ABSTRACT
The development of systems that are more accurate and time-efficient in predicting safety and efficacy of target products in humans are critically important in reducing the cost and duration of pharmaceutical development. To circumvent some of the limitations imposed by the use of animal models, ex vivo systems, such as precision-cut lung slices (PCLS), have been proposed as an alternative for evaluating safety, immunogenicity and efficacy of vaccines and pharmaceuticals. In this study, we have established a human PCLS system and methodology for PCLS cultivation that can provide long-term viability and functionality in culture. Using these techniques, we found that cultured PCLS remained viable for at least 14 d in culture and maintained normal metabolic activity, tissue homeostasis and structural integrity. To investigate whether cultured PCLS remained functional, lipopolysaccharide (LPS) was used as a target stimulating compound. We observed that after an 18-hour incubation with LPS, cultured PCLS produced a set of pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6 and IL-10 as well as the enzyme COX-2. Furthermore, cultured PCLS were shown to be capable of generating re-call immune responses, characterized by cytokine production, against antigens commonly found in routine vaccinations against influenza virus and tetanus toxoid. Taken together, these results suggest that human PCLS have the potential to be used as an alternative, high-throughput, ex vivo system for evaluating the safety, and potentially immunogenicity, of vaccines and pharmaceuticals.
Introduction
For decades, animal models have remained the mainstay for evaluating toxicological, allergic, pro-inflammatory and immunological properties of vaccines and other pharmaceutical compounds in pre-clinical studies. However, studies in animals are often expensive and time-consuming, and may not accurately predict the outcome of target products in humans. The development of ex vivo human systems that allow for high-throughput screening of potential toxicological and allergic reactions to a vaccine or another pharmaceutical product could be instrumental in predicting product safety in humans and could reduce the cost and duration of new product development.
Human precision-cut lung slices (PCLS) may have the potential to be used as an ex vivo platform. Lungs are the portal of entry for many respiratory infections, such as viruses, bacteria, and fungus, and are highly enriched with different types of immune cells. Therefore, lung tissue could offer some unique advantages when testing compounds directed against such infections. Previous work on this technology have shown that PCLS can easily be prepared in abundance just from a single human lung lobe and can be prepared from human as well as different laboratory animal species, including mice, rats, guinea pigs, sheep and monkeys.Citation1-5 Multiple studies have focused on assessing various characteristics of cultured PCLS, such as measurement of bronchoconstrictionCitation6-8 and vascular responsesCitation9,10 induced by various stimuli. In addition, PCLS have been used to evaluate allergy,Citation11 asthma,Citation6 toxicology,Citation12-14 and infectious disease responses.Citation15 The anatomical architecture of PCLS also allows for studying early inflammatory and immune responses that occur in the lung. These processes are characterized by activation or suppression of certain mediators, which can be used as tools for evaluation of immune modulating effects of target products.Citation13,16-18
In the current study, we characterize a human PCLS cultivation system that was established to assess the ability of human PCLS to develop inflammatory and immune responses to antigen stimulation. Results demonstrated that PCLS remained viable, structurally intact and able to elicit functional responses up to 14 d in culture. In addition, we showed that stimulation of PCLS with a seasonal influenza vaccine or tetanus toxoid were able to generate inflammatory cytokine responses above background levels. Taken together, these data indicate the feasibility of using human PCLS as a model to assess potential toxicological, inflammatory and immunological responses specific to target vaccines and pharmaceuticals.
Results
Metabolic activity and viability of PCLS during cultivation
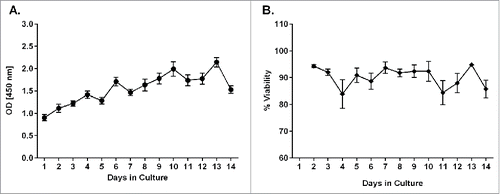
After optimizing the method for preparing and culturing PCLS from human donors, several assays were established to monitor the PCLS viability and metabolic activity over a 14-day time period in culture. Metabolic activity of human PCLS, as measured by the WST-1 assay, was lowest on day 1 after preparation (). However, the activity appeared to increase with time in culture, indicating a slight recovery phase following tissue preparation. A significant increase (p ≤ 0.05) in metabolic activity was detected by day 6 in culture after which the activity level appeared to remain steady through study day 14 (). A slight decrease in activity was observed on study day 14; however, it was not a statistically significant reduction. PCLS prepared and cultivated under established culture conditions also maintained tissue homeostasis as measured by the LDH assay. The average viability of PCLS at each culture time point remained above 80% over the 14-day cultivation period (). No significant changes in the viability of the tissue slices were observed during the test period.
Figure 1. Metabolic activity and viability measurements during cultivation of human PCLS. At different time points during cultivation of PCLS, the metabolic activity was measured using a WST-1 assay (A) and the viability was measured using an LDH assay (B). Each data point represents the mean ± SD of 4 PCLS from 5 to 8 lungs.
