ABSTRACT
Neisseria meningitidis serogroup B (MenB) is an important cause of invasive meningococcal disease. The development of safe and effective vaccines with activity across the diversity of MenB strains has been challenging. While capsular polysaccharide conjugate vaccines have been highly successful in the prevention of disease due to meningococcal serogroups A, C, W, and Y, this approach has not been possible for MenB owing to the poor immunogenicity of the MenB capsular polysaccharide. Vaccines based on outer membrane vesicles have been successful in the prevention of invasive MenB disease caused by the single epidemic strain from which they were derived, but they do not confer broad protection against diverse MenB strains. Thus, alternative approaches to vaccine development have been pursued to identify vaccine antigens that can provide broad protection against the epidemiologic and antigenic diversity of invasive MenB strains. Human factor H binding protein (fHBP) was found to be such an antigen, as it is expressed on nearly all invasive disease strains of MenB and can induce bactericidal responses against diverse MenB strains. A bivalent vaccine (Trumenba®, MenB-FHbp, bivalent rLP2086) composed of equal amounts of 2 fHBP variants from each of the 2 immunologically diverse subfamilies of fHBP (subfamilies A and B) was the first MenB vaccine licensed in the United States under an accelerated approval pathway for prevention of invasive MenB disease. Due to the relatively low incidence of meningococcal disease, demonstration of vaccine efficacy for the purposes of licensure of bivalent rLP2086 was based on vaccine-elicited bactericidal activity as a surrogate marker of efficacy, as measured in vitro by the serum bactericidal assay using human complement. Because bacterial surface proteins such as fHBP are antigenically variable, an important component for evaluation and licensure of bivalent rLP2086 included stringent criteria for assessment of breadth of coverage across antigenically diverse and epidemiologically important MenB strains. This review describes the rigorous approach used to assess broad coverage of bivalent rLP2086. Alternative nonfunctional assays proposed for assessing vaccine coverage are also discussed.
Introduction
Neisseria meningitidis, the causative agent of invasive meningococcal disease (IMD), most often presents in clinical disease cases as meningitis and/or septicemia,Citation1,2 with peaks in disease incidence observed in infants, adolescents, and young adults.Citation2 Approximately 20% of survivors experience permanent sequelae including neurologic impairment, hearing loss, or limb amputation.Citation3,4
Six serogroups defined by their capsular polysaccharides (A, B, C, W, X, and Y) cause almost all meningococcal disease globally.Citation5 During recent years, meningococcal serogroup B (MenB) has caused a large proportion of IMD cases in the United States,Citation6 Europe,Citation7 Canada,Citation8 Australia,Citation9 and New Zealand.Citation10 Moreover, in the past decade, MenB outbreaks have been observed at several university campuses in the United StatesCitation11 and in various settings within Europe, including family clusters, childcare settings, and schools.Citation12-16 These outbreaks may be prolonged and can result in significant morbidity and mortality.Citation17-19 While disease caused by meningococcal serogroups A, C, W, and Y can be prevented effectively with capsular polysaccharide conjugate vaccines,Citation2 such vaccines are not possible for MenB due to the structural similarity of the MenB polysaccharide capsule with polysialic acid structures on human neuronal cells and the associated low immunogenicity of the MenB polysaccharide.Citation20
Outer membrane vesicle (OMV) vaccines have been successfully used to control local MenB epidemics caused by single clones.Citation21,22 However, in contrast to capsular polysaccharide conjugate vaccines, which provide broad, serogroup-specific protection, the coverage provided by OMV vaccines is generally limited to the targeted epidemic meningococcal strain due to the sequence heterogeneity of the dominant OMV vaccine antigen, porin A (PorA).Citation23-26 This limitation of OMV vaccines and the predominance of MenB in several large geographic regions emphasized the need for development of an efficacious vaccine to confer broad protection against diverse invasive disease strains of MenB.
To address broad coverage against IMD, researchers sought to identify immunogenic surface proteins present in a large proportion of circulating MenB disease strains and also capable of stimulating protective immune responses against the diversity of MenB isolates.Citation27,28 In this context, factor H binding protein (fHBP) was identified as a surface lipoprotein present on nearly all disease-causing strains, inducing bactericidal responses against MenB strains expressing antigenically diverse fHBP variants.Citation29,30 Importantly, fHBP amino acid sequence variants segregate into 2 antigenic and immunologically diverse subfamilies, designated A and B.Citation30,31 Each strain of MenB expresses a single variant of fHBP.
Early research efforts identified that a vaccine containing an fHBP variant from each of the 2 subfamilies (A and B) would greatly enhance the breadth of bactericidal antibody responses against MenB strains. Thus, bivalent rLP2086 (Trumenba®, MenB-FHbp; Pfizer Inc, Collegeville, PA), composed of equal amounts of recombinant, lipidated fHBP antigens from subfamily A (variant A05) and subfamily B (variant B01), was developed. Bivalent rLP2086 was subsequently the first MenB vaccine approved under an accelerated approval program by the US Food and Drug Administration in October 2014 for the prevention of MenB disease in adolescents and young adults 10 to 25 y of age.Citation32,33
Similar to development programs for meningococcal serogroup C and meningococcal serogroups ACWY polysaccharide conjugate vaccines, large prelicensure clinical efficacy studies have not been feasible for MenB vaccines due to the relatively low overall incidence of disease.Citation34,35 Therefore, evaluations of vaccine efficacy for licensure are based instead on demonstration of vaccine-elicited serum bactericidal antibody responses as a surrogate marker of efficacy measured by in vitro serum bactericidal assays using human complement (hSBAs).Citation36 As an hSBA titer ≥ 1:4 correlates with protection against meningococcal disease,Citation34,36-38 hSBA responses have been accepted as surrogate measures of vaccine efficacy for licensure across meningococcal serogroups.Citation37,39 The approach also was supported by the experiences and data from postimplementation surveillance studies of polysaccharide conjugate vaccines and MenB OMV vaccines that were licensed or implemented on the basis of hSBA results.Citation36,40,41
This review will describe the approach used to demonstrate broad coverage of bivalent rLP2086 with hSBAs using antigenically and epidemiologically relevant MenB strains. Additional discussion is provided regarding alternative nonfunctional assays that have been proposed for assessing vaccine coverage.
