ABSTRACT
The consequences of the 2013–16 Ebola Zaire virus disease epidemic in West Africa were grave. The economies, healthcare systems and communities of Guinea, Sierra Leone and Liberia were devastated by over 18 months of active Ebola virus transmission, followed by sporadic resurgences potentially related to sexual transmission by survivors with viral persistence in body fluids following recovery. The need to develop and implement strategies to prevent and mitigate future outbreaks is now beyond dispute. The potential for unpredictable outbreaks of indeterminate duration, and control challenges posed by the possibility of sporadic re-emergence, mean that implementation of an effective vaccination program for outbreak containment necessitates a vaccine providing durable immunity. Heterologous prime-boost vaccine regimens deliver the same or similar antigens through different vaccine types, the first to prime and the second to boost the immune system. Ad26.ZEBOV/MVA-BN-Filo is an investigational Ebola Zaire vaccine regimen that uses this heterologous prime-boost approach. Preliminary Phase 1 data suggest that Ad26.ZEBOV/MVA-BN-Filo confers durable immunity for at least 240 d and is well-tolerated with a good safety profile. This regimen may therefore be suitable for prophylactic use in a regional or targeted population vaccination strategy, and could potentially aid prevention and control of future Ebola outbreaks.
Introduction
The 2013–16 outbreak in West Africa was caused by the Ebola Zaire species, one of 5 species of Ebolavirus. This particular species has been responsible for 4 of the 5 most serious Ebola outbreaks,Citation1 however this was the first emergence of the Zaire species recorded in a high-density urban population,Citation2 resulting in its rapid and widespread transmission. The resulting epidemic was of unprecedented scale, causing over 28,000 total cases (suspected, probable and confirmed), and more than 11,000 deaths.Citation3
Ebolavirus is a member of the Filoviridae family of viruses and causes Ebola virus disease (EVD), also known as Ebola hemorrhagic fever (EHF), in both human and non-human primates. Human-to-human transmission of the virus results from contact with infected body fluids and secretions.Citation4 The virus can persist in some body fluids for months or years following clearance of viraemia.Citation5 In particular, viral persistence in the semen has been recorded up to 488 d.Citation6
The Ebola virus epidemic of 2013 is thought to have begun in an 18-month-old boy in Guinea, having been transmitted to him from an infected wild animal, probably a bat.Citation7,8 The virus then spread to the neighboring countries of Liberia and Sierra Leone, where weak health systems and poor surveillance hindered risk assessment and potentially contributed to its accelerated transmission.Citation9,10 Sporadic flare-ups occurred in Sierra Leone, Guinea and Liberia in early 2016, in all cases following declaration of the end of the epidemic in each country.Citation11-13 At least some of these flare-ups have been proven to have arisen as a consequence of re-emergence of previously active transmission chains, perhaps resulting from transmission by a persistently infected survivor.Citation14,15
Although the West African epidemic has now been declared over, a new outbreak could arise at any time. It is now clear that Ebola spread resulting from human-to-human transmission, including infection by survivors and mother-to-child transmission, can give rise to an epidemic of much greater scale and impact than previously anticipated. Trends suggest that future outbreaks could be even more serious than the West African epidemic if effective control measures are not put in place.Citation16 Adequate preparation, encompassing early detection, management and prevention, is vital to control any future outbreaks and prevent a repetition of the human and economic impact of the West African epidemic.