Metabolic activity and viability of PCLS after treatment with LPS or Triton X-100
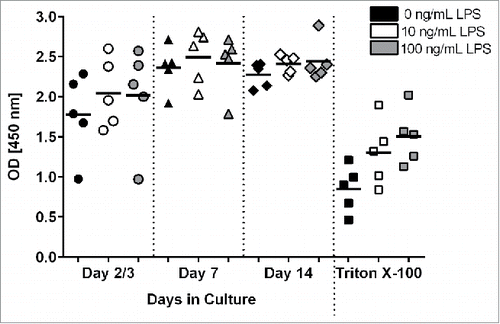
Human PCLS were treated with 0, 10 or 100 ng/mL LPS for 18 hours to investigate the effect of LPS stimulation on the vitality of the tissue slices at different times during cultivation. Using the WST-1 assay, metabolic activity of PCLS stimulated with LPS was shown to be comparable in activity to slices cultured in medium alone (). In addition, there were no differences in optical density (OD) values when comparing the low (10 ng/mL) versus high (100 ng/mL) concentrations of LPS during stimulation. Again, the data shows a slightly lower, more variable response early (day 2/3) in culture that recovers by day 7 and is then maintained through day 14. In contrast, there was a significant (p = 0.05) decrease in the metabolic activity after exposure of PCLS to Triton X-100, a commonly used detergent with known toxic effects. A similar observation was made when viability was measured using the LDH assay after LPS or Triton X-100 treatment (data not shown).
Figure 2. Measurement of metabolic activity and viability in LPS- and Triton X-100 -treated PCLS. On days 2 or 3, 7, and 12 or 13 of cultivation, PCLS were treated with different concentrations of LPS or medium only for 18 hours. As a control, PCLS were treated with 0.25 - 0.3 mM Triton X-100 for 1 hour. Metabolic activity was measured by a WST-1 assay. Each symbol on the graph represents PCLS from a given donor with the lines representing the mean value for the PCLS treated with each condition.
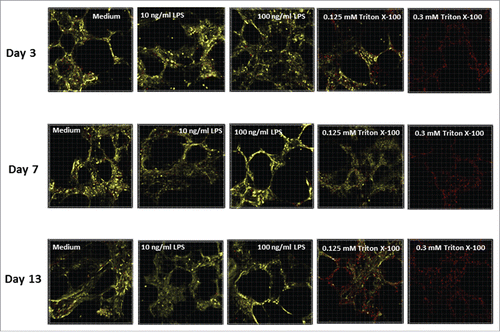
The effect of treating PCLS with LPS or Triton X-100 during cultivation was also investigated using Calcein AM/ethidium homodimer-1 (EthD-1) staining of live tissue. At least 2 slices per time point were examined and analyzed using a confocal laser scanning microscope. For each treatment, random duplicates or triplicates from 30-μm thick 3D stacks were recorded. Representative images obtained from one lung are shown in . The results suggest that the viability (shown by yellow staining) of untreated PCLS (medium alone) was steady over the entire culture period of 13 d. In addition, no increase in the dead cell nuclei (red spots) or decrease in the vital cytoplasm (yellow staining) were observed in the stained tissue following treatment with LPS at either concentration. These data agree with the results of the LDH and WST-1 assays. Furthermore, the confocal microscopy data showed that the alveolar structure of the lung tissue was preserved during the long-term cultivation in both LPS-treated and untreated PCLS. In contrast, treatment with Triton X-100 reduced the PCLS vitality in a dose-dependent manner, with a complete loss of vitality achieved in the 0.3 mM Triton X-100 treated slices.
Figure 3. Representative images of human PCLS stained with Calcein AM and Ethd-1 at different days of cultivation. PCLS were treated with 2 concentrations of LPS (10 or 100 ng/mL), 2 concentrations of Triton X-100 (0.125 or 0.3 mM) or medium alone. PCLS were stained by Calcein AM and Ethd-1 and evaluated by confocal microscopy. Three stacks of 31 pictures (30-µm thickness) per slice were randomly selected on days 3, 7 and 13. The assay was conducted in duplicate.
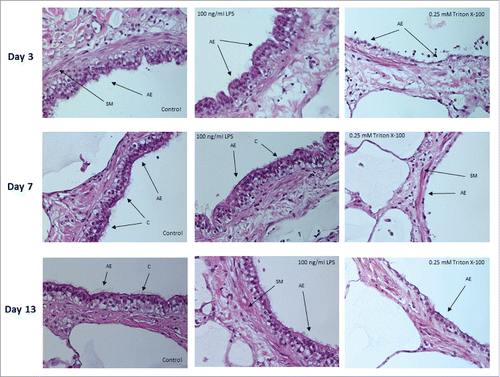
Hematoxylin and eosin (H&E) staining of tissue sections from PCLS were performed to confirm that cultured PCLS maintained structural, architectural and histological characteristics of normal human lung tissue with and without LPS stimulation. On different days of cultivation, PCLS were exposed to medium only, LPS or Triton X-100 prior to being fixed, sectioned and stained. Images indicate that the structural characteristics of the lung parenchyma (pneumocytes, alveolar septal structure and blood vessels) as well as the airways (bronchiolar epithelium, bronchiolar wall, smooth muscle layer and vessels) remained intact during long-term cultivation (). Results also showed that LPS stimulation had no effect on the structural integrity of the PCLS. In contrast, PCLS that had been exposed to 0.25 mM Triton X-100 for 1 hour were shown to be structurally damaged ().