Serum bactericidal assay using human complement
Use of hSBAs to assess vaccine-elicited protection from meningococcal disease
hSBAs measure the functional ability of serum antibodies to kill meningococcal test strains in a complement-dependent fashion ().Citation36,42,43 An hSBA titer is defined as the highest serum dilution that kills at least 50% of the bacteria in the assay.Citation36 An hSBA titer ≥ 1:4 is the accepted correlate of protection, although a titer <1:4 is not always indicative of disease
Figure 1. Serum bactericidal assay with human complement. Figure adapted from Ghandi, et al. Postgraduate Medicine, 2016.Citation82
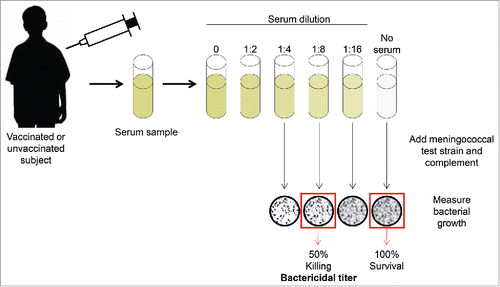
An hSBA titer ≥ 1:4 was initially identified as the correlate of protection using homologous human complement in the assays (ie, the complement source was derived from the same individual whose serum was tested in the assay). The assays were later adapted to use exogenous human complement and test sera (ie, the human complement source was derived from someone other than the individual whose serum was tested in the assay).Citation37,44,45 The use of human complement has logistic difficulties, such as obtaining sera from human donors, prescreening the sera to ensure that the complement is active and compatible with the hSBA, and ensuring that the complement source does not result in nonspecific killing of the bacteria in the assay. Therefore, SBAs have also been performed with baby rabbit complement (rSBAs), as this reagent is readily available in high quantities and shows low nonspecific bactericidal activity.Citation34,42,46,47 However, only human complement is appropriate for testing bactericidal activity of human sera against MenB,Citation34 as the use of rabbit complement results in deceptively higher bactericidal titers to MenB strains than the use of human complement,Citation47,48 and rSBA responses do not correlate well with disease protection over time.Citation46
Bactericidal activity as assessed in hSBAs with a single test strain can correlate well with protection against all disease strains of a given serogroup when the vaccine antigen is the serogroup-specific capsular polysaccharide, which is identical in, and expressed by, all strains within the targeted serogroup (eg, meningococcal serogroup C).Citation34,42 In these cases, vaccine efficacy can be inferred to apply to the whole serogroup, and thus, breadth of coverage can be demonstrated by hSBAs using a single representative serogroup-specific strain. However, when the vaccine antigen is not serogroup specific, as with many of the sequence-variable proteins expressed on the bacterial cell surface, assessment of breadth of coverage against disease strains is more complex and requires a different approach. Moreover, these proteins may not be expressed by all disease strains, and if they are expressed, expression levels can vary among different strains.Citation49 All of these factors are known to contribute to a lack of bactericidal activity in SBAs to a specific test strain.Citation49 The choice of test strains to determine both vaccine-elicited protection and breadth of coverage is therefore critical for protein antigen vaccines and is discussed in more detail below.
Assessing breadth of coverage of bivalent rLP2086
Fundamental to the use of hSBA responses as surrogates for vaccine efficacy is the selection of appropriate test strains and immunologic response endpoints. At the time of bivalent rLP2086 development, no standardized hSBAs were available to evaluate the breadth of protection of MenB vaccines based on surface protein antigens exhibiting sequence and expression variability.
For the purpose of evaluating the breadth of coverage of bivalent rLP2086, it was first necessary to conduct global surveillance and antigenic characterization of disease-causing strains to identify hSBA test strains appropriate for evaluation of protein-based vaccines for the prevention of MenB disease.
Approximately 1800 invasive MenB disease strains from reference laboratories in Europe and the United States were collected, systematically assembled, and characterized.Citation27 All isolates were found to contain the gene encoding fHBP. Based on amino acid sequences, fHBP variants segregated into 2 immunologically distinct subfamilies (designated A and B). The amino acid sequence identity of variants within each subfamily was found to be ≥ 83%, while variant sequence identity between subfamilies was just 60–75%.Citation27,29 Collectively, the 10 most prevalent fHBP variants in the strain pool were found to be expressed by nearly 80% of all strains.Citation27 Notably, the breadth of fHBP bactericidal activity is predominantly subfamily specific, with limited immunologic protection across subfamilies.Citation29,31
Extensive exploratory hSBAs were then conducted using clinical isolates that represented the breadth of both the antigenic and epidemiologic diversity of the MnB serogroup. Results from these analyses revealed that bivalent rLP2086 induced bactericidal antibodies that killed MnB isolates, independent of the sequence diversity of the fHBP expressed by the bacteria strain ().Citation31,50 Pivotal licensure studies required the breadth of coverage to be assessed in a hypothesis-driven manner and necessitated an approach to select strains without bias. Therefore, a random approach was used to select the primary MenB test strains for use in hSBAs, while taking into account specific selection criteria to ensure that test strains appropriately represented the antigenic diversity of MenB isolates.Citation27 Selection criteria included: (i) expression of fHBP variants that differed from the vaccine antigens; (ii) expression of fHBP variants prevalent in contemporary IMD strains from the United States and Europe; (iii) representative in vitro surface expression levels of the fHBP vaccine antigens across all strains; and (iv) demonstration of low baseline bactericidal hSBA titers with selected strains. The last of these criteria is of particular note, as those most at risk for meningococcal disease have virtually nonexistent bactericidal activity against the majority of strains.
Table 1. MenB test strains used for the assessment of bivalent rLP2086.
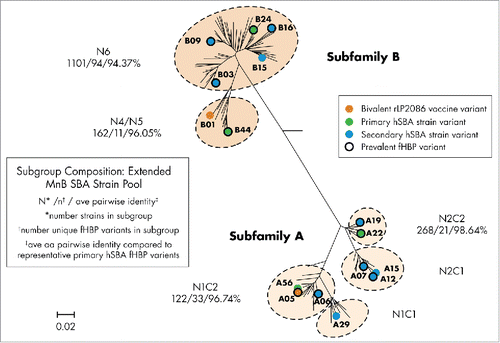
The bivalent rLP2086 clinical program rigorously validated hSBAs using 4 primary MenB test strains identified by the approach described above. Four test strains, each expressing fHBP variants different from (ie, heterologous to) the vaccine antigens were considered sufficient to provide indication of both efficacy and breadth of vaccine coverage. These 4 test strains [(PMB80 (A22/88.9%; fHBP variant/sequence identity to vaccine antigen of same subfamily), PMB2001 (A56/98.1%), PMB2948 (B24/86.2%) and PMB2707 (B44/91.6%)] were used in hSBAs in phase 2 and 3 studies designed to support licensure of bivalent rLP2086 in the United States and Europe ().Citation27,51-57 The relevance of these 4 test strains to be representative of all fHBP-expressing MenB strains was established using a pairwise identity approach. The fHBP family can be divided into 6 subgroups; the relationship of each of the 4 test strains was assessed relative to the fHBP variants within its matched subfamily (ie, A and B) and its closest related subgroup (). As a result, the pairwise identity observed for a test strain and the fHBP sequences from the same subgroup that it represents ranges from 94.37% to 98.64%, indicating high sequence conservation between the test strains and other variants. The total minimun pairwise identity of variants to the vaccine antigens is 88.5% for A05 and 84.8% for B01, thus providing an opportunity to assess the breadth of coverage of the vaccine without the requirement of developing assays for every strain during clinical testing.
Figure 2. Phylogenetic relationship of different fHBP subgroups in the extended MenB SBA strain pool. Representation of the phylogeny of fHBP is based on a clustalW alignment and drawn with MEGA 4.2. The relative phylogenetic position of subfamily A and B fHBP variants, as well as the 6 major subgroups of fHBP, are outlined. The phylogenetic position of fHBP variants expressed by the 4 primary and 10 secondary SBA test strains, as well as 2 vaccine variants, is highlighted. The numbers beneath each of the fHBP subgroups represent the number of isolates, number of unique fHBP variants, and average amino acid percent identity from the respective subgroups. The scale bar represents phylogenetic distance based on the deduced fHBP protein sequence. Phylogenetic relationship of MenB fHBP variants first described by Murphy, et al. J Infect Dis. 2009.Citation30
Although the serologic correlate for protection against IMD is an hSBA titer of ≥ 1:4, the lower limit of quantification (LLOQ) for hSBA using these 4 primary MenB test strains was determined to be a titer equal to 1:8 for strains expressing fHBP variants A56, B24, and B44, and a titer equal to 1:16 for the strain expressing fHBP variant A22. Because these LLOQ titers (rather than titers of ≥ 1:4) were employed for calculation of hSBA results during phase 2 and phase 3 clinical studies, the reported study results provide additional assurances for estimates of protection afforded by immunization with bivalent rLP2086.