Role of vaccination in managing potential future outbreaks
The prevention of Ebola virus transmission must form a key element of any control plan. The availability of a vaccine conferring durable immunity would undoubtedly aid the implementation of such a plan. Several vaccine candidates are currently in development. These include ChAd3-EBO, a chimpanzee Adenovirus vector vaccine,Citation17 and rVSV-ZEBOV, which utilizes a Recombinant Vesicular Stomatitis Virus (rVSV). Interim results from studies of rVSV-vectored vaccines expressing Ebola virus glycoprotein have demonstrated a rapid immune response. The preliminary data suggest that the vaccine is efficacious in the context of a reactive ring vaccination strategy and could aid containment of an active outbreak when used in such a setting;Citation18 furthermore, Agnandji et al (2016) have indicated that further evaluation is needed to assess safety and efficacy.Citation19 Additionally, Ad26.ZEBOV/MVA-BN-Filo is an investigational heterologous prime-boost regimen that has demonstrated strong immunogenicity together with a good safety profile in preliminary Phase 1 data.Citation20
Different vaccination strategies may be used to prevent and mitigate the spread of Ebola, depending on the circumstances. The three strategies that have been modeled for the Ebola epidemic are ring vaccination, targeted vaccination of high-risk individuals (such as front-line workers and healthcare workers) and population vaccination (). During an outbreak where ring vaccination is the strategy used to disrupt chains of transmission and prevent the outbreak spreading, rapid onset of protection is important and duration of immunity is a lower priority. In at-risk populations in which the virus is not actively circulating, a prophylactic population vaccination strategy using a vaccine regimen that confers sustained immunity may be more appropriate.Citation21
Table 1. Overview of potential Ebola vaccination strategies.
Ebola transmission modeling data suggest that ring vaccination at the start of an outbreak would probably not be sufficient to contain the spread, particularly where individual cases and contacts are difficult to identify.Citation22 This was the case in West Africa early in the course of the epidemic, and is likely to be in the future, particularly in densely populated areas and those with mobile populations. Therefore, regional population vaccination is likely to be necessary to bring sustained transmission under control. In addition, targeted vaccination of at-risk groups such as front-line workers is a vital adjunct to such a strategy in order to protect the members of the community at highest risk of exposure. Because the timing of front-line workers' exposure to the virus is difficult to predict, durability of protection is an important consideration in implementing such a program. To implement an effective regional population vaccination strategy and provide suitable protection for front-line workers, a vaccine conferring sustained immunity and with a good safety and tolerability profile will be needed.Citation23 The utilization of a heterologous prime-boost strategy, such as in the Ad26.ZEBOV/MVA-BN-Filo vaccine regimen, represents an innovative approach to fulfilling this need.
Heterologous prime-boost approach to enhancing durability of immune response
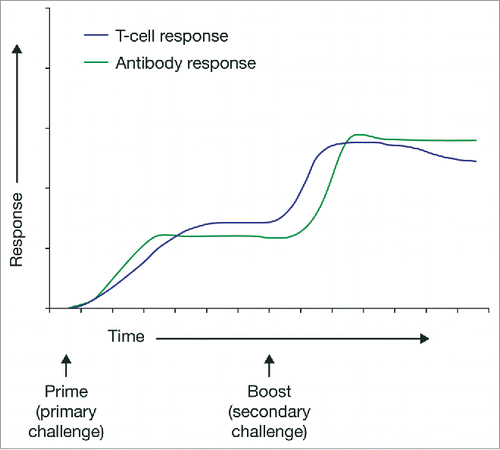
Multiple-dose prime-boost vaccination regimens are well established. Several homologous prime-boost regimens, whereby the same vaccine is used for the prime and boost doses, are routinely recommended. Examples include the tetanus, hepatitis B, measles and injectable polio vaccines.Citation24 More recently, heterologous prime-boost regimens have been developed with the goal of producing stronger and longer-lasting immunity to a disease. Heterologous prime-boost delivers the same or similar antigens for the disease through different vaccine types, the first to prime the immune system and the second to boost the immune system. Evidence has emerged that such a heterologous prime-boost approach may in some cases be even more effective in producing durable immunity than homologous boosting ().Citation25,26 Although single-dose vaccinations are preferred due to ease of deployment, the heterologous prime-boost approach can potentially offer significant durability benefits compared with single-dose alternatives, justifying its more complex administration. In the case of the Ad26.ZEBOV/MVA-BN-Filo regimen, the priming vaccine component is Ad26.ZEBOV, and the boosting vaccine component is MVA-BN-Filo.