Figure 4. Histology of lung airways in sections of PCLS. Images of airway epithelium from PCLS treated with medium only (control) or 100 ng/mL of LPS for 18 hours. Comparable PCLS were also treated with 0.25 mM Triton X-100 for one hour. Experiments were performed on days 3, 7 and 13 of cultivation. SM = smooth muscle, C = cilia, AE = airway epithelium. Original magnification 400 x.
Production of cytokines, chemokines and COX-2 by human PCLS treated with LPS or Triton X-100
Human PCLS exposed to 100 ng/mL of LPS were also evaluated for the production of select cytokines and chemokines as well as the COX-2 enzyme on different days of cultivation. Following LPS exposure, levels of cytokines were measured in PCLS culture supernatants. Treatment with LPS was shown to stimulate secretion of several cytokines and chemokines, including TNF-α, IL-1β, IL-6, IL-8, IL-10 and MIP-1β (). IL-2, IL-4, IFN-γ and IL-13 were found to be mostly below the assay detection limit (data not shown). Of the pro-inflammatory cytokines detected, IL-8 and IL-6 were produced at the highest levels, followed by MIP-1β and TNF-α. IL-10 and IL-1β were produced at the lowest levels. In addition, COX-2 was produced at moderate levels following LPS stimulation (). In general, greater levels of these secreted proteins were detected when PCLS were stimulated with LPS on day 2 or 3 of cultivation compared with days 7 and 13. Levels of these soluble mediators in culture supernatants from PCLS treated with Triton X-100 were generally lower compare with LPS-treated PCLS, or were below the detection limit (data not shown).
Table 1A. Secretion of cytokines and chemokines by PCLS in response to LPS.
Table 1B. Level of COX-2 expression in PCLS stimulated by LPS.
Re-call immune responses in PCLS against seasonal influenza vaccine and tetanus toxoid
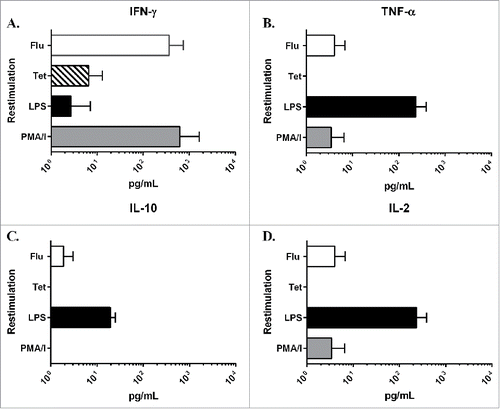
Cultured human PCLS were assessed for their ability to mount re-call immune responses against antigens used in routine vaccinations. The re-call responses were measured by the levels of cytokines in culture supernatants of PCLS exposed to the vaccine antigens as compare with a medium only control. For positive controls, the mitogen phorbol-12-myristate-13-acetate (PMA) as well as LPS were used. The two vaccine antigens used were the 2010–2011 seasonal influenza vaccine as well as tetanus toxin. The results showed that in response to stimulation with the influenza vaccine, there was a robust IFN-γ response followed by lower amounts of TNF-α and IL-2 (). This pattern of cytokine production was consistent in all 4 donors tested. The response to the tetanus toxoid antigen was lower in magnitude as compare with the influenza vaccine response, but was also dominated by IFN-γ production. These results demonstrate that cultured PCLS can generate antigen-specific immune responses.
Figure 5. Level of cytokines in culture supernatants from restimulated PCLS. PCLS were treated on day 2 of cultivation with either LPS, PMA/I, the 2010–2011 seasonal influenza vaccine (Flu) or tetanus toxoid (Tet) for 24 hours. After incubation, the level of cytokine in the culture supernatant was measured using the MSD Multi-Spot assay system: A) IFN-γ, B) TNF-α, C) IL-10 and D) IL-2. Values in the graphs represent the average pg/mL ± SD of PCLS from 4 different donor lungs normalized to a medium only control sample.
