration in this age group. Another limitation was the study sample size that was powered for immunogenicity and so did not evaluate new onset chronic or autoimmune diseases and other unsolicited rare AEs, nor report on long-term safety outcomes. Long-term post-licensure surveillance of adverse drug reactions (ADRs) did not uncover any significant safety concerns with Cervarix®.Citation31 Safety monitoring of potential immune-mediated disorders and pregnancy outcomes such as congenital abnormalities, were comparable to background rates.Citation31 Long-term safety analysis since the licensure of Gardasil® across 15 studies, including roughly one million vaccinees and specific population groups has highlighted no increased frequency of SAEs including new onset autoimmune diseases and abnormal pregnancies.Citation32 Our study did not include participants with autoimmune disorders or HIV infection, however as described previously, our results are in line with global studies of diverse groups.Citation14,16,29
The results presented here are supported by safety evidence of both vaccines across diverse ethnic groups, and in specific populations. In the future, studies assessing prevalence of infection and the HPV types contributing to the remainder of cervical cancer cases will be needed to inform development of higher valency HPV vaccines. It will be important to draw on the knowledge obtained regarding the adjuvants and reactogenicity, especially when considering immune interference with the main 2 HPV types,Citation33 as well as higher dosing of VLPs and alternative routes of administration such as intradermal vaccination that may achieve greater immunogenicity.Citation34 These methods would aim to support a cost effective and reduced HPV schedule, while maintaining safety and public confidence.
Recruitment was lower than anticipated to the study due to the short window of opportunity to recruit before the national roll out of the program and the available study population of participating GP surgeries in Hertfordshire and Gloucestershire. Anecdotally, families preferred to wait for the vaccine once introduced, when no samples would be needed and were especially cautious to enrol at the time due to negative press about the HPV vaccine. However, there was sufficient power to answer primary immunological objectives, which informed the sample size calculation, and meant it was not possible to identify less frequent adverse events (AEs), however safety comparisons can be drawn with large surveillance studies and those powered to evaluate safety. A systematic review of 12 RCTs, involving ∼30,000 participants, provided consensus on Cervarix® giving rise to more solicited AEs, especially those at the injection site, compared with Gardasil®. However, SAEs were rarely considered associated with either vaccine and their safety profiles were comparable with other vaccines given in this age group.Citation35
The data in this manuscript demonstrate that the Cervarix® and Gardasil® vaccines, when given in a 3-dose schedule at 0,1 and 6 months, have reactogenicity profiles acceptable for their use in the UK national immunisation program. The most significant finding of this study, in line with similar studies, was that Cervarix® elicited a higher proportion of participants with an event of severe tenderness and tenderness of longer duration at the injection site than Gardasil. There were no consistent differences in swelling or redness nor systemic reactions, except for headache and tiredness where there was a difference between vaccines observed at a single dose. Using non-overlapping confidence intervals, dose specific differences for each vaccine were only observed for any level of swelling induced by Cervarix® though this was not observed for moderate/severe swelling when further examined. These results add to the body of evidence in support of the use of both HPV vaccines, although Cervarix® is shown to elicit more tenderness this is largely accepted as a reaction following vaccination, as for other routine immunisations as detailed in this manuscript. Our results demonstrate the safety of both Cervarix® and Gardasil® in a representative cohort of girls for the UK vaccination schedule as well as confirming the suitability of a 3-dose schedule of Gardasil® at 0,1 and 6 months. This was within the limits of the manufacturers recommendation though not as the advised 0, 2, 6 months, thus the results add confidence to the administration of Gardasil® in this way to the target population.
Materials and methods
Regulatory and ethics approval
The required regulatory approvals for conducting clinical research in the UK were obtained including approvals from the UK Research Ethics Service (Research Ethics Committee reference 09/H0720/25) and the Medicines and Healthcare products Regulatory Agency (MHRA). The EudraCT number for this study is 2008–006773–32. All delegated responsibilities and investigator duties were conducted in line with the Declaration of Helsinki and Good Clinical Practice guidelines, for which study staff received appropriate training.