The surrogate measure for licensure of bivalent rLP2086 required demonstration of bactericidal immune response by a high proportion of vaccine recipients using 5 different endpoints.Citation33 Study success was achieved only if predefined criteria were met for all 5 endpoints. Four of the 5 endpoints were based on vaccine-elicited 4-fold increases from baseline in hSBA responses to each primary test strain, thus providing evidence of vaccine benefit for recipients with low baseline titers as well as those with higher hSBA titers prior to vaccination. The fifth endpoint measure was a composite response, demonstrating the proportions of vaccine recipients exhibiting hSBA titers ≥ LLOQ against not only 1, but all 4 primary test strains combined. These endpoints were included as prespecified exploratory endpoints in 3 of the phase 2 studies used for licensureCitation55,56,58 and as the primary immunogenicity endpoints in phase 3 studies that became post-marketing commitments under the accelerated approval process.Citation59,60 Successful achievement of the 5 endpoints described above supported accelerated licensure of bivalent rLP2086 in the United States and provided strong evidence of breadth of coverage across the diversity of invasive MnB strains.
Performance of hSBA with 10 additional test strains provided further evidence of breadth of vaccine coverage against disease strains circulating in the United States and Europe (). These results confirmed that the observed immune responses to the 4 primary strains were indicative of broad protection against MenB disease-causing strains that express diverse fHBP sequence variants.Citation27,61,62 As with the 4 primary strains, these 10 additional hSBA test strains all expressed fHBP variant sequences different from (ie, heterologous to) the vaccine antigens and were selected in a similar manner as the 4 primary test strains.Citation63 Strains with low to medium expression levels were chosen to mimic the normal distribution of fHBP expression among isolates.Citation27 Taken together, these 14 MnB test strains (4 primary and 10 additional) used in the clinical development of the bivalent rLP2086 vaccine represent both immunologic subfamilies of fHBP and address the potential diversity of fHBP in invasive disease isolates. Notably, the hSBA responses elicited by bivalent rLP2086 to these test strains all were assessed using sera from individual vaccine recipients rather than pooled serum from multiple subjects, thus permitting an estimate of the breadth of vaccine coverage at the population level by reporting on the proportion of vaccinated subjects with functional bactericidal antibodies.
Further evidence of the breadth of coverage for bivalent rLP2086 is available by performance of hSBA using MnB isolates collected during recent disease outbreaks. Several documented clusters or outbreaks of MenB disease have occurred during the last several years at US university campuses.Citation64 Additional analyses, including previously unpublished data, examined MenB strains from 5 of these clusters or outbreaks in exploratory hSBAs using sera from individuals who participated in an earlier clinical study and who received bivalent rLP2086 according to a 3-dose regimen of 0, 2, and 6 months (). Three of these outbreaks were caused by strains expressing the prevalent B24 fHBP variant (Universities B, C, and D). Two of the strains expressing B24 fHBP belonged to the common clonal complex that is associated with a prolonged outbreak in Oregon, and the third represented a less prevalent clonal complex (unpublished data andCitation65). The other 2 isolates were strains expressing rare fHBP variants B153 (University A) and B133 (University E), both of which were identified for the first time among MenB isolates in the United States. A strain expressing the B153 variant has recently been observed in a collection of invasive MenB isolates from England and Wales, whereas the B133 variant has been noted in isolates from Germany, England, Wales, and the Netherlands (unpublished data andCitation66,67).
Table 2. Bactericidal activity of bivalent rLP2086 immune sera against university outbreak strains in the United States.Footnote*
The proportion of bivalent rLP2086 vaccine recipients with hSBA titers of ≥1:4 to the B24 outbreak strains ranged from 20–78% after the second vaccination and from 40–100% after the third vaccination (Universities B, C, and D; ). For the B153 variant (University A), the proportions of hSBA responders were 67% and 78% after the second and third vaccine doses, respectively, and for the B133 variant (University E), corresponding values were 80% and 80%, respectively (). The B133 variant had a higher proportion of subjects with prevaccination hSBA titers ≥ 1:4 (27%) than had been observed for the other outbreak strains, while hSBA titers of >1:4 were observed for 80% of vaccinees after both doses 2 and 3 (; ). As observed with outbreak strains from Universities B, C, and D, which all express the B24 fHBP variant, differences in hSBA responses still occur. These observations further highlight the need for an unbiased selection of MenB test strains for hSBAs as well as the need for more extensive hSBA evaluations to accurately discern population response rates to MenB vaccination.
Figure 3. Activity of rLP2086 immune sera against fHBP B133 isolates in exploratory hSBAs. Isolates tested were from the University E outbreak (PMB5543 and PMB5544) and from the Netherlands (PMB5507). Sera are from 15 subjects vaccinated with bivalent rLP2086. Sera were collected before vaccination, after the first vaccination (PD1), after the second vaccination (PD2), and after the third vaccination (PD3). Responder rates based on individual titers ≥1:4 are indicated. Open rectangles represent geometric mean titers with 95% CIs (error bars). fHBP = factor H binding protein; hSBA = serum bactericidal assay using human complement; PD = postdose.
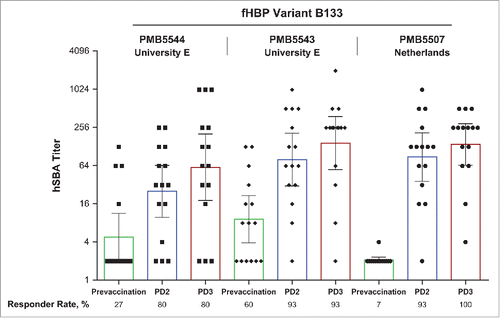
As the B133 variant was newly identified in the United States, additional hSBA testing was conducted on other B133-expressing isolates (). A second isolate [PMB5543 (B133)] from the outbreak at University E yielded post-dose 2 and 3 responder rates of 93%, although prevaccination titers were observed to be higher (60%). Baseline hSBA titers determined using a third strain expressing fHBP variant B133 [PMB5507 (B133)], which was obtained from the Netherlands during routine surveillance in Europe, were low (7%) but increased to 93% and 100% following 2 and 3 vaccine doses, respectively. Together, these hSBA results using recent outbreak strains provide assurance of the breadth of bivalent rLP2086 coverage predicted by the hSBA responses obtained with the 4 primary strains used in phase 2 and 3 clinical studies.Citation51-57
Alternative nonfunctional assays to infer vaccine breadth of coverage
Although the benefits of hSBA titers as an immunologic correlate of vaccine efficacy are well established, some limitations do exist. For instance, hSBAs are labor intensive and logistically challenging when a large number of strains have to be tested due to the need for considerable amounts of sera and sources of assay compatible complement.Citation68 Differences among laboratories in performing hSBAs can also hinder the ability to compare responses and assessments of breadth of coverage between meningococcal vaccines.Citation69 Therefore, alternative assays have been proposed to predict vaccine coverage (not efficacy) that are technically easier to perform than SBAs; these approaches are summarized in the following section.