Discussion
Lung slices have been used to study pulmonary physiology, pharmacology, pathogenesis and toxicity.Citation19 An advantage of this technology is that it maintains the structural integrity of the tissue and its cell populations allowing for a more comprehensive assessment of local responses. In an effort to overcome one of the limitations of this system, the present study assessed whether human PCLS held in culture for up to 14 d could be used to study cytotoxic, inflammatory and immune responses against selected stimuli. Results demonstrated that the kinetics of the metabolic activity in PCLS remained constant over the entire cultivation period, following a short recovery phase. This is in contrast to other publications that report a limited viability of PCLS between 1–6 d in culture.Citation7,20,21 We further demonstrated that the viability of human PCLS, as shown by the LDH assay, remained above 80% for the full 14-day cultivation period. This type of long-term cultivation could increase the experimental window for using PCLS, maximizing data sets generated in this model. In addition, since PCLS can be prepared in abundance from just one human lung lobe, being able to cultivate viable PCLS from a single donor would also help combat the unpredictability of available human lung material.
Previous in vitro studies have shown that LPS is a potent activator of monocytes, including alveolar macrophages, and can induce inflammatory responses in PCLS.Citation7,22 However, it was also shown that exposure to LPS could directly cause tissue damage by triggering apoptotic cell death in bronchial epithelialCitation23 and endothelialCitation24 cells. In addition, LPS was reported to induce apoptosis in human alveolar macrophages in a dose-dependent manner.Citation25 In our study, we demonstrated a lack of change in metabolic activity and viability of the PCLS after LPS treatment during long-term cultivation. These findings were supported by Calcein AM/EthD-1 staining of PCLS where the number of dead cells in the slices did not increase nor was there a loss in tissue viability after treatment with LPS. In addition to metabolic activity and viability, we confirmed that lung airway epithelium exposed to LPS remained intact showing no structural damage.
While the metabolic activity and PCLS viability remained stable, we did detect a number of pro-inflammatory cytokine and chemokines produced in response to LPS stimulation. Previous studies have shown increased production of TNF-α, IL-1β and IL-6 as well as COX-2 induction by PCLS after in vitro stimulation with LPS.Citation2,7 In addition to these proteins, we also detected increases in IL-8, IL-10 and MIP-1β production. Unlike Switalla et al., we did not detect IL-12p40 or IFN-γ production in culture supernatants, potentially due to a different duration of LPS stimulation (18 vs. 24 hours).Citation18
We then explored if PCLS in culture could specifically respond to common antigen stimulation. It has already been shown that human lung slices exposed to live influenza virus induce a strong pro-inflammatory cytokine and chemokine response.Citation15 This response included induction of IL-6 and IFN-γ, monocyte chemokines MIP-1α/β and MCP-1, the neutrophil chemokine IL-8 and the lymphocyte chemokine IP-1.Citation15 Immunohistochemistry data showed that macrophages and alveolar epithelial cells were responsible for production of these soluble mediators. To assess if this would apply to a vaccine antigen, we incubated PCLS with a seasonal influenza vaccine and tetanus toxin. Results showed that a robust response dominated by IFN-γ production as well as TNF-α and IL-2 was detected in these PCLS. The influenza vaccine induced a stronger cytokine response compare with the tetanus toxin, which may be due to the fact that influenza is a respiratory pathogen whereas tetanus is not. However, the cell populations in our PCLS model responsible for the production of cytokines in response to stimuli like LPS and the seasonal influenza vaccine still need to be identified. Knowing the cell populations involved in these responses would be useful in understanding local host-pathogen immune responses in the lung and critical in evaluating if this model can be used to characterize adaptive as well as innate immune responses.
As PCLS represent a promising method to evaluate local toxicity in the lung, steps have been taken to validate the PCLS technique.Citation26 However, the use of this model in evaluating immune responses requires overcoming some obstacles such as the lack of cell trafficking within the slices. There also appears to be a general decrease in cytokine production during cultivation indicating a change in the cell population or cell function within PCLS over time in culture. One potential way to overcome this physiological change would be to explore the use of cryopreservation as described in Rosner et al.Citation27 Their results showed minimal cell death, mainly along the edges, but also some reduction in the metabolic activity of the tissue following a freeze-thaw cycle. However, further optimization of such a cryopreservation technique could help to practically address the short window of viability of PCLS in culture. It would also allow multiple assays to use the same donor material, which could reduce variability in results and assist with establishing trends within the data sets. As lungs are a common entry point for infections, lung slices are being used by others to study host-pathogen interactions, such as influenzaCitation15 and respiratory syncytial virus.Citation28 Data presented here indicate the possibility a studying recall responses from immune donors, which may contribute to improved vaccine design and efficacy.
Materials and methods
Human donors
Human lungs were obtained through the organ and tissue procurement program of the National Disease Research Interchange (NDRI). Lungs of transplant donors that were not deemed suitable for transplantation were received from within the United States. Lungs were flushed and stored in preservation solution UW (University of Wisconsin solution) or HTK (Histidine-tryptophan-ketoglutarate) and express delivered using a courier service. Lungs were kept in preservation solution on ice for the entire transit (average 13.8 hour). Donors in this study were diverse regarding age, gender, ethnicity, medical history and cause of death.