Subjects
Adolescent girls aged 12–15 years; registered at participating GP surgeries in Hertfordshire and Gloucestershire were invited to take part. Written informed consent was obtained from parents. Individuals were ineligible if pregnant or if they became pregnant throughout the trial, as well as breast-feeding mothers and those with previous allergy to any of the vaccine components. In addition, prior or current vaccination with a HPV vaccine contraindicated participation. Vaccination was delayed if the participants' temperature was >38°C at the study visit. Recruitment began in October 2009 with trial completion after the final blood collection in December 2011.
Sample size/statistical Analysis
Participants were randomized 1:1 to receive Cervarix® or Gardasil® in a 3-dose schedule at 0, 1 and 6 months, as per the routine immunisation schedule for Cervarix® at the time, though not per the exact manufacturers recommendation of providing Gardasil® at 0, 2 and 6 months. This enabled a simpler blinded comparison study by negating the need for an additional visit involving vaccination with placebo at different time points in each arm. Logistically it was also thought of as a way to limit the disruption to the current schedule with the introduction of a different vaccine. Randomization was by computer generated block lists and was blinded to participants until completion of the 3-dose schedule as well as to laboratory staff during testing.
Sample size was based on the immunological objectives of the study and not on reactogenicity outcomes, though was limited to half the recruitment number anticipated.Citation2 To maintain 80% power with this sample size the precision of 95% confidence intervals for antibody estimates were reduced from the initial power calculation of 200 participants in each arm, and the range of differences increased from 10–15 to 15–20% as the difference needed for the detection of a difference between vaccines considering immunogenicity. The sample size was sufficient to address some differences in cross-neutralising antibodies elicited by the 2 vaccines. Reactogenicity was compared between the groups according to a) the maximum severity of each symptom in the week following each dose as well as over all 3 doses, b) the day after vaccination for each dose that the maximum severity was reached (in those with the symptom), c) the number of days the symptom lasted for each dose (in those with the symptom). The maximum severity outcome (a) was assessed by whether there was any symptom as well as whether this reached a severe level with proportions calculated with 95% confidence intervals and groups compared by Fisher's exact test. Comparisons between doses for a) is assessed informally according to non-overlapping 95% confidence intervals which is a conservative method given the large number of comparisons.
Vaccines/samples
Cervarix® is a bivalent vaccine adjuvanated with AS04; containing HPV16 and 18 VLPs. Gardasil® is a tetravalent vaccine including HPV6, 11, 16 and 18 virus-like particles (VLPs), with an aluminum adjuvant (). Vaccination was by intramuscular injection of the deltoid.
Table 1. Details the characteristics of the vaccines used in this study.
Table 2. Depicts the number of vaccinees experiencing any level of local or systemic symptoms after any dose for both vaccines. The percentage represents the proportion of participants who experienced at least one event over the 3 doses of HPV vaccine.
Table 3. Depicts the percentage of participants who experienced at least one event of moderate-severe or severe local and systemic symptoms across all 3 doses and any events recorded per dose. The data are presented by vaccine and a p value denotes whether a difference was observed at each dose and across the full vaccine schedule (significant p values in bold). Safety data was not complete for all doses, hence variations in denominator value across the table. Loss of appetite was missing 1–2 meals to having no appetite at all, fatigue as sleeping more than usual or most of the time and pain as discomfort to pain resulting in participants unwilling to use their arm. Temperature was considered mild if >37.5°C, moderate if >38.5°C and severe if ≥ 39°C. Redness and swelling were considered mild if >0 mm, moderate if >30 mm and severe if >50 mm in diameter. Headache was scored as mild, moderate or severe.
Reactogenicity data collection
Following each of the 3 vaccinations, participants were given a 7-day health diary to record any local reactions, systemic symptoms and GP/hospital consultations. The day of vaccination was recorded as day 0. Solicited local reactions included, redness, swelling, and tenderness. Systemic symptoms comprised; temperature, headache, fatigue and loss of appetite, scored according to severity. Participants were monitored for 30mins after vaccination to ensure medical supervision for any unexpected adverse events.