Meningococcal antigen typing system
4CMenB (Bexsero®, MenB-4C; Novartis Inc, Cambridge, MA) is the only other MenB vaccine licensed in the United States for use in adolescents and young adults 10 to 25 y of age;Citation32 4CMenB is also approved in other regions.Citation70 4CMenB is comprised of 4 main components: a subfamily B fHBP (variant B24), 2 meningococcal proteins (Neisserial adhesin A [NadA]; Neisserial heparin binding antigen [NHBA]) expressed as recombinant fusion proteins, and OMVs (PorA serosubtype P1.4).Citation32 fHBP appears to be the major target of vaccine-induced serum bactericidal activity for both 4CMenB and bivalent rLP2086.Citation71
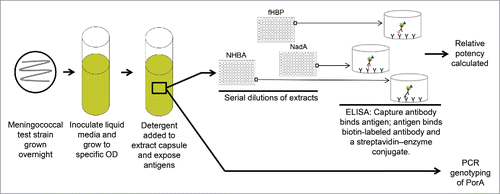
Clinical studies of 4CMenB have predominantly used the Meningococcal Antigen Typing System (MATS) assay to assess vaccine coverage (). This method consists of 3 antigen-specific sandwich enzyme-linked immunosorbent assays (ELISAs) that measure the reactivity of lysed bacterial extracts prepared from the test strain of interest with rabbit polyclonal antibodies against fHBP, NHBA, or NadA; PorA is evaluated using genotyping.Citation72,73 Reference strains (ie, H44/76, NGH38, 5/99) against which the activity of test strains are compared express the same fHBP, NHBA, and NadA antigens present in 4CMenB.Citation71,72 The relative ELISA potency for each antigen is then compared with a corresponding bactericidal threshold that is the minimum value predictive of killing in the hSBA. The positive bactericidal thresholds for each antigen are determined separately in hSBAs with vaccine immune sera.
Figure 4. MATS assay. Bacteria are grown overnight. A bacterial suspension is grown to a specific OD and detergent is added to the suspension to extract the capsule and expose the antigens. Serial dilutions of the extract are tested by ELISA. fHBP, NHBA, and NadA coverage is assessed by defining a relative potency of the tested strain versus a reference strain for each antigen and then extrapolating a relative potency to a positive bactericidal threshold. PCR genotyping is used to identify PorA. ELISA = enzyme-linked immunosorbent assay; fHBP = factor H binding protein; MATS = Meningococcal Antigen Typing System; NadA = Neisserial adhesin A; NHBA = Neisserial heparin binding antigen; OD = optical density; PCR = polymerase chain reaction; PorA = porin A.
The main advantage of MATS is that it is comparably faster and less resource intensive to perform than hSBAs.Citation68 However, because the MATS system was designed specifically to measure coverage pertaining to 4CMenB antigens, ELISAs used to assess coverage of NHBA or NadA antigens and PorA genotyping cannot be used to measure strain coverage of other MenB vaccines with different antigen compositions.Citation72 In addition, MATS results are assessed against hSBAs performed using pooled serum,Citation73 which is less informative than using individual samples as population responses to vaccination cannot be inferred. Further limitations of MATS include uncertainties with linking MATS results to specific age groups or administration schedules, as well as the recognized challenges for estimation of potential vaccine benefit in the absence of direct testing of individual serum samples by hSBA; these challenges were highlighted in a recent study by Basta and colleagues that used hSBAs to examine 4CMenB's response to the University A outbreak strain.Citation74
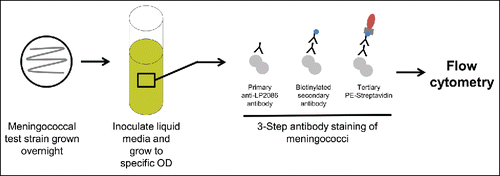
MEASURE flow cytometry assay
The Meningococcal Antigen Surface Expression (MEASURE) assay has been used to assess whether surface expression levels of vaccine antigen on MenB strains predict whether a MenB strain can be killed in hSBA by bivalent rLP2086 immune sera (). MEASURE is a flow cytometry method that uses a monoclonal antibody that is specific to an epitope common to fHBP variants from both subfamilies, thereby allowing a phenotypic assessment of fHBP expression and selective quantification of surface-expressed fHBP concentrations on intact bacteria that are prepared using standard hSBA procedures.Citation27,75,76 Because only the cell surface exposed portion of the antigen is accessible to serum bactericidal antibodies, MEASURE selectively quantifies the concentration of fHBP expressed on the surface.Citation27 Qualification of the MEASURE assay found that the distribution of fHBP expression levels is independent of subfamily or subgroup, but differences in expression levels between fHBP variant groups and MenB strains were found.Citation27 The MEASURE assay was found to be specific, robust, precise, and transferable to other laboratories.Citation76 Unlike some other meningococcal epidemiologic markers, fHBP surface expression levels determined by the flow cytometric-based MEASURE assay were predictive of strain susceptibility in the hSBA.Citation31 In addition, the applicability of MEASURE to predict bactericidal activity and breadth of coverage of bivalent rLP2086 to globally collected disease strains has been suggested.Citation76 MEASURE shares some of the shortcomings of the MATS assay because it does not provide information on the proportion of the population that responds to vaccination or that is protected against a given strain. In addition, vaccine-elicited benefit in a population above baseline coverage cannot be inferred.
Figure 5. MEASURE assay. Bacteria are grown overnight using standard hSBA procedures. Bacteria are then stained using a 3-step antibody staining method. Flow cytometry is used to selectively quantify the concentration of surface-expressed fHBP. fHBP = factor H binding protein; hSBA = serum bactericidal assay using human complement; MEASURE = Meningococcal Antigen Surface Expression; OD = optical density.
Selected reaction monitoring mass spectrometry
Selected reaction monitoring mass spectrometry (SRM-MS) is a technique for the specific and accurate quantification of a selected protein in a complex biological mixture. SRM-MS has been used for the absolute quantification of total cellular fHBP protein in a large panel of MenB disease strains representative of the genetic diversity of N meningitidis isolates.Citation77 This method attempts to correlate fHBP detected by SRM-MS with SBA activity. In principal, SRM-MS is analogous to the flow cytometry-based MEASURE assay but is less direct, because it detects levels of total antigen in a bacterial lysate rather than levels exposed on the bacterial surface. Moreover, attempts to correlate SBA activity with this approach are based on bactericidal assays with rabbit complement and mouse sera, rather than SBAs with human sera, thereby making it irrelevant for human vaccine studies.