Culture medium and reagents
Dulbecco's Modified Eagle's Medium (DMEM) Nutrient Mixture F-12 Ham with L-glutamine, 15 mM HEPES without phenol red, pH 7.2–7.4 was supplied by Sigma Aldrich (St. Louis, MO). It was supplemented with 7.5% w/v sodium bicarbonate, 100 U/ml penicillin, and 100 µg/ml streptomycin, but no fetal bovine serum. The medium was also supplemented with antibiotic-antimycotic solution (Sigma Aldrich), gentamicin (Gibco, Life Technologies, Grand Island, NY), fungizone (Gibco), and ITS-X (Gibco).
Preparation and cultivation of PCLS
The selected lobe of the donor lung was isolated and inflated with 1.5% low-gelling agarose (Sigma Aldrich) in culture medium. Agarose-inflated lobes were placed on ice for 30 minutes to allow agarose to solidify and then cut into 1-cm thick slices. Tissue cores were prepared out of 1-cm thick slices using an 8-mm coring tool (Alabama Research and Development, Munford, AL). Cores were sliced at approximately 600-μm thickness using a Krumdieck tissue slicer MD6000 (Alabama Research and Development) containing cold phosphate buffered saline (PBS). Tissue slices were collected from the slicer and placed into 10-cm diameter sterile Petri dishes with culture medium. The medium was changed once to remove remaining agarose before overnight incubation under tissue culture conditions (37°C, 5% CO2 and 100% humidity). Next day, the slices were placed into 24-well tissue culture plates, one slice per well, in 1 ml of culture medium, and maintained under tissue culture conditions. Culture medium was changed daily.
Measuring LDH activity
Levels of LDH activity in PCLS were determined in culture supernatants using an assay kit obtained from Clontech (Mountain View, CA), according to the manufacturer's instructions. Results are presented as % viability using PCLS in culture medium treated with 1% Triton X-100 as a 100% cell death control (maximum LDH release). The results were compared using ANOVA and the Kruskal-Wallis test (GraphPad Prism 6). Differences were considered statistically significant at a level of p ≤ 0.05.
WST-1 reduction
The metabolic activity of PCLS was determined using the WST-1 reagent obtained from Clontech (Mountain View, CA). Medium was removed from PCLS in culture and replaced with a freshly prepared WST-1 solution (diluted 1:10 in culture medium). After incubating for 1 hour at 37°C, the absorbance of the formazan solution in supernatants was measured in a spectrophotometer at 450 nm. The results were compared using ANOVA and the Kruskal-Wallis test (GraphPad Prism 6). Differences were considered statistically significant at a level of p ≤ 0.05.
Incubation of PCLS with LPS and Triton X-100
At different days in culture, PCLS were stimulated with 0, 10 or 100 ng/mL LPS (Sigma-Aldrich, St. Louis, MO) under tissue culture conditions for 18 hours. As a positive control for cytotoxicity, PCLS were treated with 0.25–0.3 mM Triton X-100 for 1 hour, washed 3 times and incubated in culture medium for further 18 hours under tissue culture conditions. Following incubation, LDH and WST-1 assays were performed and calcein AM/EthD-1 staining was conducted. In addition, PCLS lysates were prepared for COX-2 detection and culture supernatants were collected for measurement of select cytokine and chemokine levels. The results were compared using ANOVA and the Kruskal-Wallis test (GraphPad Prism 6). Differences were considered statistically significant at a level of p ≤ 0.05.
Calcein AM/EthD-1 staining
Calcein AM and EthD-1 reagents were purchased from Invitrogen (Carlsbad, CA). PCLS were placed in 48-well tissue culture plates with 300 μL DMEM, and then washed once with 500 μL DMEM. Calcein AM and EthD-1 reagents were allowed to equilibrate to room temperature, and then 150 μL DMEM containing 4 μM calcein AM and 4 μM EthD-1 were added to PCLS followed by incubation for 45–60 minutes at 37°C. PCLS were subsequently washed once with DMEM and placed into chambered cover glasses with 200 µl DMEM. Analysis was performed using a confocal laser scanning microscope Meta 510 (Zeiss, Jena, Germany). From each treated slice, random duplicates or triplicates from 30-µm thick 3D stacks were recorded (10X objective, excitation wavelengths 488 nm and 543 nm, emission filters LP 560 nm and BP 505–550 nm).
H&E staining
PCLS were fixed in 10% formalin and then processed and embedded in paraffin by the Comparative Pathology Laboratory of the University of Delaware. Thin sections (6 μm thickness) were cut onto glass slides and stained with hematoxylin, washed in water, differentiated in 70% alcohol containing HCl, washed again in water, and stained with 0.5% eosin. Sections were washed in 1% acetic acid, dehydrated, cleared, and mounted for light microscopy.