Ad26.ZEBOV/MVA-BN-Filo clinical development
Several Phase 1–3 clinical studies are currently being conducted in support of potential eventual registration for the Ad26.ZEBOV/MVA-BN-Filo Ebola vaccine regimen. Since 2008, Janssen have pursued the development of an adenovirus-based Filovirus vaccine. The Ad26.ZEBOV/MVA-BN-Filo study program was accelerated in 2014 as part of the public health response to the West African epidemic. To this end, 4 Phase 1 and 3 Phase 2 studies are assessing safety, tolerability and immunogenicity of the regimen in healthy adults and populations including subjects with HIV, elderly people, adolescents and young children. An expanded safety and immunogenicity trial in Sierra Leone, the EBOVAC-Salone study, is underway,Citation27 and 2 supporting Phase 3 studies are currently ongoing with different vaccine lots of Ad26.ZEBOV and MVA-BN-Filo for quality assessment of the vaccines. Finally, a prospective registry has been established to follow subjects who received Ad26.ZEBOV and/or MVA-BN-Filo in clinical studies, to document their long-term safety profile.
Preliminary safety and immunogenicity data
The Phase 1 clinical studies being conducted in the US, Europe and Africa all share the overall aim of establishing sufficient safety and immunogenicity data to rapidly progress the selected prime-boost sequence and schedule(s) toward licensure. Studies in the UK (EBL1001) and US (EBL1002) are intended to establish preliminary safety and immunogenicity, identify optimal short schedules and investigate durability of immune responses, while the studies in Africa (EBL1003 and EBL1004) aim to replicate data of first in human (FIH) study in countries unaffected by the West African epidemic and confirm preliminary safety and immunogenicity.
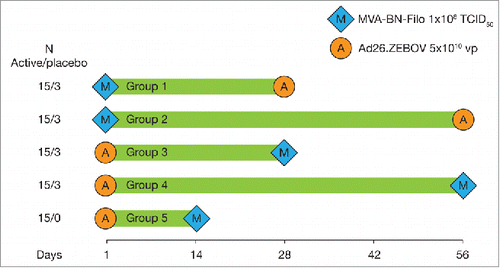
The EBL1001 trial is a single-center, randomized, placebo-controlled, observer blind trial held in the UK. MVA-BN-Filo and Ad26.ZEBOV were administered in different sequences and schedules in healthy 18–50 y olds (n = 87) (). EBL1001 data have been reported in a preliminary communication by Iain Milligan and colleagues.Citation20 Preliminary safety data in healthy volunteers are indicative of a well-tolerated vaccine regimen with a good safety profile. No safety concerns with either vaccine were identified, and no vaccine-related serious adverse events occurred. The most frequent adverse events were mild in intensity and short in duration.
Figure 2. Dosing schedule for groups receiving heterologous prime-boost MVA-BN-Filo and Ad26.ZEBOV in EBL1001 study (Clinicaltrials.gov: NCT02313077). Note: Groups 1–4 were randomized and blinded to active vaccine/placebo; group 5 was open-label.
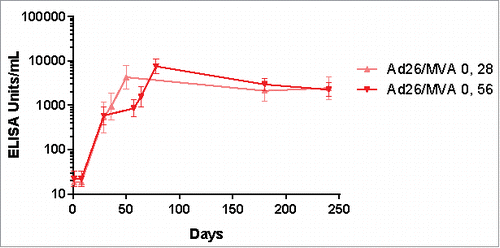
In terms of immunogenicity, Ad26.ZEBOV priming has been observed to induce antibody and T cell responses to Ebola virus glycoprotein, detectable as early as Day 14 post-prime in 80% of participants. Post-prime immune responses were stronger and more rapid following Ad26.ZEBOV priming versus MVA.BN-Filo priming (; Fig. S1). Substantial post-boost strengthening of antibody and T cell responses was observed in participants receiving all tested heterologous prime-boost regimens. Responses were sustained for at least 240 d post-prime, with no apparent effect of varying the prime-boost interval (56 vs. 28 days) on the durability of immune responses (). Follow-up is ongoing. Notably, 8 months following prime vaccination, 100% of individuals in the study maintained Ebola-specific antibodies, and furthermore T cell responses persisted in 77–80% of individuals receiving the Ad26.ZEBOV/MVA-BN-Filo regimen ().