IgG ELISA
Immunoglobulin G (IgG) ELISAs have been employed for assessment of population-based immunity, but the results are not generally accepted as a correlate of individual protection and are not used as surrogates of efficacy for licensure.Citation47 This approach most recently has been assessed with respect to meningococcal A vaccines.Citation78 An IgG meningococcal serogroup A polysaccharide antibody concentration of > 2 μg/mL was considered indicative of protection against MenA disease based on the results of a Finnish clinical study of a meningococcal polysaccharide vaccine conducted nearly 40 y ago, but differences were noted among some populations in the African meningitis belt, where antibody concentrations were generally higher.Citation69 It has been suggested that much of the antibody detected by this ELISA technique is nonfunctional, potentially because it is induced by cross-reacting bacteria or due to low avidity of the antigen-antibody complex. Limitations regarding the use of ELISA for meningococcal-specific IgG rather than SBA for serogroup C disease have also been discussed in other published studies.Citation79,80
Conclusions
Bivalent rLP2086, composed of recombinant, lipidated fHBP protein variants representing each fHBP subfamily, was designed to provide broad protection against antigenically and epidemiologically diverse MenB strains that cause invasive disease. fHBP is a conserved meningococcal virulence factor that is expressed in nearly all invasive MenB strains. In completed clinical studies, bivalent rLP2086 has demonstrated an acceptable safety profile and has elicited hSBA titers above the accepted correlate of protection in a high percentage of individuals when given alone or with concomitant vaccines.Citation51-57
Disease caused by MenB is generally rare, making assessment of MenB vaccine efficacy with clinical disease endpoints challenging and requiring measurement of hSBA responses as surrogate markers of efficacy.Citation27,34,35 The individual immune responses elicited by vaccination with bivalent rLP2086, and measured in hSBAs performed with a panel of MenB strains representative of antigenically and epidemiologically diverse invasive disease isolates, support the notion of broad protection afforded by bivalent rLP2086 against MenB disease.Citation50 The totality of hSBA data from completed, randomized, controlled studies met rigorously and prospectively established primary endpoints and success criteria, including a composite hSBA response to primary test strains.Citation51-54,56,57,59,60,81 Supported by additional hSBA analyses with 10 additional epidemiologically diverse MenB disease strains,Citation61,62 these vaccine-elicited responses provide assurance of the broad protection afforded by bivalent rLP2086 against IMD.
hSBA titers of ≥ 1:4 are recognized as the serological correlate of protection against meningococcal disease and the assay results are accepted as surrogate measures of efficacy for vaccine license. The assay also remains the most comprehensive, informative, and biologically relevant means to assess breadth of vaccine coverage. While the logistical challenges to perform quality hSBAs in a consistent manner have led to the development of alternative methods to assess immunogenicity and breadth of coverage, these methods do not measure vaccine-elicited functional antibodies, are not used as surrogates for vaccine licensure, and to date have limited utility in assessing breadth of coverage against diverse disease strains. These limitations of alternative assays highlight the importance for prelicensure studies using hSBAs with rigorously selected, unbiased test strains that express antigen sequences different from the vaccine antigens.
Abbreviations
| 4CMenB | = | Bexsero®, MenB-4C |
| Bivalent rLP2086 | = | Trumenba®, MenB-FHbp |
| ELISA | = | enzyme-linked immunosorbent assay |
| fHBP | = | factor H binding protein |
| hSBA | = | serum bactericidal assay using human complement |
| IMD | = | invasive meningococcal disease |
| IgG | = | immunoglobulin G |
| LLOQ | = | lower limit of quantification |
| LP2086 | = | lipoprotein 2086 |
| LOD | = | limit of detection |
| MATS | = | Meningococcal Antigen Typing System |
| MEASURE | = | Meningococcal Antigen Surface Expression assay |
| MenB | = | meningococcal serogroup B |
| NadA | = | Neisserial adhesin A |
| NHBA | = | Neisserial heparin binding antigen |
| OD | = | optical density |
| OMV | = | outer membrane vesicle |
| PCR | = | polymerase chain reaction |
| PD | = | postdose |
| PorA | = | porin A |
| rSBA | = | serum bactericidal assay using rabbit complement |
| SBA | = | serum bactericidal assay |
| SRM-MS | = | selected reaction monitoring mass spectrometry |
Disclosure of potential conflicts of interest
RGKD, JCH, LH, PL, TRJ, SLH, JLP, JJE, KUJ, and ASA are current employees of Pfizer Inc and may hold stock options.
Acknowledgments
We thank the Centers for Disease Control and Prevention (CDC), Atlanta, GA, for providing MenB isolates causing outbreaks at US colleges. The isolates were provided to the CDC by the California, New Jersey, and Rhode Island Departments of Health and the Oregon Health Authority. Editorial/medical writing support was provided by Tricia Newell, PhD, of Complete Healthcare Communications, LLC (Chadds Ford, PA), and was funded by Pfizer Inc.
References
- Rosenstein NE, Fischer M, Tappero JW. Meningococcal vaccines. Infect Dis Clin North Am 2001; 15(1):155-169; PMID:11301813; http://dx.doi.org/10.1016/S0891-5520(05)70273-7
- Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE, Centers for Disease Control and Prevention. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62:1-28; PMID:23515099
- Centers for Disease Control and Prevention. Meningococcal disease: technical & clinical information. Available at: http://www.cdc.gov/meningococcal/clinical-info.html. Accessed July 13, 2015.
- Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine 2012; 30 Suppl 2: B3-9; PMID:22607896; http://dx.doi.org/10.1016/j.vaccine.2011.12.062
- Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, Corton C, Care R, Poolman JT, Zollinger WD, et al. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis 2013; 19:566-573; PMID:23628376; http://dx.doi.org/10.3201/eid1904.111799
- Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) report, Emerging Infections Program Network Neisseria meningitidis, 2013-provisional. Available at: http://www.cdc.gov/abcs/reports-findings/survreports/mening13.pdf. Accessed September 13, 2016.
- European Centre for Disease Prevention and Control. Surveillance of invasive bacterial diseases in Europe, 2012. Available at: http://ecdc.europa.eu/en/publications/Publications/Surveillance%20of%20IBD%20in%20Europe%202012.pdf. Accessed October 22, 2015.
- Li YA, Tsang R, Desai S, Dehhan H. Enhanced surveillance of invasive meningococcal disease in Canada, 2006-2011. Can Communicable Dis Rep 2014; 40:160-169.
- Dey A, Knox S, Wang H, Beard FH, McIntyre PB. Summary of national surveillance data on vaccine preventable diseases in Australia, 2008-2011. Commun Dis Intell Q Rep 2016; 40:S1-S70; PMID:27087017
- Lopez L, Sherwood J. The epidemiology of meningococcal disease in New Zealand in 2013. Institute of Enviornmental Science and Research (ESR). Available at: https://surv.esr.cri.nz/PDF_surveillance/MeningococcalDisease/2013/2013AnnualRpt.pdf. Accessed October 1, 2015.