Disclosure of potential conflicts of interest
Study registered on the ClinicalTrials.gov website (NCT00956553). All authors do not have any conflicts of interest to declare.
Acknowledgments
We are grateful to the vaccine research nurses Lynne Joslin, Norah Ashwood, Diane Webb, Anne Maher and Wendy Nedoma for the exceptional execution of this study. Special thanks go to the study participants for their interest in this study. We are indebted to Teresa Gibbs, Deborah Cohen and Elizabeth Sheasby for the excellent administration of this study and Dr Anu Ohrling and Dr Chee Yung for their clinical expertise. We are grateful to Sanofi Pasteur MSD, UK for the generous gift of some of the Gardasil® vaccine doses used in this study. We thank the Virus Reference Department (VRD), led by Dr Simon Beddows for sample testing and analysis.
References
- Human papillomavirus (HPV) immunisation programme review: 2008 to 2014 - Publications - GOV.UK (Internet). Available from: https://www.gov.uk/government/publications/human-papillomavirus-hpv-immunisation-programme-review-2008-to-2014
- Draper E, Bissett SL, Howell-Jones R, Waight P, Soldan K, Jit M, et al. A Randomized, Observer-Blinded Immunogenicity Trial of Cervarix® and Gardasil® Human Papillomavirus Vaccines in 12–15 Year Old Girls. Ellis RD, editor. PLoS One (Internet) 2013 (cited 2016 Nov 18); 8(5):e61825. Available from: http://dx.plos.org/10.1371/journal.pone.0061825; PMID:23650505; https://doi.org/10.1371/journal.pone.0061825
- Smith A, Yarwood J, Salisbury DM. Tracking mothers' attitudes to MMR immunisation 1996–2006. Vaccine (Internet) 2007 (cited 2016 Nov 18); 25(20):3996-4002. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0264410X07002241; PMID:17395344; https://doi.org/10.1016/j.vaccine.2007.02.071
- Walsh CD, Gera A, Shah M, Sharma A, Powell JE, Wilson S. Public knowledge and attitudes towards Human Papilloma Virus (HPV) vaccination. BMC Public Health (Internet) 2008 (cited 2016 Nov 18); 8(1):368. Available from: http://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-8-368; PMID:18947430; https://doi.org/10.1186/1471-2458-8-368
- Noakes K, Yarwood J, Salisbury D. Parental response to the introduction of a vaccine against human papilloma virus. Hum Vaccin (Internet) 2015 (cited 2016 Nov 18); 2(6):243-8. Available from: http://www.landesbioscience.com/journals/vaccines/abstract.php?id= 3391; https://doi.org/10.4161/hv.2.6.3391
- Medicines Agency E. HPV vaccines: EMA confirms evidence does not support that they cause CRPS or POTS (Internet). 2016 (cited 2016 Nov 19). Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/HPV_vaccines_20/European_Commission_final_decision/WC500196773.pdf
- Miranda S, Chaignot C, Collin C, Dray-Spira R, Weill A, Zureik M. Vaccination anti-HPV et risque de maladies auto-immunes : étude de cohorte française. Rev Epidemiol Sante Publique 2016; 64:S10; https://doi.org/10.1016/j.respe.2016.01.037
- Arnheim-Dahlström L, Pasternak B, Svanström H, Sparén P, Hviid A. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. BMJ 2013; 347:f5906; PMID:24108159; https://doi.org/10.1136/bmj.f5906
- Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin (Internet) 2009 (cited 2016 Nov 18); 5(10):705-19. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19684472; PMID:19684472; https://doi.org/10.4161/hv.5.10.9518
- Smith JF, Brownlow M, Brown M, Kowalski R, Esser MT, Ruiz W, Barr E, Brown DR, Bryan JT. Antibodies from Women Immunized with Gardasil ® Cross-Neutralize HPV 45 Pseudovirions. Hum Vaccin (Internet) 2007 (cited 2016 Nov 18); 3(4):109-15. Available from: http://www.tandfonline.com/doi/abs/10.4161/hv.3.4.4058; PMID:17611417; https://doi.org/10.4161/hv.3.4.4058