Figure 3. EBOV GP-specific antibody responses to different orders of prime and boost administration, with 14-, 28- and 56-day prime- boost interval (EBL1001 study data, shown up to 21 d post-boost), assessed by ELISA (enzyme-linked immunosorbent assay). GMC, geometric mean concentration; GP: glycoprotein.
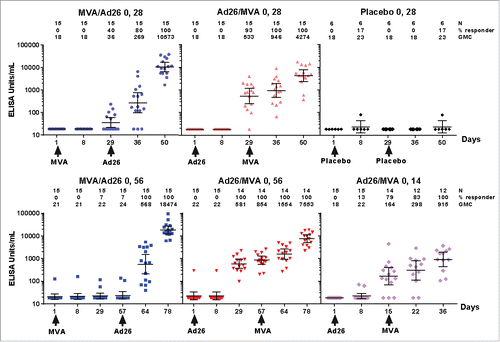
Figure 4. EBL1001 duration of anti-Ebola GP antibody response (assessed by ELISA) up to 240 d post-prime ELISA: Enzyme-linked immunosorbent assay; GP: glycoprotein.
In summary, Phase 1 findings so far reported indicate that Ad26.ZEBOV prime immunization readily induces an immune response which is enhanced further by MVA-BN-Filo boosting, that the Ad26.ZEBOV/MVA-BN-Filo heterologous prime-boost regimen induces durable immunity to Ebola Zaire, and that both the prime and boost are well tolerated with a good safety profile.
Concluding remarks
The West African epidemic of Ebola Zaire was unprecedented in scale. It is not enough to wait for another runaway epidemic to emerge, at the immense cost of thousands of lives and millions of dollars, to invest in preparation for the prevention and mitigation of future outbreaks. Lessons from the epidemic include the importance of co-ordinating and adequately resourcing control measures, the crucial importance of robust and resilient health systems, and key role of community engagement in enabling contact tracing and early reporting.Citation28 It is now apparent that spread of Ebola through human-to-human transmission can greatly worsen the scale of outbreaks, and that viral persistence and sexual transmission from survivors can perpetuate an epidemic and result in flare-ups long after the outbreak is thought to have ended.Citation29
Data from preliminary studies suggest that the heterologous prime-boost Ad26.ZEBOV/MVA-BN-Filo vaccine regimen confers durable immunity with a good safety and tolerability profile. This regimen may therefore be well-suited to prophylactic use, potentially underpinning preventive vaccination strategies in the event of a new outbreak. The protection of frontline workers such as healthcare providers, as recommended in the WHO target product profile for Ebola vaccines,Citation30 is key to mitigating the impact of a developing outbreak. Because the timescale during which frontline workers may be at risk of exposure to the virus cannot easily be determined in advance, durability of protection is a key consideration for such a strategy. Furthermore, the lessons of the West African outbreak, including the newfound understanding of the risks associated with prolonged viral persistence, highlight potential risks to frontline workers even after active transmission is thought to have ended.Citation31 Protection of frontline workers is necessary, but may not be sufficient to achieve control and prevent a future outbreak from becoming another widespread epidemic.Citation21 An effective targeted population vaccination program may be required, and once again sustained, robust immunity will be necessary for success.Citation32
Janssen have dramatically scaled up manufacturing capabilities for both components of the regimen in preparation for the next outbreak, leveraging existing platforms to facilitate rapid production in response to an emergency. The vaccine has demonstrated standard cold-chain (2–8°C) compatibility and suitability for long-term refrigerated storage in field conditions. Efforts to determine how best to deploy the heterologous prime-boost regimen are ongoing; the stability findings suggest that the vaccine components are appropriate for field deployment as part of such a regimen and for the potential implementation of a large-scale prophylactic population vaccination strategy. In addition, taking consideration of the possibility that the next major filovirus outbreak may involve a species other than Ebola Zaire, a multivalent Ad26/MVA-BN-Filo vaccine regimen is in ongoing development; first-in-human trials are scheduled to begin in the fourth quarter of 2016.