Cytokine and chemokine measurements
Levels of pro-inflammatory cytokines and chemokines were measured in culture supernatants from PCLS stimulated with 100 ng/mL LPS. More specifically, PCLS were treated on days 2 or 3, 7 and 12 or 13 of cultivation with LPS for 18 hours. Culture supernatants were then collected and frozen at −80°C. Cytokine levels in culture supernatants were measured using the Meso Scale Discovery (MSD) technology (Meso Scale Diagnostics, Rockville, MD). Assays were performed according to the manufacturer's recommendations using the MSD Multi-Spot assay (10-spot for IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-13, IFN-γ and TNF-α) or Multi-Array assay (small spot for IL-6, IL-8, and MIP-1β) system. Cytokines and chemokines were quantified using calibrators provided by MSD. Plates were read using a multiplex reader, SECTOR Imager 2400A (MSD). Data were analyzed using the MSD Discovery Workbench software. Values in the table represent the average pg/mL or ng/mL ± standard deviation (SD) of 4 PCLS from 3 different donor lungs.
To assess potential recall responses, PCLS were treated on day 2 of cultivation with either LPS (10 ng/mL), PMA (2 µg/mL) with ionomycin (I, 2 µg/mL), the 2010–2011 seasonal influenza vaccine (Fluzone®; BEI Resources, Manassas, VA; used at 1.8 µg/mL) or tetanus toxoid (List Biologicals Inc., Campbell, California; used at 5 µg/mL). After 24 hours of incubation at 37°C, supernatants were collected and assayed for pro-inflammatory cytokines using the MSD Multi-Spot assay system. Cytokines were quantified using calibrators provided by MSD. Plates were read using a multiplex reader, SECTOR Imager 2400A (MSD). Data were analyzed using the MSD Discovery Workbench software. Values in the table represent the average pg/mL ± SD of PCLS from 4 different donor lungs normalized to a medium only control sample.
COX-2 measurement
Levels of COX-2 expression in lysates of PCLS treated with 100 ng/mL LPS were determined. More specifically, PCLS were treated on days 2 or 3, 7, and 12 or 13 of cultivation with LPS for 18 hours. After incubation, 4 slices from each condition were placed into a 1.5-ml Eppendorf tube. Lysis buffer (150 mM NaCl, 20 mM Tris, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, Protease Inhibitor Cocktail [Sigma Aldrich] 1:100; pH 7.5) was added to each tube along with 4 zirconium beads and slices were homogenized using a bullet blender for 5 minutes at 4°C. The lysates were then centrifuged for 15 minutes at 14,000 rpm at 4°C and supernatants were removed and frozen at −80°C. COX-2 concentration in the lysates was measured by a sandwich immunoassay based on the use of a COX-2 antibody pair (R&D Systems, Minneapolis, MN) and MSD standard black plates. Plates were coated with 0.5 µg/mL of the COX-2 capture antibody and detected using 1 μg/mL of a biotinylated anti-COX-2 detection antibody and 1 µg/mL of SULFO-TAG-Streptavidin. The assay was performed according to the manufacturer's recommendation (MSD). Plates were read using a multiplex reader SECTOR Imager 2400A (MSD) and data were analyzed using the MSD Discovery Workbench software. Lysates were measured in triplicate and data are shown as the average pg/mL COX-2 ± SD from 3 different donor lungs.
Abbreviations
| COX-2 | = | cyclooxygenase-2 |
| DMEM | = | Dulbecco's Modified Eagle Medium |
| Ethd-1 | = | ethidium homodimer-1 |
| H&E | = | hematoxylin and eosin |
| HEPES | = | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HTK | = | histidine-tryptophan-ketoglutarate |
| I | = | ionomycin |
| LDH | = | lactate dehydrogenase |
| LPS | = | lipopolysaccharide |
| MSD | = | Meso Scale Discovery |
| NDRI | = | National Disease Research Interchange |
| OD | = | optical density |
| PBS | = | phosphate-buffered saline |
| PCLS | = | precision-cut lung slices |
| PMA | = | phorbol-12-myristate-13-acetate |
| UW | = | University of Wisconsin solution |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
The authors would like to thank Dr. Natasha Kushnir (Fraunhofer USA - Center for Molecular Biotechnology) for editorial assistance.