- Romanowski B, Schwarz TF, Ferguson LM, Peters K, Dionne M, Schulze K, Ramjattan B, Hillemanns P, Catteau G, Dobbelaere K, et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared to the licensed 3-dose schedule. Hum Vaccin (Internet) 2011 (cited 2016 Nov 18); 7(12):1374-86. Available from: http://www.tandfonline.com/doi/abs/10.4161/hv.7.12.18322; PMID:22048171; https://doi.org/10.4161/hv.7.12.18322
- Kim SC, Song YS, Kim YT, Kim YT, Ryu KS, Gunapalaiah B, Bi D, Bock HL, Park JS. Human papillomavirus 16/18 AS04-adjuvanted cervical cancer vaccine: immunogenicity and safety in 15–25 years old healthy Korean women. J Gynecol Oncol (Internet) 2011 (cited 2016 Nov 18); 22(2):67. Available from: http://synapse.koreamed.org/DOIx.php?id = 10.3802/jgo.2011.22.2.67; PMID:21860731; https://doi.org/10.3802/jgo.2011.22.2.67
- Kojic EM, Kang M, Cespedes MS, Umbleja T, Godfrey C, Allen RT, Firnhaber C, Grinsztejn B, Palefsky JM, Webster-Cyriaque JY, et al. Immunogenicity and Safety of the Quadrivalent Human Papillomavirus Vaccine in HIV-1-Infected Women. Clin Infect Dis (Internet) 2014 Jul 1 (cited 2016 Nov 18); 59(1):127-35. Available from: http://cid.oxfordjournals.org/lookup/doi/10.1093/cid/ciu238; PMID:24723284; https://doi.org/10.1093/cid/ciu238
- Leung TF, Liu AP-Y, Lim FS, Thollot F, Oh HML, Lee BW, Rombo L, Tan NC, Rouzier R, Friel D, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine administered according to 2- and 3-dose schedules in girls aged 9–14 years: Results to month 12 from a randomized trial. Hum Vaccin Immunother (Internet) 2015 (cited 2016 Nov 18); 11(7):1689-702. Available from: http://www.tandfonline.com/doi/full/10.1080/21645515.2015.1050570; PMID:26062002; https://doi.org/10.1080/21645515.2015.1050570
- Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, Fourneau MA, Colau B, Suzich J, Losonksy G, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine (Internet) 2006 (cited 2016 Nov 19); 24(33–34):5937-49. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16828940; PMID:16828940; https://doi.org/10.1016/j.vaccine.2006.06.005
- Sow PS, Watson-Jones D, Kiviat N, Changalucha J, Mbaye KD, Brown J, Bousso K, Kavishe B, Andreasen A, Toure M, et al. Safety and Immunogenicity of Human Papillomavirus-16/18 AS04-Adjuvanted Vaccine: A Randomized Trial in 10–25-Year-Old HIV-seronegative african girls and young women. J Infect Dis (Internet) 2013 (cited 2016 Nov 18); 207(11):1753-63. Available from: http://jid.oxfordjournals.org/lookup/doi/10.1093/infdis/jis619; PMID:23242542; https://doi.org/10.1093/infdis/jis619
- Skinner SR, Szarewski A, Romanowski B, Garland SM, Lazcano-Ponce E, Salmerón J, Del Rosario-Raymundo MR, Verheijen RH, Quek SC, da Silva DP, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 4-year interim follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet (Internet) 2014 (cited 2016 Nov 18); 384(9961):2213-27. Available from: http://linkinghub.elsevier.com/retrieve/pii/S014067361460920X; PMID:25189358; https://doi.org/10.1016/S0140-6736(14)60920-X
- Petäjä T, Keränen H, Karppa T, Kawa A, Lantela S, Siitari-Mattila M, Levänen H, Tocklin T, Godeaux O, Lehtinen M, et al. Immunogenicity and Safety of Human Papillomavirus (HPV)-16/18 AS04-Adjuvanted Vaccine in Healthy Boys Aged 10–18 Years. J Adolesc Heal (Internet) 2009 (cited 2016 Nov 18); 44(1):33-40. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1054139X08004345; https://doi.org/10.1016/j.jadohealth.2008.10.002