An effective vaccine with an acceptable safety profile could, with suitably reliable distribution arrangements, form an integral part of control measures in the case of a future outbreak.Citation23 Based on the preliminary safety and immunogenicity data presented here, Ad26.ZEBOV/MVA-BN-Filo has demonstrated considerable promise as a regimen well-suited to such a strategy.
Abbreviations
| EBOV GP | = | Ebola Virus Glycoprotein |
| EVD | = | Ebola Virus Disease |
| EHF | = | Ebola Hemorrhagic Fever |
| FIH | = | First In Human |
| rVSV | = | Recombinant Vesicular Stomatitis Virus |
Disclosure of potential conflicts of interest
All authors are employees of Janssen Pharmaceuticals.
Supplemental_Material.zip
Download Zip (47 KB)Acknowledgments
EBL1001 is sponsored by Janssen Vaccines & Prevention B.V. (formerly known as Crucell Holland B.V.), hereafter referred to as “Janssen.”
The Ad26.ZEBOV/MVA-BN-Filo vaccine regimen is in development in collaboration with Bavarian Nordic A/S, Denmark and in partnership with the Division of Microbiology and Infectious Diseases (DMID) of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) (Contract No. HHSN272200800056C). Development has been further enhanced by the formation of Ebola vaccine development consortia (EBOVAC1 and EBOVAC2) that have been granted funding from the European Innovative Medicines Initiative (IMI). Partnering organisations include Janssen, the London School of Hygiene & Tropical Medicine, the University of Oxford, l'Institut National de la Santé et de la Recherche Médicale (INSERM), Le Center Muraz, Bavarian Nordic A/S, Vibalogics, Grameen Foundation and World Vision of Ireland. The authors would like to thank the EBL1001 principal investigator (M. Snape); D. Watson-Jones, B. Greenwood, R. Thiebaut, and N. Meda for their important contributions to the clinical development of the vaccine regimen; as well as all EBL1001 investigators, study participants, study center staff and Janssen study personnel.
Editorial support was provided by Kaedy Bryson and Ian Grieve on behalf of Zoetic Science, an Ashfield company, funded by Janssen.
Funding
EBL1001 received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement numbers 115854 and 115861. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation program and the European Federation of Pharmaceutical Industries and Association. The development program has been funded in whole or in part with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority (BARDA), under Contract No. HHSO100201500008C. In addition, further funding has been granted by the Joint Vaccine Acquisition Program (JVAP) in the United States.
References
- Pringle CR. Virus taxonomy—San Diego 1998. Arch Virol 1998; 143:1449-60; PMID:9742051; http://dx.doi.org/10.1007/s007050050389
- Brainard J, Pond K, Hooper L, Edmunds K, Hunter P. Presence and persistence of ebola or Marburg virus in patients and survivors: a rapid systematic review. PLoS Negl Trop Dis 2016; 10:e0004475; PMID:26927697; http://dx.doi.org/10.1371/journal.pntd.0004475
- Centres for Disease Control and Prevention. Ebola (Ebola virus disease) About Ebola Virus Disease. 2016a. http://www.cdc.gov/vhf/ebola/about.html. Last accessed: 26 August 2016.