References
- Henjakovic M, Martin C, Hoymann HG, Sewald K, Ressmeyer AR, Dassow C, Pohlmann G, Krug N, Uhlig S, Braun A. Ex vivo lung function measurements in precision-cut lung slices (PCLS) from chemical allergen-sensitized mice represent a suitable alternative to in vivo studies. Toxicol Sci 2008; 106:444-53; PMID:18775882; http://dx.doi.org/10.1093/toxsci/kfn178
- Martin C, Uhlig S, Ullrich V. Cytokine-induced bronchoconstriction in precision-cut lung slices is dependent upon cyclooxygenase-2 and thromboxane receptor activation. Am J Respir Cell Mol Biol 2001; 24:139-45; PMID:11159047; http://dx.doi.org/10.1165/ajrcmb.24.2.3545
- Ressmeyer AR, Larsson AK, Vollmer E, Dahlen SE, Uhlig S, Martin C. Characterisation of guinea pig precision-cut lung slices: comparison with human tissues. Eur Respir J 2006; 28:603-11; PMID:16737991; http://dx.doi.org/10.1183/09031936.06.00004206
- Schleputz M, Rieg AD, Seehase S, Spillner J, Perez-Bouza A, Braunschweig T, Schroeder T, Bernau M, Lambermont V, Schlumbohm C, et al. Neurally mediated airway constriction in human and other species: a comparative study using precision-cut lung slices (PCLS). PLoS One 2012; 7:e47344; PMID:23056631; http://dx.doi.org/10.1371/journal.pone.0047344
- Seehase S, Lauenstein HD, Schlumbohm C, Switalla S, Neuhaus V, Forster C, Fieguth HG, Pfennig O, Fuchs E, Kaup FJ, et al. LPS-induced lung inflammation in marmoset monkeys - an acute model for anti-inflammatory drug testing. PLoS One 2012; 7:e43709; PMID:22952743; http://dx.doi.org/10.1371/journal.pone.0043709
- Banerjee A, Trivedi CM, Damera G, Jiang M, Jester W, Hoshi T, Epstein JA, Panettieri RA, Jr. Trichostatin A abrogates airway constriction, but not inflammation, in murine and human asthma models. Am J Respir Cell Mol Biol 2012; 46:132-8; PMID:22298527; http://dx.doi.org/10.1165/rcmb.2010-0276OC
- Henjakovic M, Sewald K, Switalla S, Kaiser D, Muller M, Veres TZ, Martin C, Uhlig S, Krug N, Braun A. Ex vivo testing of immune responses in precision-cut lung slices. Toxicol Appl Pharmacol 2008; 231:68-76; PMID:18504053; http://dx.doi.org/10.1016/j.taap.2008.04.003
- Seehase S, Schleputz M, Switalla S, Matz-Rensing K, Kaup FJ, Zoller M, Schlumbohm C, Fuchs E, Lauenstein HD, Winkler C, et al. Bronchoconstriction in nonhuman primates: a species comparison. J Appl Physiol (1985) 2011; 111:791-8; http://dx.doi.org/10.1152/japplphysiol.00162.2011
- Rieg AD, Rossaint R, Uhlig S, Martin C. Cardiovascular agents affect the tone of pulmonary arteries and veins in precision-cut lung slices. PLoS One 2011; 6:e29698; PMID:22216346; http://dx.doi.org/10.1371/journal.pone.0029698
- Rieg AD, Suleiman S, Perez-Bouza A, Braunschweig T, Spillner JW, Schroder T, Verjans E, Schalte G, Rossaint R, Uhlig S, et al. Milrinone relaxes pulmonary veins in guinea pigs and humans. PLoS One 2014; 9:e87685; PMID:24498166; http://dx.doi.org/10.1371/journal.pone.0087685
- Cooper PR, Zhang J, Damera G, Hoshi T, Zopf DA, Panettieri RA, Jr. C-027 inhibits IgE-mediated passive sensitization bronchoconstriction and acts as a histamine and serotonin antagonist in human airways. Allergy Asthma Proc 2011; 32:359-65; PMID:22195688; http://dx.doi.org/10.2500/aap.2011.32.3460
- Lauenstein L, Switalla S, Prenzler F, Seehase S, Pfennig O, Forster C, Fieguth H, Braun A, Sewald K. Assessment of immunotoxicity induced by chemicals in human precision-cut lung slices (PCLS). Toxicol In Vitro 2014; 28:588-99; PMID:24412833; http://dx.doi.org/10.1016/j.tiv.2013.12.016
- Nassimi M, Schleh C, Lauenstein HD, Hussein R, Hoymann HG, Koch W, Pohlmann G, Krug N, Sewald K, Rittinghausen S, et al. A toxicological evaluation of inhaled solid lipid nanoparticles used as a potential drug delivery system for the lung. Eur J Pharm Biopharm 2010; 75:107-16; PMID:20206256; http://dx.doi.org/10.1016/j.ejpb.2010.02.014
- Paranjpe M, Neuhaus V, Finke JH, Richter C, Gothsch T, Kwade A, Buttgenbach S, Braun A, Muller-Goymann CC. In vitro and ex vivo toxicological testing of sildenafil-loaded solid lipid nanoparticles. Inhal Toxicol 2013; 25:536-43; PMID:23905970; http://dx.doi.org/10.3109/08958378.2013.810315
- Wu W, Booth JL, Duggan ES, Wu S, Patel KB, Coggeshall KM, Metcalf JP. Innate immune response to H3N2 and H1N1 influenza virus infection in a human lung organ culture model. Virology 2010; 396:178-88; PMID:19913271; http://dx.doi.org/10.1016/j.virol.2009.10.016
- Cooper PR, Lamb R, Day ND, Branigan PJ, Kajekar R, San Mateo L, Hornby PJ, Panettieri RA, Jr. TLR3 activation stimulates cytokine secretion without altering agonist-induced human small airway contraction or relaxation. Am J Physiol Lung Cell Mol Physiol 2009; 297:L530-7; PMID:19542247; http://dx.doi.org/10.1152/ajplung.00133.2009
- Neuhaus V, Schwarz K, Klee A, Seehase S, Forster C, Pfennig O, Jonigk D, Fieguth HG, Koch W, Warnecke G, et al. Functional testing of an inhalable nanoparticle based influenza vaccine using a human precision cut lung slice technique. PLoS One 2013; 8:e71728.