- Kim YJ, Kim KT, Kim JH, Cha SD, Kim JW, Bae DS, Nam JH, Ahn WS, Choi HS, Ng T, et al. Vaccination with a Human Papillomavirus (HPV)-16/18 AS04-Adjuvanted Cervical Cancer Vaccine in Korean Girls Aged 10–14 Years. J Korean Med Sci (Internet) 2010 (cited 2016 Nov 18); 25(8):1197. Available from: http://synapse.koreamed.org/DOIx.php?id=10.3346/jkms.2010.25.8.1197; PMID:20676333; https://doi.org/10.3346/jkms.2010.25.8.1197
- Zhu F, Li J, Hu Y, Zhang X, Yang X, Zhao H, Wang J, Yang J, Xia G, Dai Q, et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese girls and women aged 9 to 45 years. Hum Vaccin Immunother (Internet) 2014 (cited 2016 Nov 18); 10(7):1795-806. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25424785; PMID:25424785; https://doi.org/10.4161/hv.28702
- Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T, Dubin G. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin (Internet) 2009 (cited 2016 Nov 18); 5(5):332-40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19221517; https://doi.org/10.4161/hv.5.5.7211
- Panagiotou OA, Befano BL, Gonzalez P, Rodríguez AC, Herrero R, Schiller JT, Kreimer AR, Schiffman M, Hildesheim A, Wilcox AJ, et al. Effect of bivalent human papillomavirus vaccination on pregnancy outcomes: long term observational follow-up in the Costa Rica HPV Vaccine Trial. BMJ 2015; 351:h4358; PMID:26346155; https://doi.org/10.1136/bmj.h4358
- Garcia-Sicilia J, Schwarz TF, Carmona A, Peters K, Malkin J-E, Tran PM, Behre U, Iturbe EB, Catteau G, Thomas F, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted cervical cancer vaccine coadministered with combined diphtheria-tetanus-acellular pertussis–inactivated poliovirus vaccine to girls and young women. J Adolesc Heal (Internet) 2010 (cited 2016 Nov 18); 46(2):142-51. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1054139X09006296; https://doi.org/10.1016/j.jadohealth.2009.11.205
- Arguedas A, Soley C, Loaiza C, Rincon G, Guevara S, Perez A, Porras W, Alvarado O, Aguilar L, Abdelnour A, et al. Safety and immunogenicity of one dose of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, when administered to adolescents concomitantly or sequentially with Tdap and HPV vaccines. Vaccine (Internet) 2010 (cited 2016 Nov 18); 28(18):3171-9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0264410X10002136; PMID:20189491; https://doi.org/10.1016/j.vaccine.2010.02.045
- Wheeler CM, Harvey BM, Pichichero ME, Simon MW, Combs SP, Blatter MM, Marshall GS, Catteau G, Dobbelaere K, Descamps D, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted Vaccine Coadministered With Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccine and/or Meningococcal Conjugate Vaccine to Healthy Girls 11 to 18 years of age. Pediatr Infect Dis J (Internet) 2011 (cited 2016 Nov 18); 30(12):e225-34. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006454-201112000-00037; PMID:21817954; https://doi.org/10.1097/INF.0b013e31822d28df
- Schmeink CE, Bekkers RLM, Josefsson A, Richardus JH, Berndtsson Blom K, David MP, Dobbelaere K, Descamps D. Co-administration of human papillomavirus-16/18 AS04-adjuvanted vaccine with hepatitis B vaccine: Randomized study in healthy girls. Vaccine (Internet) 2011 (cited 2016 Nov 18); 29(49):9276-83. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0264410X11012680; PMID:21856349; https://doi.org/10.1016/j.vaccine.2011.08.037