- Centres for Disease Control and Prevention. Ebola (Ebola virus disease) Case counts. 2016b. https://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/. Last accessed: 26 August 2016.
- Chughtai AA, Barnes M, Macintyre CR. Persistence of Ebola virus in various body fluids during convalescence: evidence and implications for disease control and transmission. Epidemiol Infect 2016; 144:1652-60; PMID:26808232; http://dx.doi.org/10.1017/S0950268816000054
- Fallah M for the Prevail III Research Team. A cohort study of survivors of Ebola virus infection in Liberia (PREVAIL III). Abstract presented at the Conference on Retroviruses and Opportunistic Infections (CROI) 2016, February 22–25, 2016, Boston, MA, USA; Abstract 74LB.
- World Health Organization. Origins of the 2014 Ebola epidemic. January 2015 (2015a). http://www.who.int/csr/disease/ebola/one-year-report/virus-origin/en. Last accessed: 26 August 2016.
- Marí Saéz A, Weiss S, Nowak K, Lapeyre V, Zimmermann F, Düx A, Kühl HS, Kaba M, Regnaut S, Merkel K, et al. Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol Med 2015; 7:17-23; http://dx.doi.org/10.15252/emmm.201404792
- World Health Organization. Report of the Ebola interim assessment panel. May 2015 (2015b). www.who.int/csr/resources/publications/ebola/ebola-interim-assessment/en/. Last accessed: 26 August 2016.
- Kamradt-Scott A. WHO's to blame? The World Health Organization and the 2014 Ebola outbreak in West Africa. Third World Quarterly 2016; 37:401-8; http://dx.doi.org/10.1080/01436597.2015.1112232
- World Health Organization. Ebola situation report. 3 February 2016 (2016a). http://apps.who.int/ebola/current-situation/ebola-situation-report-3-february-2016. Last accessed: 26 August 2016.
- World Health Organization. Hundreds of contacts identified and monitored in new Ebola flare-up in Guinea. 22 March 2016 (2016b). http://www.who.int/csr/disease/ebola/guinea-flareup-update/en/. Last accessed: 26 August 2016.
- World Health Organization. Emergency response to Ebola flare underway in Liberia. Case investigation widens to Guinea. 04 April 2016 (2016c). http://www.who.int/csr/disease/ebola/liberia-flareups-update/en/. Last accessed: 26 August 2016.
- Arias A, Watson SJ, Asogun D, Tobin EA, Lu J, Phan MVT, Jah U, Wadoum REG, Meredith L, Thorne L, et al. Rapid outbreak sequencing of Ebola virus in Sierra Leone identifies transmission chains linked to sporadic cases. Vir Evol 2016; 2:vew016
- Blackley DJ, Wiley MR, Ladner JT, Fallah M, Lo T, Gilbert ML, Gregory C, D'ambrozia J, Coulter S, Mate S, et al. Reduced evolutionary rate in reemerged Ebola virus transmission chains. Sci Adv 2016; 2:e1600378; PMID:27386513; http://dx.doi.org/10.1126/sciadv.1600378
- Ayukekbong JA. The 2014–2015 Ebola saga: lessons for the future. J Epidemiol Community Health 2016; 70(1):1-2; PMID:26372790; http://dx.doi.org/10.1136/jech-2015-206138
- Ledgerwood JE, DeZure AD, Stanley DA, Novik L, Enama ME, Berkowitz NM, Hu Z, Joshi G, Ploquin A, Sitar S, et al. Chimpanzee adenovirus vector Ebola vaccine – preliminary report. N Engl J Med 2015; 373:776; PMID:26287857
- Henao-Restrapo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, Carroll MW, Doumbia M, Draguez B, Duraffour S, et al. Lancet 2015; 386:857-86; PMID:26248676; http://dx.doi.org/10.1016/S0140-6736(15)61117-5