- MacNeil JR, Rubin L, Folaranmi T, Ortega-Sanchez IR, Patel M, Martin SW. Use of Serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:1171-1176; http://dx.doi.org/10.15585/mmwr.mm6441a3
- O'Connor L, Ward M, Bennett D, Mulhall R, O'Lorcain P, Cunney R, McDermott R, Neville E, Heslin J, FitzGerald R, et al. A prolonged outbreak of invasive meningococcal disease in an extended Irish Traveller family across three Health Service Executive (HSE) areas in Ireland, 2010 to 2013. Euro Surveill 2015; 20; pii:21139; PMID:26062560
- Tzanakaki G, Kesanopoulos K, Yazdankhah SP, Levidiotou S, Kremastinou J, Caugant DA. Conventional and molecular investigation of meningococcal isolates in relation to two outbreaks in the area of Athens, Greece. Clin Microbiol Infect 2006; 12:1024-1026; PMID:16961641; http://dx.doi.org/10.1111/j.1469-0691.2006.01521.x
- Stewart A, Coetzee N, Knapper E, Rajanaidu S, Iqbal Z, Duggal H. Public health action and mass chemoprophylaxis in response to a small meningococcal infection outbreak at a nursery in the West Midlands, England. Perspect Public Health 2013; 133:104-109; PMID:23467531; http://dx.doi.org/10.1177/1757913912439928
- Chatt C, Gajraj R, Hawker J, Neal K, Tahir M, Lawrence M, Gray SJ, Lucidarme J, Carr AD, Clark SA, et al. Four-month outbreak of invasive meningococcal disease caused by a rare serogroup B strain, identified through the use of molecular PorA subtyping, England, 2013. Euro Surveill 2014; 19:pii:20949; PMID:25394258
- Acheson P, Barron R, Borrow R, Gray S, Marodi C, Ramsay M, Waller J, Flood T. A cluster of four cases of meningococcal disease in a single nuclear family. Arch Dis Child 2012; 97:248-249; PMID:22247241; http://dx.doi.org/10.1136/archdischild-2011-301074
- Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 2007; 369:2196-2210; PMID:17604802; http://dx.doi.org/10.1016/S0140-6736(07)61016-2
- Dastouri F, Hosseini AM, Haworth E, Khandaker G, Rashid H, Booy R. Complications of serogroup B meningococcal disease in survivors: a review. Infect Disord Drug Targets 2015; 14:205-212; http://dx.doi.org/10.2174/1871526515999150320155614
- Viner RM, Booy R, Johnson H, Edmunds WJ, Hudson L, Bedford H, Kaczmarski E, Rajput K, Ramsay M, Christie D. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): a case-control study. Lancet Neurol 2012; 11:774-783; PMID:22863608; http://dx.doi.org/10.1016/S1474-4422(12)70180-1
- Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 1983; 2:355-357.
- Kelly RE, Jr., Shamberger RC, Mellins RB, Mitchell KK, Lawson ML, Oldham K, Azizkhan RG, Hebra AV, Nuss D, Goretsky MJ, et al. Prospective multicenter study of surgical correction of pectus excavatum: design, perioperative complications, pain, and baseline pulmonary function facilitated by internet-based data collection. J Am Coll Surg 2007; 205:205-216; PMID:17660066; http://dx.doi.org/10.1016/j.jamcollsurg.2007.03.027
- Lennon D, Reid S, Stewart J, Jackson C, Crengle S, Percival T. Reducing inequalities with vaccines: New Zealand's MeNZB vaccine initiative to control an epidemic. J Paediatr Child Health 2012; 48:193-201; PMID:21996021; http://dx.doi.org/10.1111/j.1440-1754.2010.01969.x
- Tappero JW, Lagos R, Ballesteros AM, Plikaytis B, Williams D, Dykes J, Gheesling LL, Carlone GM, Hoiby EA, Holst J, et al. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 1999; 281:1520-1527; PMID:10227322; http://dx.doi.org/10.1001/jama.281.16.1520
- Holst J, Feiring B, Naess LM, Norheim G, Kristiansen P, Hoiby EA, Bryn K, Oster P, Costantino P, Taha MK, et al. The concept of “tailor-made”, protein-based, outer membrane vesicle vaccines against meningococcal disease. Vaccine 2005; 23:2202-2205; PMID:15755595; http://dx.doi.org/10.1016/j.vaccine.2005.01.058
- Martin DR, Ruijne N, McCallum L, O'Hallahan J, Oster P. The VR2 epitope on the PorA P1.7-2,4 protein is the major target for the immune response elicited by the strain-specific group B meningococcal vaccine MeNZB. Clin Vaccine Immunol 2006; 13:486-491; PMID:16603616; http://dx.doi.org/10.1128/CVI.13.4.486-491.2006
- Bjune G, Hoiby EA, Gronnesby JK, Arnesen O, Fredriksen JH, Halstensen A, Holten E, Lindbak AK, Nokleby H, Rosenqvist E, et al. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 1991; 338:1093-1096; PMID:1682541; http://dx.doi.org/10.1016/0140-6736(91)91961-S
- Zlotnick GW, Jones TR, Liberator P, Hao L, Harris S, McNeil LK, Zhu D, Perez J, Eiden J, Jansen KU, et al. The discovery and development of a novel vaccine to protect against Neisseria meningitidis serogroup B disease. Hum Vaccin Immunother 2015; 11:5-13; PMID:25483509; http://dx.doi.org/10.4161/hv.34293
- Green LR, Eiden J, Hao L, Jones T, Perez J, McNeil LK, Jansen KU, Anderson AS. Approach to the discovery, development, and evaluation of a novel neisseria meningitidis serogroup B vaccine. Methods Mol Biol 2016; 1403:445-469; PMID:27076147; http://dx.doi.org/10.1007/978-1-4939-3387-7_25
- Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, Ooi P, Smith RP, Weise P, Wetherell M, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun 2004; 72:2088-2100; PMID:15039331; http://dx.doi.org/10.1128/IAI.72.4.2088-2100.2004
- Murphy E, Andrew L, Lee KL, Dilts DA, Nunez L, Fink PS, Ambrose K, Borrow R, Findlow J, Taha MK, et al. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J Infect Dis 2009; 200:379-389; PMID:19534597; http://dx.doi.org/10.1086/600141
- Jiang HQ, Hoiseth SK, Harris SL, McNeil LK, Zhu D, Tan C, Scott AA, Alexander K, Mason K, Miller L, et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 2010; 28:6086-6093; PMID:20619376; http://dx.doi.org/10.1016/j.vaccine.2010.06.083
- Folaranmi T, Rubin L, Martin SW, Patel M, MacNeil JR. Use of serogroup B meningococcal vaccines in persons aged ≥ 10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:608-612.
- TRUMENBA (Meningococcal Group B Vaccine). Full Prescribing Information, Wyeth Pharmaceuticals Inc, a subsidiary of Pfizer Inc, Philadelphia, PA, 2014.
- Vaccines and Related Biologic Products Advisory Committee. Approaches to licensure of meningococcal vaccines for prevention of serogroup B invasive meningococcal disease. Briefing document for the Vaccines and Related Biologic Products Advisory Committee Meeting. April 7, 2011. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM248586.pdf. Accessed October 30, 2015.
- Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, Eskola J, Fermon F, Klugman KP, Ramsay M, Sow S, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr 2013; 11:17; PMID:24016339; http://dx.doi.org/10.1186/1478-7954-11-17
- Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection–serum bactericidal antibody activity. Vaccine 2005; 23:2222-2227; PMID:15755600; http://dx.doi.org/10.1016/j.vaccine.2005.01.051
- Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med 1969; 129:1307-1326.
- Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol 2003; 10:780-786; PMID:12965904
- Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine 2001; 20 Suppl 1: S58-67; PMID:11587814; http://dx.doi.org/10.1016/S0264-410X(01)00299-7
- Frasch CE, Borrow R, Donnelly J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine 2009; 27(suppl 2):B112-116; PMID:19464093; http://dx.doi.org/10.1016/j.vaccine.2009.04.065
- Ramsay ME, Andrews N, Kaczmarski EB, Miller E. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet 2001; 357:195-196; PMID:11213098; http://dx.doi.org/10.1016/S0140-6736(00)03594-7
- Borrow R, Carlone GM. Serogroup B and C serum bactericidal assays. Methods Mol Med 2001; 66:289-304; PMID:21336762
- World Health Organization. Standardization and validation of serological assays for the evaluation of immune responses to Neisseria meningitidis serogroup A/C vaccines. Geneva, Switzerland: March 8–9, 1999. WHO/V&B/99/19.