- Switalla S, Lauenstein L, Prenzler F, Knothe S, Forster C, Fieguth HG, Pfennig O, Schaumann F, Martin C, Guzman CA, et al. Natural innate cytokine response to immunomodulators and adjuvants in human precision-cut lung slices. Toxicol Appl Pharmacol 2010; 246:107-15; PMID:20434477; http://dx.doi.org/10.1016/j.taap.2010.04.010
- Liberati TA, Randle MR, Toth LA. In vitro lung slices: a powerful approach for assessment of lung pathophysiology. Expert Rev Mol Diagn 2010; 10:501-8; PMID:20465504; http://dx.doi.org/10.1586/erm.10.21
- Sanderson MJ. Exploring lung physiology in health and disease with lung slices. Pulm Pharmacol Ther 2011; 24:452-65; PMID:21600999; http://dx.doi.org/10.1016/j.pupt.2011.05.001
- Umachandran M, Ioannides C. Stability of cytochromes P450 and phase II conjugation systems in precision-cut rat lung slices cultured up to 72 h. Toxicology 2006; 224:14-21; PMID:16701934; http://dx.doi.org/10.1016/j.tox.2006.03.020
- Martin TR, Mathison JC, Tobias PS, Leturcq DJ, Moriarty AM, Maunder RJ, Ulevitch RJ. Lipopolysaccharide binding protein enhances the responsiveness of alveolar macrophages to bacterial lipopolysaccharide. Implications for cytokine production in normal and injured lungs. J Clin Invest 1992; 90:2209-19.
- Vernooy JH, Dentener MA, van Suylen RJ, Buurman WA, Wouters EF. Intratracheal instillation of lipopolysaccharide in mice induces apoptosis in bronchial epithelial cells: no role for tumor necrosis factor-alpha and infiltrating neutrophils. Am J Respir Cell Mol Biol 2001; 24:569-76; PMID:11350826; http://dx.doi.org/10.1165/ajrcmb.24.5.4156
- Wang HL, Akinci IO, Baker CM, Urich D, Bellmeyer A, Jain M, Chandel NS, Mutlu GM, Budinger GR. The intrinsic apoptotic pathway is required for lipopolysaccharide-induced lung endothelial cell death. J Immunol 2007; 179:1834-41; PMID:17641050; http://dx.doi.org/10.4049/jimmunol.179.3.1834
- Bingisser R, Stey C, Weller M, Groscurth P, Russi E, Frei K. Apoptosis in human alveolar macrophages is induced by endotoxin and is modulated by cytokines. Am J Respir Cell Mol Biol 1996; 15:64-70; PMID:8679223; http://dx.doi.org/10.1165/ajrcmb.15.1.8679223
- Hess A, Wang-Lauenstein L, Braun A, Kolle SN, Landsiedel R, Liebsch M, Ma-Hock L, Pirow R, Schneider X, Steinfath M, et al. Prevalidation of the ex-vivo model PCLS for prediction of respiratory toxicity. Toxicol In Vitro 2016; 32:347-61; PMID:26778741; http://dx.doi.org/10.1016/j.tiv.2016.01.006
- Rosner SR, Ram-Mohan S, Paez-Cortez JR, Lavoie TL, Dowell ML, Yuan L, Ai X, Fine A, Aird WC, Solway J, et al. Airway contractility in the precision-cut lung slice after cryopreservation. Am J Respir Cell Mol Biol 2014; 50:876-81; PMID:24313705; http://dx.doi.org/10.1165/rcmb.2013-0166MA
- Ebsen M, Mogilevski G, Anhenn O, Maiworm V, Theegarten D, Schwarze J, Morgenroth K. Infection of murine precision cut lung slices (PCLS) with respiratory syncytial virus (RSV) and chlamydophila pneumoniae using the Krumdieck technique. Pathol Res Pract 2002; 198:747-53; PMID:12530578; http://dx.doi.org/10.1078/0344-0338-00331