- Agnandji S, Huttner A, Zinser ME, Njuguna P, Dahlke C, Fernandes JF, Yerly S, Dayer JA, Kraehling V, Kasonta R, et al. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med 2016; 374:1647-60; PMID:25830326; http://dx.doi.org/10.1056/NEJMoa1502924
- Milligan ID, Gibani MM, Sewell R, Clutterbuck EA, Campbell D, Plested E, Nuthall E, Voysey M, Silva-Reyes L, McElrath MJ, et al. Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia ankara-vectored ebola vaccines: A randomized clinical trial. JAMA 2016; 315(15):1610-23; PMID:27092831; http://dx.doi.org/10.1001/jama.2016.4218
- Coltart CE, Johnson AM, Whitty CJ. Role of healthcare workers in early epidemic spread of Ebola: policy implications of prophylactic compared to reactive vaccination policy in outbreak prevention and control. BMC Med 2015; 13:271; PMID:26482396; http://dx.doi.org/10.1186/s12916-015-0477-2
- Kucharski AJ, Eggo RM, Watson CH, Camacho A, Funk S, Edmunds WJ. Effectiveness of ring vaccination as control strategy for Ebola virus disease. Emerg Infect Dis 2016; 22:105-8; PMID:26691346; http://dx.doi.org/10.3201/eid2201.151410
- CIDRAP & Wellcome Trust. Recommendations for accelerating the development of Ebola vaccines. February 2015. Available at: http://www.cidrap.umn.edu/recommendations-accelerating-development-ebola-vaccines-0. Last accessed: 26 August 2016.
- World Health Organization. WHO recommendations for routine immunization – summary tables. February 2015 (2015c). http://www.who.int/immunization/policy/immunization_tables/en/. Last accessed: 26 August 2016.
- Lu S. Heterologous Prime-Boost Vaccination. Curr Opin Immunol 2009; 21:346-51; PMID:19500964; http://dx.doi.org/10.1016/j.coi.2009.05.016
- Moorthy BM, Fast P, Greenwood B. Heterologous prime-boost immunisation in Ebola vaccine development, testing and licensure. Report of a WHO Consultation held on 21 November 2014, Geneva, Switzerland. http://www.who.int/immunization/research/meetings_workshops/WHO_primeboost_Ebola_21nov14_meeting_report.pdf. Last accessed 26 August 2016.
- EBOVAC Projects. The Trials – EBOVAC-Salone. 2016. Available at: http://www.ebovac.org/the-trials/the-trials-phase-3/. Last accessed 26 August 2016.
- United Nations. Making a difference: The global Ebola response outlook 2015. https://ebolaresponse.un.org/sites/default/files/ebolaoutlook_full.pdf. Last accessed 26 August 2016.
- Vinson JE, Drake JM, Rohani P, Park AW. The potential for sexual transmission to compromise control of Ebola virus outbreaks. Biol Lett 2016; 12:20151079; PMID:27277951; http://dx.doi.org/10.1098/rsbl.2015.1079
- World Health Organization. WHO Ebola vaccine target product profile. 05 January 2016 (2016d). http://www.who.int/immunization/research/target-product-profile/ebolavaccine/en/. Last accessed: 26 August 2016.
- MacDermott NE, Bausch DG. Virus persistence and recrudescence after Ebola virus disease: what are the risks of healthcare workers? J Hosp Infect 2016; 94(2):113-115; PMID:27499080; http://dx.doi.org/10.1016/j.jhin.2016.07.004
- Osterholm M, Moore K, Ostrowsky J, Kimball-Baker K, Farrar J, Wellcome Trust-CIDRAP Ebola Vaccine Team B. The Ebola Vaccine Team B: a model for promoting the rapid development of medical countermeasures for emerging infectious disease threats. Lancet Infect Dis 2016; 16:e1-9; PMID:26526664; http://dx.doi.org/10.1016/S1473-3099(15)00416-8