- Gotschlich EC, Goldschneider I, Artenstein MS. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med 1969; 129:1367-1384; PMID:4977283; http://dx.doi.org/10.1084/jem.129.6.1367
- Vipond C, Care R, Feavers IM. History of meningococcal vaccines and their serological correlates of protection. Vaccine 2012; 30 Suppl 2: B10-17; PMID:22607894; http://dx.doi.org/10.1016/j.vaccine.2011.12.060
- Gill CJ, Ram S, Welsch JA, Detora L, Anemona A. Correlation between serum bactericidal activity against Neisseria meningitidis serogroups A, C, W-135 and Y measured using human versus rabbit serum as the complement source. Vaccine 2011; 30:29-34; PMID:22075087; http://dx.doi.org/10.1016/j.vaccine.2011.10.068
- Borrow R, Miller E. Surrogates of protection. In: Frosch M, Maiden M, eds. Handbook of meningococcal disease. Weinheim: Wiley-VCH; 2006:323-351.
- Zollinger WD, Mandrell RE. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun 1983; 40:257-264; PMID:6403466
- Michaelsen TE, Garred P, Aase A. Human IgG subclass pattern of inducing complement-mediated cytolysis depends on antigen concentration and to a lesser extent on epitope patchiness, antibody affinity and complement concentration. Eur J Immunol 1991; 21:11-16; PMID:1703960; http://dx.doi.org/10.1002/eji.1830210103
- Harris SL, Donald RGK, Hawkins JC, Tan C, O'Neill RE, McNeil LK, Perez J, Anderson AS, Jansen KU, Jones TR. Neisseria meningitidis serogroup B vaccine, bivalent rLP2086, induces broad serum bactericidal activity against diverse invasive disease strains including outbreak strains. Pediatr Infect Dis J 2016; In press.
- Marshall HS, Richmond PC, Nissen MD, Wouters A, Baber J, Jiang Q, Anderson AS, Jones TR, Harris SL, Jansen KU, et al. A phase 2 open-label safety and immunogenicity study of a meningococcal B bivalent rLP2086 vaccine in healthy adults. Vaccine 2013; 31:1569-1575; PMID:23352429; http://dx.doi.org/10.1016/j.vaccine.2013.01.021
- Nissen MD, Marshall HS, Richmond PC, Jiang Q, Harris SL, Jones TR, Jansen KU, Perez JL. A randomized, controlled, phase 1/2 trial of a Neisseria meningitidis serogroup B bivalent rLP2086 vaccine in healthy children and adolescents. Pediatr Infect Dis J 2013; 32:364-371; PMID:23114369; http://dx.doi.org/10.1097/INF.0b013e31827b0d24
- Richmond PC, Marshall HS, Nissen MD, Jiang Q, Jansen KU, Garces-Sanchez M, Martinon-Torres F, Beeslaar J, Szenborn L, Wysocki J, et al. Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 2012; 12:597-607; PMID:22569484; http://dx.doi.org/10.1016/S1473-3099(12)70087-7
- Richmond PC, Nissen MD, Marshall HS, Lambert SB, Roberton D, Gruber WC, Jones TR, Arora A. A bivalent Neisseria meningitidis recombinant lipidated factor H binding protein vaccine in young adults: results of a randomised, controlled, dose-escalation phase 1 trial. Vaccine 2012; 30:6163-6174; PMID:22871351; http://dx.doi.org/10.1016/j.vaccine.2012.07.065
- Vesikari T, Wysocki J, Beeslaar J, Eiden J, Jiang Q, Jansen KU, Jones TR, Harris SL, O'Neill RE, York LJ, et al. Immunogenicity, safety, and tolerability of bivalent rLP2086 meningococcal group B vaccine administered concomitantly with diphtheria, tetanus, and acellular pertussis and inactivated poliomyelitis vaccines to healthy adolescents. J Pediatric Infect Dis Soc 2016; 5:180-187; PMID:26803328; http://dx.doi.org/10.1093/jpids/piv064
- Senders S, Bhuyan P, Jiang Q, Absalon J, Eiden JJ, Jones TR, York LJ, Jansen KU, O'Neill RE, Harris SL, et al. Immunogenicity, tolerability and safety in adolescents of bivalent rLP2086, a meningococcal serogroup B vaccine, coadministered with quadrivalent human papilloma virus vaccine. Pediatr Infect Dis J 2016; 35:548-554; PMID:26835974; http://dx.doi.org/10.1097/INF.0000000000001072
- Reiner DM, Bhuyan P, Eiden JJ, Ginis J, Harris S, Jansen KU, Jiang Q, Jones TR, O'Neill RE, York LJ, et al. Immunogenicity, safety, and tolerability of the meningococcal serogroup B bivalent rLP2086 vaccine in adult laboratory workers. Vaccine 2016; 34:809-813; PMID:26707218; http://dx.doi.org/10.1016/j.vaccine.2015.12.016
- Vesikari T, Ostergaard L, Diez-Domingo J, Wysocki J, Flodmark CE, Beeslaar J, Eiden J, Jiang Q, Jansen KU, Jones TR, et al. Meningococcal serogroup B bivalent rLP2086 vaccine elicits broad and robust serum bactericidal responses in healthy adolescents. J Pediatric Infect Dis Soc 2016; 5:152-160; PMID:26407272; http://dx.doi.org/10.1093/jpids/piv039
- Vesikari V, Senders S, Absalon J, Eiden JJ, Jansen KU, Beeslaar J, York LJ, Jones TR, Maansson R, Harris SL, et al. Phase 3 trial of the immunogenicity, safety, and lot consistency of bivalent rLP2086, a meningococcal serogroup B vaccine, in adolescents. Presented at: Pediatric Academic Societies Annual Meeting; April 30-May 3, 2016; Baltimore, MD, USA.
- Ostergaard L, Ward BJ, Beeslaar J, Eiden J, KU J, Absalon J, York L, Jones TR, Harris S, O'Neill RE, et al. Phase 3 trial of the immunogenicity and safety of bivalent rLP2086, a meningococcal serogroup B vaccine, in young adults. Presented at: Pediatric Academic Societies Annual Meeting; April 30–May 3, 2016, Baltimore, MD, USA.
- Østergaard L, Ward BJ, Beeslaar JF, Eiden JJ, Jansen KU, Absalon J, York LJ, Jones TR, Harris SL, O'Neill R, et al. Phase 3 trial of immunogenicity of bivalent rLP2086, a meningococcal serogroup B vaccine, in young adults: bactericidal activity against a panel of antigenically diverse strains. Presented at: European Society for Paediatric Infectious Diseases Annual Meeting; May 10–14, 2016; Brighton, UK.
- Vesikari V, Senders S, Absalon J, Eiden JJ, Jansen KU, Beeslaar J, York LJ, Jones TR, Maansson R, Harris SL, et al. Phase 3 trial of immunogenicity of bivalent rLP2086, a meningococcal serogroup B vaccine, in adolescents: bactericidal activity against a panel of antigenically diverse strains. Presented at: European Society for Paediatric Infectious Diseases Annual Meeting; May 10–14, 2016; Brighton, UK.