- Leroux-Roels G, Haelterman E, Maes C, Levy J, De Boever F, Licini L, David MP, Dobbelaere K, Descamps D. Randomized Trial of the Immunogenicity and Safety of the Hepatitis B Vaccine Given in an Accelerated Schedule Coadministered with the Human Papillomavirus Type 16/18 AS04-Adjuvanted Cervical Cancer Vaccine. Clin Vaccine Immunol (Internet) 2011 (cited 2016 Nov 18); 18(9):1510-8. Available from: http://cvi.asm.org/cgi/doi/10.1128/CVI.00539-10; PMID:21734063; https://doi.org/10.1128/CVI.00539-10
- Pedersen C, Breindahl M, Aggarwal N, Berglund J, Oroszlán G, Silfverdal SA, Szüts P, O'Mahony M, David MP, Dobbelaere K, et al. Randomized Trial: Immunogenicity and safety of coadministered human papillomavirus-16/18 AS04-Adjuvanted vaccine and combined Hepatitis A and B vaccine in girls. J Adolesc Heal (Internet) 2012 (cited 2016 Nov 18); 50(1):38-46. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1054139X11003533; https://doi.org/10.1016/j.jadohealth.2011.10.009
- Toft L, Storgaard M, Muller M, Sehr P, Bonde J, Tolstrup M, Østergaard L, Søgaard OS. Comparison of the Immunogenicity and Reactogenicity of Cervarix and Gardasil Human Papillomavirus Vaccines in HIV-Infected Adults: A Randomized, Double-Blind Clinical Trial. J Infect Dis (Internet) 2014 (cited 2016 Nov 18); 209(8):1165-73. Available from: http://jid.oxfordjournals.org/lookup/doi/10.1093/infdis/jit657; PMID:24273179; https://doi.org/10.1093/infdis/jit657
- Vishram B, Byrne L, White J, Edelstein M. Human Papillomavirus (HPV) vaccination coverage in adolescent females in England: 2014/15. 2015 (cited 2016 Nov 18); Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/487514/HPV_2014_15_ReportFinal181215_v1.1.pdf
- Angelo M-G, Zima J, Tavares Da Silva F, Baril L, Arellano F. Post-licensure safety surveillance for human papillomavirus-16/18-AS04-adjuvanted vaccine: more than 4 years of experience. Pharmacoepidemiol Drug Saf (Internet) 2014 (cited 2016 Nov 18);23(5):456-65. Available from:http://doi.wiley.com/10.1002/pds.3593; PMID:24644078; https://doi.org/10.1002/pds.3593
- Vichnin M, Bonanni P, Klein NP, Garland SM, Block SL, Kjaer SK, Sings HL, Perez G, Haupt RM, Saah AJ, et al. An Overview of Quadrivalent Human Papillomavirus Vaccine Safety. Pediatr Infect Dis J (Internet) 2015 (cited 2016 Nov 18); 34(9):983-91. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006454-201509000-00017; PMID:26107345; https://doi.org/10.1097/INF.0000000000000793
- Van Damme P, Leroux-Roels G, Simon P, Foidart J-M, Donders G, Hoppenbrouwers K, Levin M, Tibaldi F, Poncelet S, Moris P, et al. Effects of varying antigens and adjuvant systems on the immunogenicity and safety of investigational tetravalent human oncogenic papillomavirus vaccines: Results from two randomized trials. Vaccine (Internet) 2014 (cited 2016 Nov 18); 32(29):3694-705. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0264410X14004095; PMID:24674663; https://doi.org/10.1016/j.vaccine.2014.03.040
- Nelson EAS, Lam HS, Choi KC, Ho WCS, Fung LWE, Cheng FWT, Sung RY, Royals M, Chan PK. A pilot randomized study to assess immunogenicity, reactogenicity, safety and tolerability of two human papillomavirus vaccines administered intramuscularly and intradermally to females aged 18–26 years. Vaccine 2013; 31(34):3452-60; PMID:23770335; https://doi.org/10.1016/j.vaccine.2013.06.034
- Gonçalves AK, Cobucci RN, Rodrigues HM, de Melo AG, Giraldo PC. Safety, tolerability and side effects of human papillomavirus vaccines: a systematic quantitative review. Brazilian J Infect Dis (Internet) 2014 (cited 2016 Nov 18); 18(6):651-9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1413867014000695; https://doi.org/10.1016/j.bjid.2014.02.005