- Tan C, Jansen KU, Anderson AS, Jones TR, Harris SL. Selection of ten additional diverse MnB SBA test strains to provide supportive immunogenicity data in Phase 3 trials of bivalent rLP2086. Presented at: International Pathogenic Neisseria Conference (IPNC); September 4–9, 2016; Manchester, UK.
- Atkinson B, Gandhi A, Balmer P. History of meningococcal outbreaks in the United States: implications for vaccination and disease prevention. Pharmacotherapy 2016; 36:880-892; PMID:27332671; http://dx.doi.org/10.1002/phar.1790
- Diermayer M, Hedberg K, Hoesly F, Fischer M, Perkins B, Reeves M, Fleming D. Epidemic serogroup B meningococcal disease in Oregon: the evolving epidemiology of the ET-5 strain. JAMA 1999; 281:1493-1497; PMID:10227318; http://dx.doi.org/10.1001/jama.281.16.1493
- Clark SA, Lekshmi A, Lucidarme J, Hao L, Tsao H, Lee-Jones L, Jansen KU, Newbold LS, Anderson AS, Borrow R. Differences between culture & non-culture confirmed invasive meningococci with a focus on factor H-binding protein distribution. J Infect 2016; 73:63-70; PMID:27025206; http://dx.doi.org/10.1016/j.jinf.2016.03.012
- Hoiseth SK, Murphy E, Andrew L, Vogel U, Frosch M, Hellenbrand W, Abad R, Vazquez JA, Borrow R, Findlow J, et al. A multi-country evaluation of Neisseria meningitidis serogroup B factor H-binding proteins and implications for vaccine coverage in different age groups. Pediatr Infect Dis J 2013; 32:1096-1101; PMID:23694830; http://dx.doi.org/10.1097/INF.0b013e31829aa63b
- Domnich A, Gasparini R, Amicizia D, Boccadifuoco G, Giuliani MM, Panatto D. Meningococcal antigen typing system development and application to the evaluation of effectiveness of meningococcal B vaccine and possible use for other purposes. J Immunol Res 2015; 2015:353461; PMID:26351645; http://dx.doi.org/10.1155/2015/353461
- Manigart O, Trotter C, Findlow H, Assefa A, Mihret W, Moti Demisse T, Yeshitela B, Osei I, Hodgson A, Quaye SL, et al. A seroepidemiological study of serogroup A meningococcal infection in the African meningitis belt. PLoS ONE 2016; 11:e0147928; PMID:26872255; http://dx.doi.org/10.1371/journal.pone.0147928
- Adis Insight (Springer). Meningococcal vaccine group B - GlaxoSmithKline. Available at: http://adisinsight.springer.com/drugs/800012858. Accessed March 10, 2016.
- Rossi R, Beernink PT, Giuntini S, Granoff DM. Susceptibility of meningococcal strains responsible for two serogroup B outbreaks on U.S. university campuses to serum bactericidal activity elicited by the MenB-4C vaccine. Clin Vaccine Immunol 2015; 22:1227-1234; PMID:26424832; http://dx.doi.org/10.1128/CVI.00474-15
- Medini D, Stella M, Wassil J. MATS: Global coverage estimates for 4CMenB, a novel multicomponent meningococcal B vaccine. Vaccine 2015; 33:2629-2636; PMID:25882169; http://dx.doi.org/10.1016/j.vaccine.2015.04.015
- Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, Moxon ER, Stella M, Comanducci M, Bambini S, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci U S A 2010; 107:19490-19495; PMID:20962280; http://dx.doi.org/10.1073/pnas.1013758107
- Basta N, Mahmoud A, Wolfson J, Ploss A, Heller B, Hanna S, Johnsen P, Izzo R, Grenfell B, Findlow J, et al. Immunogenicity of a meningococcal B vaccine during a university outbreak. N Engl J Med 2016; 375:220-228; PMID:27468058; http://dx.doi.org/10.1056/NEJMoa1514866
- McNeil LK, Murphy E, Zhao XJ, Guttmann S, Harris SL, Scott AA, Tan C, Mack M, DaSilva I, Alexander K, et al. Detection of LP2086 on the cell surface of Neisseria meningitidis and its accessibility in the presence of serogroup B capsular polysaccharide. Vaccine 2009; 27:3417-3421; PMID:19200847; http://dx.doi.org/10.1016/j.vaccine.2009.01.075
- McNeil LK, Zlotnick GW, Camposano E, Logan SM, Novikova EG, Zhao XJ, Anderson AS, Pride MW, Jansen KU. Development of a meningococcal antigen surface expression (MEASURE) assay for the phenotypic characterization of fHBP expression by Neisseria meningitidis. Presented at: European Meningococcal Disease Society, 2011; Ljubljana, Slovenija.
- Biagini M, Spinsanti M, De Angelis G, Tomei S, Ferlenghi I, Scarselli M, Rigat F, Messuti N, Biolchi A, Muzzi A, et al. Expression of factor H binding protein in meningococcal strains can vary at least 15-fold and is genetically determined. Proc Natl Acad Sci U S A 2016; 113:2714-2719; PMID:26888286; http://dx.doi.org/10.1073/pnas.1521142113
- Peltola H, Makela H, Kayhty H, Jousimies H, Herva E, Hallstrom K, Sivonen A, Renkonen OV, Pettay O, Karanko V, et al. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med 1977; 297:686-691; PMID:408682; http://dx.doi.org/10.1056/NEJM197709292971302
- Sikkema DJ, Friedman KE, Corsaro B, Kimura A, Hildreth SW, Madore DV, Quataert SA. Relationship between serum bactericidal activity and serogroup-specific immunoglobulin G concentration for adults, toddlers, and infants immunized with Neisseria meningitidis serogroup C vaccines. Clin Diagn Lab Immunol 2000; 7:764-768; PMID:10973451
- King WJ, MacDonald NE, Wells G, Huang J, Allen U, Chan F, Ferris W, Diaz-Mitoma F, Ashton F. Total and functional antibody response to a quadrivalent meningococcal polysaccharide vaccine among children. J Pediatr 1996; 128:196-202; PMID:8636811; http://dx.doi.org/10.1016/S0022-3476(96)70389-X
- Vesikari T, Forsten A, Bianco V, Van der Wielen M, Miller JM. Antibody persistence up to 5 years after vaccination of toddlers and children between 12 months and 10 years of age with a quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine. Hum Vaccin Immunother 2016; 12:132-139; PMID:26575983; http://dx.doi.org/10.1080/21645515.2015.1058457
- Gandhi A, Balmer P, York LJ. Characteristics of a new meningococcal serogroup B vaccine, bivalent rLP2086 (MenB-FHbp; Trumenba(R)). Postgrad Med 2016; 128:548-556; PMID:27467048; http://dx.doi.org/10.1080/00325481.2016.1203238
- Taha M-K, Hawkins JC, Liberator PL, Deghmane A-E, Andrew L, Hao L, Jones TR, McNeil LK, O'Neill RE, Perez JL, et al. Bactericidal activity of sera from subjects vaccinated with bivalent rLP2086 against meningococcal serogroup B outbreak strains from France. Submitted to Vaccine; 2016.
